Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Regioselective catalytic acetoxylation of limonene
Marc
von Czapiewski
and
Michael A. R.
Meier
*
Laboratory of Applied Chemistry, Institute of Organic Chemistry, Karlsruhe Institute of Technology (KIT), Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany. E-mail: m.a.r.meier@kit.edu; Web: http://www.meier-michael.com
First published on 30th April 2014
Abstract
Two efficient strategies for a direct catalytic and regioselective acetoxylation of terpenes are described. Acetoxylated limonene derivatives were synthesized via palladium-catalyzed C–H activation utilizing para-benzoquinone (BQ) as reoxdidation agent and acetic acid as solvent and reactant. Addition of dimethyl sulfoxide (DMSO) to the catalytic system led to highly selective functionalization of the exocyclic double bond of limonene. This catalytic acetoxylation of limonene was further optimized with regard to a more sustainable and environmentally-friendly procedure. On the other hand, the use of an aerobic tandem catalytic system using iron(II) phthalocyanine (Fe(Pc)) as co-catalyst, which acts as electron transfer mediator (ETM), enabled a highly selective acetoxylation of the endocyclic double bond of limonene with high conversions. Moreover, diacetoxylated products were prepared by a reaction sequence applying the aforementioned catalytic systems.
Introduction
The development of sustainable processes using renewable feedstocks is an important and urgent challenge, not only due to the depletion of fossil resources.1 With an increasing demand of fossil-derived raw materials the price of crude oil will increase dramatically, thus promoting the search for suitable alternatives.2 Renewable raw materials (e.g. starch, fats and oils, cellulose, and others), represent a valuable alternative to fossil resources. Already today, nine million tons of renewable raw material derived products are used within the European industry per year, demonstrating their remarkable potential.3 Among the available renewable materials, terpenes constitute a valuable and cheap biomass resource, which are available in large scales from various essential oils or as by-product from diverse industrial processes.4–6 Terpenes are secondary metabolites synthesized by plants, fungi and microorganisms. Furthermore, terpenes are of great interest for humans, due to their versatile application possibilities, e.g. as flavors, fragrances, nutrients, pheromones or as pharmacological substrates.7–9 The remarkable structural diversity of terpenes, containing cycloaliphatic, aromatic or diene moieties offers versatile functionalization possibilities.10 Thus, in order to synthesize diverse value added terpene compounds, the development of efficient and catalytic derivatization processes are of great interest. Especially, a selective oxidation of terpenes is highly desirable from an economic and ecologic point of view, since these terpene derivatives are used in a wide range of applications. Wilson and Shaw reported the synthesis of oxygenated limonene derivates using a selenium dioxide/hydrogen peroxide system. The resulting products bear hydroxy- and epoxy functional groups, which are obtained by oxidation at the corresponding allylic position or double bond.11 Da Silva et al. reported an oxyfunctionalization protocol for the autoxidation of limonene using acetic acid or acetonitrile as solvent and a cobalt(II) chloride catalyst. Within this catalytic procedure, the respective internal epoxidized limonene, carvone and carveolis were obtained in moderate yields after four hours at 60 °C.12 Another interesting approach for the oxyfunctionalization of limonene is realized by a tandem catalytic system using palladium(II) chloride/copper(II) chloride in the presence of lithium chloride and acetic acid. Gusevskaya and Gonsalves described an efficient catalytic process that enabled the preparation of trans-carveyl acetate with high conversion of 87% after four hours at 80 °C. However, small amounts of different oxidized and isomerized products were formed as well.13 Firdoussi et al. reported the synthesis of regioselectively acetoxylated limonene derivates under mild reaction conditions and long reaction times of 48 hours. By using a tandem catalytic system consisting of palladium(II) chloride/copper(II) chloride and sodium acetate in acetic acid as solvent, high conversion of 90% were obtained. However, due to allylic acetoxylation at the endocyclic double bond, three different products were formed. The same group also described a catalytic system making use of palladium(II) acetate as catalyst and stoichiometric amounts of p-benzoquinone as reoxidation agent.14 Under mild reaction conditions of 20 °C, two internally acetoxylated products were formed in good conversion of 80%. Unfortunately, long reaction times (72 h) were required to obtain the corresponding products.15 White and colleagues reported the synthesis of acetoxylated α-olefins by a highly selective allylic C–H oxidation process. The remarkable selectivity of this catalytic procedure is accomplished by a sulfoxide-promoted oxidation using palladium(II) acetate as catalyst and p-benzoquinone. Depending on the employed sulfoxide agent, linear or branched acetoxylated products were obtained. The aforementioned catalytic systems usually require stoichiometric amounts of an oxidizing agent (e.g. p-benzoquinone) to facilitate the regeneration of palladium(II) species and to avoid the precipitation of the catalyst. Interestingly, Piera and Bäckvall recently published an oxidation protocol for the aerobic 1,4-diacetoxylation of 1,3 dienes by a tandem catalytic system that involved an electron transfer mediator (ETM), which allowed for an efficient oxidation reaction. One major advantage of such an aerobic tandem procedure is the use of catalytic amounts of the oxidizing agent, e.g. p-benzoquinone, which is regenerated during the catalytic process by an iron(II) phthalocyanine co-catalyst (ETM).16,17 Thus, it would be highly interesting to make use of such an oxidation protocol to achieve an efficient and selective functionalization of terpene substrates. Therefore, in order to allow an efficient and regioselective functionalization of limonene, we report two oxidation methods: the palladium-catalyzed C–H activation process using dimethyl sulfoxide and an aerobic tandem catalytic system using an ETM. Moreover, the two catalytic procedures were used in a sequential reaction procedure to synthesize diacetoxylated limonene derivatives. The thus prepared limonene derivatives are valuable precursors for step-growth polymerizations. Additionally, these limonene derivatives are valuable precursors for organic synthesis, i.e. for transition metal catalyzed allylic substitution reactions.Experimental section
Materials
(S)-(−)-Limonene (96%, Sigma Aldrich), palladium(II) acetate (98%, Sigma Aldrich), iron(II) phthalocyanine (dye content ~ 90%, Sigma Aldrich), hydroquinone (99%, Sigma Aldrich), p-benzoquinone (>98%, Sigma Aldrich), acetic acid (>96%, Roth), dimethyl sulfoxide (DMSO, >99, 5%, Roth), sodium acetate (anhydrous, Sigma Aldrich), sodium sulfate (>99% anhydrous, Acros Organics), tetrahydrothiophene (99%, Sigma Aldrich), α,α,α-trifluorotoluene (>99%, Sigma Aldrich),sodium hydrogen carbonate (>95%, Sigma Aldrich), tetradecane (>99%, Sigma Aldrich), silica gel 60 (0.040–0.063, Sigma Aldrich), TLC silica gel F254 (Merck), chloroform-d (CDCl3, 99.8 atom % D, Euroiso-top), potassium permanganate (>99%, Sigma Aldrich), potassium carbonate (99.7%, Sigma Aldrich), sodium hydroxide (98%, Sigma Aldrich), molecular sieve (3Å, Sigma Aldrich), acetonitrile, dimethylformamide, dimethylacetamine, n-hexane, ethyl acetate. All solvents were used without further purification.General procedure for the synthesis of acetoxylated products via palladium-catalyzed process using DMSO and acetic acid
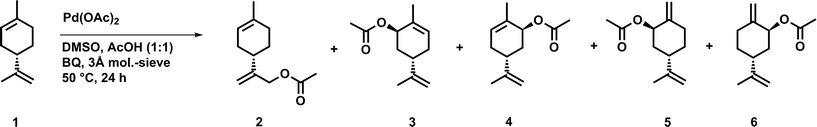
0.20 g (S)-(−)-limonene 1 (1.47 mmol) were dissolved in 1.5 mL DMSO and 1.5 mL acetic acid. Additionally, 0.16 g p-benzoquinone (1.47 mmol, 1.0 eq.), 0.25 g molecular sieves (3 Å), and 29.0 mg n-tetradecane (0.15 mmol, 10.0 mol%) as internal standard were added and the mixture was stirred for five minutes at 50 °C. Then, 13.2 mg palladium(II) acetate (0.06 mmol, 4.0 mol%) was added and the mixture was stirred for 24 hours at 50 °C. The crude reaction mixture was washed with water, sodium hydrogen carbonate and brine, dried over sodium sulfate and evaporated to dryness. Afterwards, the crude product was purified by column chromatography (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford an orange-yellow liquid. The product was obtained as a mixture of the major product 2 and small amounts of the by-products (enantiomers 3 and 4 as well as 5 and 6).
5) to afford an orange-yellow liquid. The product was obtained as a mixture of the major product 2 and small amounts of the by-products (enantiomers 3 and 4 as well as 5 and 6).
General procedure for the synthesis of acetoxylated products via aerobic tandem catalytic system
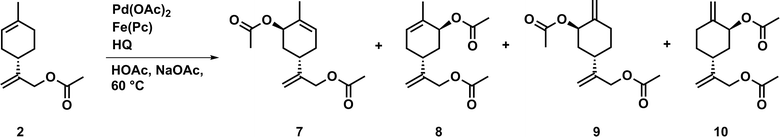
0.20 g (S)-(−)-limonene 1 (1.47 mmol), 41.7 mg iron(II) phthalocyanine (0.07 mmol, 5.0 mol%), 32.3 mg hydroquinone (0.29 mmol, 20 mol%), 60.2 mg sodium acetate (0.74 mmol, 0.5 eq.), and 29.0 mg n-tetradecane (0.15 mmol, 10.0 mol%) as internal standard were dissolved in 3.0 mL acetic acid and stirred for five minutes at 60 °C. Subsequently, 16.5 mg palladium(II) acetate (0.07 mmol, 5.0 mol%) was added and the mixture was stirred for 24 hours at 60 °C. The crude reaction mixture was washed with water, sodium hydrogen carbonate, and brine, dried over sodium sulfate and evaporated to dryness. Afterwards, the crude product was purified by column chromatography (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford a slightly yellow liquid. The product was obtained as a mixture of the major products of enantiomers 3 and 4 as well as 5 and 6 and a small amount of by-product 2.
5) to afford a slightly yellow liquid. The product was obtained as a mixture of the major products of enantiomers 3 and 4 as well as 5 and 6 and a small amount of by-product 2.
General procedure for the synthesis of diacetoxylatedproducts
0.20 g 2 (1.03 mmol, 1.0 eq.), 29.3 mg iron(II) phthalocyanine (0.05 mmol, 5.0 mol%), 22.7 mg hydroquinone (0.21 mmol, 20 mol%), 42.2 mg sodium acetate (0.52 mmol, 0.5 eq.) were dissolved in 3.0 mL acetic acid and stirred for five minutes at 60 °C. Subsequently, 11.6 mg palladium(II) acetate (0.05 mmol, 5.0 mol%) was added and the mixture was stirred for 5 days at 60 °C. The crude reaction mixture was washed with water, sodium hydrogen carbonate, and brine, dried over sodium sulfate and evaporated to dryness. Afterwards, the crude product was purified by column chromatography (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford a yellow liquid in 37% yield (95.0 mg). The obtained product was isolated as a mixture of enantiomers 7 and 8 as well as 9 and 10.
5) to afford a yellow liquid in 37% yield (95.0 mg). The obtained product was isolated as a mixture of enantiomers 7 and 8 as well as 9 and 10.
(S)-2-(4-Methylcyclohex-3-en-1-yl)allyl acetate 2. Orange-yellow liquid, yield (148 mg, 52%); Rf: = 0.35 (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5);
5);
1H-NMR: (600 MHz, CDCl3) δ/ppm 5.41–5.28 (m, 1H, CH), 5.05 (s, b, 1H, CH2), 4.97 (s, b, 1H, CH2), 4.58 (s, b, 2H, CH2), 2.28–2.12 (m, 1H, CH), 2.13–1.98 (m, 2H, CH2), 2.12–1.88 (m, 2H, CH2), 2.09 (s, 3H, CH3), 1.96–1.82 (m, 1H, CH2), 1.65 (s, 3H, CH3), 1.53–1.46 (m, 1H, CH2).
13CNMR: (75 MHz, CDCl3) δ/ppm 170.9, 148.4,.133.9, 120.4, 111.1, 66.3, 37.2, 31.2, 30.5, 28.0, 23.5, 21.1.
SEC/ESI-MS of [C12H18O2Na+]: calculated: 217.12, found: 217.08.
IR (ATR platinum diamond): 2916.1, 1736.6, 1646.3, 1436.7, 1370.8, 1220.5, 1149.6, 1097.1, 1022.8, 967.2, 912.3, 797.4, 759.1, 604.0, 523.1, 429.8 (Fig. 1).
Mixture of enantiomers (1R, 5S)-2-methyl-5-(pro-1-en-2-yl-)cyclohex-2-en-1-yl acetate 3 and (1S, 5R)-2-methyl-5-(pro-1-en-2-yl-)cyclohex-2-en-1-yl acetate 4. Light yellow liquid, yield (154 mg, 64%); Rf: = 0.40 (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5);
5);
1H-NMR: (600 MHz, CDCl3) δ/ppm 5.75–5.72 (m, 1H, CH), 5.26 (t, J = 3.1 Hz, 1H, CH), 4.97 (t, J = 1.7 Hz, 1H, CH2), 4.87 (t, J = 1.8 Hz, 1H, CH2), 2.35–2.29 (m, 1H, CH), 2.24–2.20 (m, 1H, CH2), 2.08 (s, 3H, CH3), 1.97–1.93 (m, 1H, CH2), 1.90–1.84 (m, 1H, CH2), 1.73 (s, 3H, CH3), 1.70–1.68 (m, 3H, CH3), 1.67–1.64 (m, 1H, CH2).
13CNMR: (75 MHz, CDCl3) δ/ppm 171.0, 148.9, 131.0, 128.0, 112.6, 70.8, 35.9, 33.8, 31.0, 21.5, 20.9, 20.8.
SEC/ESI-MS of [C12H18O2Na+]: calculated: 217.12, found: 217.08.
IR (ATR platinum diamond): 2935.4, 1733.2, 1643.7, 1437.8, 1368.0, 1232.2, 1149.9, 1073.6, 1045.4, 1016.1, 965.5, 950.6, 911.1, 887.8, 807.6, 673.6, 609.2, 538.0, 469.4, 437.0 (Fig. 1).
Mixture of enantiomers (1R, 5S)-2-methylene-5-(prop-1-en-2-yl)cyclohexyl acetate 5 and (1S, 5R)-2-methylene-5-(prop-1-en-2-yl)cyclohexyl acetate 6. Light yellow liquid, yield (154 mg, 54%); Rf: = 0.40 (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5);
5);
1H-NMR: (600 MHz, CDCl3) δ/ppm 5.41 (t, J = 3.1 Hz, 1H, CH) 4.76–4.74 (m, 1H, CH2), 4.73–4.71 (m, 1H, CH2), 4.71–4.69 (m, 2H, CH2), 2.45–2.39 (m, 1H, CH), 2.39–2.35 (m, 1H, CH2), 2.29–2.26 (m, 1H, CH2), 2.06 (s, 3H, CH3), 2.05–2.00 (m, 1H, CH2), 1.90–1.84 (m, 1H, CH2), 1.71 (s, 3H, CH3), 1.57–1.51 (m, 1H, CH2), 1.35–1.26 (m, 1H, CH2).
13CNMR: (75 MHz, CDCl3) δ/ppm 170.3, 149.0, 145.2, 109.3, 109.3, 74.4, 39.1, 37.0, 32.5, 30.9, 21.3, 21.0.
ESI-MS of [C12H18O2Na+]: calculated: 217.12, found: 217.08.
IR (ATR platinum diamond): 2935.4, 1733.2, 1643.7, 1437.8, 1368.0, 1232.2, 1149.9, 1073.6, 1045.4, 1016.1, 965.5, 950.6, 911.1, 887.8, 807.6, 673.6, 609.2, 538.0, 469.4, 437.0 (Fig. 1).
Mixture of enantiomers 2-((1S, 5R)-5-acetoxy-4-methylcyclohex-3-en-1-yl)allyl acetate 7 and 2-((1R, 5S)-5-acetoxy-4-methylcyclohex-3-en-1-yl)allyl acetate 8. Yellow liquid, yield (95.0 mg, 37%); Rf: = 0.21 (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5);
5);
1H-NMR: (300 MHz, CDCl3) /δ/ppm 5.75–5.68 (m, 1H, CH), 5.27–5.21 (m, 1H, CH), 5.08 (s, 1H, CH2), 4.96 (s, 1H, CH2), 4.56 (s, 2H, CH2), 2.43–2.30 (m, 1H, CH), 2.29–2.19 (m, 2H, CH2), 2.07 (s, 3H, CH3), 2.06 (s, 3H, CH3), 2.04–1.95 (m, 1H, CH2), 1.69–1.64 (m, 1H, CH2), 1.68 (s, b, 3H, CH3).
13CNMR: (75 MHz, CDCl3) δ/ppm 170.9, 170.7, 147.2, 131.2, 127.5, 112.0, 70.4, 66.2, 33.9, 32.2, 31.2, 21.4, 21.0, 20.6.
ESI-MS: calculated: 252.14; found: 252.13.
IR (ATR platinum diamond): 2935.2, 1730.2, 1649.5, 1438.0, 1368.7, 1224.6, 1155.0, 1097.4, 1025.0, 953.7, 911.3, 836.5, 807.9, 605.0, 549.4, 469.9, 437.8 (Fig. 1).
Mixture of enantiomers 2-((1S, 3R)-3-acetoxy-4-methylenecyclohexyl)allyl acetate 9 and 2-((1R, 3S)-3-acetoxy-4-methylenecyclohexyl)allyl acetate 10. Yellow liquid, yield (95.0 mg, 37%); Rf: = 0.21 (hexane/ethyl acetate 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5);
5);
1H-NMR: (300 MHz, CDCl3) δ/ppm 5.39 (t, J = 3.0 Hz, 1H, CH), 5.06 (s, 1H, CH2), 4.96 (s, 1H, CH2), 4.96 (s, 1H, CH2), 4.86 (s, b, 1H, CH2), 4.54 (s, 2H, CH2), 2.54–2.43 (m, 2H, CH2), 2.08 (s, 3H, CH3), 2.04 (s, 3H, CH3), 1.94–1.82 (m, 2H, CH2), 1.78–1.69 (m, 1H, CH), 1.62–1.51 (m, 1H, CH2), 1.40–1.24 (m, 1H, CH2).
13CNMR:(75 MHz, CDCl3) δ/ppm 170.8, 170.2, 147.3, 144.8, 112.9, 111.8, 74.1, 66.1, 37.3, 35.4, 32.6, 30.8, 21.5, 20.8.
ESI-MS: calculated: 252.14; found: 252.13.
IR (ATR platinum diamond): 2935.2, 1730.2, 1649.5, 1438.0, 1368.7, 1224.6, 1155.0, 1097.4, 1025.0, 953.7, 911.3, 836.5, 807.9, 605.0, 549.4, 469.9, 437.8 (Fig. 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using helium as carrier gas (flow rate 1.0 ml min−1).
1) using helium as carrier gas (flow rate 1.0 ml min−1).
Results and discussion
The aim of this investigation was the catalytic functionalization of terpenes in order to synthesize regioselectively acetoxylated compounds that also have the potential to be used for the synthesis of renewable platform chemicals, especially for step-growth polymerization procedures. In this context, (S)-(−)-limonene (1), which can be obtained from renewable feedstock, was explored towards its reactivity in two different catalytic oxidations procedures.Acetoxylation at the exocyclic double bond of (S)-(−)-limonene
At first, a palladium-catalyzed C–H activation process using DMSO in the presence of p-benzoquinone (BQ) as reoxidation agent in acetic acid as solvent was investigated. The reaction was optimized with regard to an acetoxylation of the exocyclic double bond of limonene (Fig. 1).The first catalytic system, which was introduced by White and co-workers and highly promotes the allylic oxidation of α-olefins,18 was transferred to (S)-(−)-limonene. The procedure uses palladium(II) acetate/BQ and a solvent mixture of DMSO and acetic acid. One major advantage of this catalytic oxidation system is the formation of linear (E)-allylic acetates, which can be obtained in good yields and with high regio- and stereoselectivity. A similar procedure has been successfully applied to unsaturated fatty acid methyl esters (FAMEs) in order to obtain the corresponding linear allylic acetates.19 Transferring the procedure of White et al., who used 10 mol% of palladium catalyst, to (S)-(−)-limonene resulted mainly in an acetoxylation of the exocyclic double bond, which afforded the corresponding functionalized product 2. As by-products, enantiomers 3 and 4 as well as enantiomers 5 and 6 were formed due to an acetoxylation of the endocyclic double bond (Fig. 1). In this process, a new stereocenter is created by two possible attacks of the acetate (a′, a′′, Fig. 2), respectively. Since NMR cannot distinguish between 3 and 4 as well as 5 and 6, it is fair to assume and in accordance with the acetoxylation mechanism that the enantiomer pairs (3/4 or 5/6, respectively) are formed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The attack of the nucleophile only occurred as depicted in Fig. 2, explaining why only one of the two possible configurations of the new stereocenters was observed (i.e. attack from top in Fig. 2) by NMR analysis.
1 ratio. The attack of the nucleophile only occurred as depicted in Fig. 2, explaining why only one of the two possible configurations of the new stereocenters was observed (i.e. attack from top in Fig. 2) by NMR analysis.
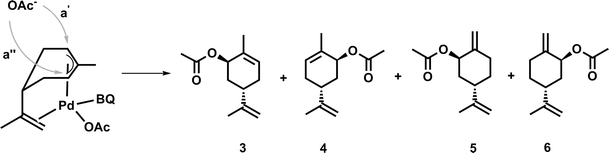 | ||
| Fig. 2 Explanation of the observed stereoselectivity in the palladium-catalyzed acetoxylation process (adopted from ref. 15). | ||
These results are also in agreement with the results reported by El Firdoussi et al., who employed (R)-(+)-limonene as substrate for palladium-catalyzed oxidation using BQ and sodium acetate. In this case, the internal acetoxylation occurred, which led to a new stereocenter. Generally, the specific formation of these enantiomers can be explained by coordination of the palladium catalyst, which coordinates to the limonene substrate via π-allyl and olefin bonding. The given stereo-configuration of the limonene substrate enabled the formation of a palladium complex, wherein the acetate can only attack from the backside (Fig. 2).15 To optimize the catalytic oxidation of 1, the amounts of palladium(II) acetate, BQ, DMSO, and acetic acid were varied to yield a maximum conversion and product selectivity. In order to minimize the catalyst loading, while still obtaining high yields of 2, a first reaction screening was performed (Table 1).
| Entry | Catalyst [mol%] | Conversiona [%] | Selectivitya [%] | ||
|---|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | |||
| Conditions: 200 mg (1.47 mmol) 1, 1.0 mL acetic acid, 1.0 mL DMSO, 2.0 eq. BQ, 250 mg 3 Å molecular sieve, 50 °C, 24 h.a Conversion and selectivity were determined by GC-MS using tetradecane as internal standard. | |||||
| 1 | 10 | 63 | 89 | 6 | 5 |
| 2 | 8 | 66 | 86 | 9 | 5 |
| 3 | 6 | 63 | 87 | 8 | 5 |
| 4 | 4 | 65 | 89 | 8 | 3 |
| 5 | 2 | 28 | 86 | 11 | 3 |
The catalyst loading was varied between 2 and 10 mol%. No significant difference, both in conversion and in product selectivity, was observed if the acetoxylation was performed with 10 to 4 mol% (Table 1). However, if the amount of catalyst was further reduced to 2 mol%, the conversion significantly decreased. Based on these results, further studies were carried out using 4 mol% of palladium(II) acetate. Since the employed oxidant (BQ) is a toxic reagent and usually used in excess, the next step was to try to reduce the required amount of BQ (Table 2).
| Entry | BQ [eq.] | Conversiona [%] | Selectivitya [%] | ||
|---|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | |||
| Conditions: 200 mg (1.47 mmol) 1, 1.0 mL acetic acid, 1.0 mL DMSO, 250 mg 3 Å molecular sieve, 4.0 mol% Pd(OAc)2, 50 °C, 24 h.a Conversion and selectivity were determined by GC-MS using tetradecane as internal standard. | |||||
| 1 | 2.0 | 64 | 89 | 6 | 5 |
| 2 | 1.5 | 63 | 83 | 9 | 8 |
| 3 | 1.0 | 60 | 85 | 10 | 5 |
| 4 | 0.5 | 44 | 86 | 11 | 3 |
| 5 | 0 | 12 | 50 | 25 | 25 |
Reducing the amount of oxidant (from two to one equivalents) has only a marginal effect on the observed conversion and selectivity. With regard to a more environmentally-friendly acetoxylation process, this optimization constitutes a remarkable improvement and clearly shows that an excess of the oxidant is not necessary. However, if the amount of oxidant was further decreased, the conversion significantly dropped. Subsequent optimization studies were performed in order to investigate the influence of the amount of solvent. According to the above mentioned reaction screenings, 4 mol% of the palladium catalyst and one equivalent of the oxidant were used (Table 3).
| Entry | AcOH [mL] | DMSO [mL] | Conversiona [%] | Selectivitya [%] | ||
|---|---|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | ||||
| Conditions: 200 mg (1.47 mmol) 1, 1.0 eq. BQ, 250 mg 3 Å molecular sieve, 4.0 mol% Pd(OAc)2, 50 °C, 24 h.a Conversion and selectivity were determined by GC-MS using tetradecane as internal standard. | ||||||
| 1 | 0.5 | 0.5 | 53 | 87 | 8 | 5 |
| 2 | 1.0 | 1.0 | 60 | 85 | 10 | 5 |
| 3 | 1.5 | 1.5 | 72 | 88 | 8 | 4 |
| 4 | 2.0 | 2.0 | 73 | 89 | 8 | 3 |
| 5 | 1.0 | 1.5 | 51 | 90 | 6 | 4 |
| 6 | 1.5 | 1.0 | 80 | 75 | 15 | 10 |
Generally, increasing the total amount of solvent (AcOH + DMSO) from 1.0 mL to 3.0 mL has a positive effect on the obtained conversion, without negatively influencing the good product selectivity (Table 3). The dilution might favor a stabilization of the active palladium species. However, if one of the solvents was used in excess, either the conversion or the product selectivity considerably decreased, suggesting that an intermediate as depicted in Fig. 2 plays indeed a key-role in this transformation. If an increased amount of DMSO was used, lower conversions were observed (Table 3, entry 5). Contrary, if an excess of acetic acid was used, the conversion was slightly improved, but the product selectivity decreased (Table 3, entry 6). As a further parameter, the reaction temperature and its impact on the conversion and product selectivity of the catalytic acetoxylation was investigated (Table 4).
| Entry | T [°C] | Conversiona [%] | Selectivitya [%] | ||
|---|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | |||
| Conditions: 200 mg (1.47 mmol) 1, 1.5 mL acetic acid, 1.5 mL DMSO, 1.0 eq. BQ, 250 mg 3 Å molecular sieve, 4.0 mol% Pd(OAc)2, 24 h.a Conversion and selectivity were determined by GC-MS using tetradecane as internal standard. | |||||
| 1 | 50 | 72 | 88 | 8 | 4 |
| 2 | 60 | 72 | 85 | 11 | 4 |
| 3 | 70 | 73 | 81 | 13 | 6 |
The obtained results revealed that increasing the temperature had no significant effect on the conversion, whereas the product selectivity slightly decreased. Therefore, performing the catalytic acetoxylation at 50 °C provided the most promising results. Applying the optimized reaction conditions to (R)-(+)-limonene yielded similar conversions as well as the same product selectivity. The remarkable selectivity, which can be achieved in the catalytic acetoxylation using DMSO as solvent, represents a highly efficient and promising procedure. Moreover, to the best of our knowledge, these results demonstrate the first direct catalytic acetoxylation (one-step) of the exocyclic double bond of limonene. Further investigations were performed with some other polar aprotic solvents in order to study their effect in the catalytic acetoxylation process (Table 5).
| Entry | Solvent [x] | Conversionc [%] | Selectivityc [%] | ||
|---|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | |||
| Conditions: 200 mg (1.47 mmol) 1, 1.5 mL acetic acid, 1.5 mL x-solvent, 1.0 eq. BQ, 250 mg 3 Å molecular sieve, 4.0 mol% Pd(OAc)2, 50 °C, 24 h.a 136 mg (1.00 mmol) 1, 2.0 ml acetic acid, 2.0 eq. BQ, 5.0 mol% THT, 5.0 mol% Pd(OAc)2, 50 °C, 24 h.b 136 mg (1.00 mmol) 1, 2.0 ml acetic acid, 2.0 eq. BQ, 5.0 mol% PhCF3, 5.0 mol% Pd(OAc)2, 50 °C, 24 h.c Conversion and selectivity were determined by GC-MS correlated using tetradecane as internal standard. | |||||
| 1 | DMA | 84 | 11 | 51 | 38 |
| 2 | DMF | 81 | 16 | 37 | 47 |
| 3 | MeCN | 30 | 47 | 32 | 21 |
| 4 | THTa | 78 | 6 | 50 | 44 |
| 5 | PhCF3b | 96 | 5 | 52 | 41 |
Interestingly, depending on the used polar and aprotic solvent, considerably different results were obtained. In reactions using DMA or DMF as solvent, the catalytic acetoxylation of 1 is highly promoted. However, mainly the functionalization at the endocyclic double bond was observed, and almost exclusively product 3 and 4 are formed. This is a very interesting result and demonstrates that the product selectivity and thus regioselectivity of this process can be inverted by simple choice of solvent. If acetonitrile was used, only a low conversion and product selectivity was observed. In a further test reaction, tetrahydrothiophene (THT) was used as additive, since Stambuli et al. described THT as highly reactive and selective ligand in acetoxylation reactions to obtain (E)-linear allylic acetates.20 Applying this catalytic process to 1, primarily the endocyclic double bond of 1 was functionalized, thus the enantiomers 3 and 4 as well as 5 and 6 were formed as major products (Table 5, entry 4). However, THT is toxic and thus replacing it with another additive, such as α,α,α-trifluorotoluene (PhCF3), was also investigated. Using PhCF3 instead of THT under same reaction conditions, an almost quantitatively conversion was achieved. In this case, the functionalization also highly occurred at the endocyclic double bond. All in all, the best results were obtained if the acetoxylation of 1 was performed with a catalyst loading of 4 mol%, stoichiometric amounts of BQ, DMSO/acetic acid as solvent in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at a temperature of 50 °C. As shown in Table 6, increasing the reaction time from 24 to 48 hours leads to almost full conversion with this optimized system without compromising its selectivity.
1 ratio at a temperature of 50 °C. As shown in Table 6, increasing the reaction time from 24 to 48 hours leads to almost full conversion with this optimized system without compromising its selectivity.
| Entry | t [h] | Conversiona [%] | Selectivitya [%] | ||
|---|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | |||
| Conditions: 200 mg (1.47 mmol) 1, 1.5 mL acetic acid, 1.5 mL DMSO, 1.0 eq. BQ, 250 mg 3 Å molecular sieve, 4.0 mol% Pd(OAc)2, 50 °C.a Conversion and selectivity were determined by GC-MS using tetradecane as internal standard. | |||||
| 1 | 2 | 7 | 72 | 28 | 0 |
| 2 | 4 | 13 | 77 | 23 | |
| 3 | 6 | 23 | 70 | 22 | 8 |
| 5 | 24 | 73 | 85 | 10 | 5 |
| 6 | 48 | 91 | 86 | 10 | 4 |
To further reduce the environmental impact of this acetoxylation procedure, we investigated acetoxylations of 1 using an aerobic tandem catalytic system. The major advantage of this catalytic procedure is the use of an ETM that is able to reoxidize the employed oxidant (e.g. BQ). For this purpose, a coupled system of palladium(II) acetate, hydroquinone (HQ) as oxidant precursor, and iron(II) phthalocyanine (Fe(Pc), ETM) were used in the presence of acetic acid and sodium acetate. In this case, oxygen (air) is the primary oxidant. First reactions were carried out using conditions reported by Bäckvall and co-workers for the catalytic diacetoxylation of dienes (Fig. 3).21,22
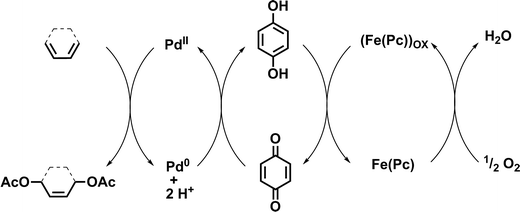 | ||
| Fig. 3 Mechanism of the aerobic 1,4-diacetoxylation of 1,3-dienes using oxygen as primary oxidant.21,22 | ||
The obtained results of the aerobic process using 1 revealed that very good conversions as well as a high selectivity can be achieved. Since the applied catalyst highly promotes C–H activation at the endocyclic double bond, it enabled the formation of the enantiomers 3 and 4 as well as 5 and 6 as major products. However, the acetoxylation also occurred at the exocyclic double bond, and thus 2 was obtained as by-product. Furthermore, with this catalytic system high conversions can be obtained in shorter reaction times, if compared to the aforementioned catalytic system involving DMSO (Table 7).
| Entry | t [h] | Conversiona [%] | Selectivitya [%] | ||
|---|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | |||
| Conditions: 200 mg (1.47 mmol) 1, 3.0 mL acetic acid, 0.5 eq. sodium acetate, 20 mol% HQ, 5.0 mol% Fe(Pc), 5.0 mol% Pd(OAc)2′, 60 °C, 24 h.a Conversion and selectivity were determined by GC-MS using tetradecane as internal standard. | |||||
| 1 | 2 | 39 | — | 39 | 61 |
| 2 | 4 | 69 | 8 | 36 | 56 |
| 3 | 6 | 78 | 9 | 37 | 54 |
| 4 | 24 | 98 | 10 | 33 | 57 |
Additionally, the required amount of oxidant in the catalytic aerobic process was tremendously reduced. Thus, a catalytic amount of oxidant instead of stoichiometric amount is sufficient to perform the acetoxylation in a very efficient manner. Compared to the reaction conditions described above, the aerobic oxidation process represents a notable improvement in the development of a sustainable and environmentally-benign catalytic acetoxylation procedure. Further studies were carried out to investigate the effect of sodium acetate and DMSO as an additive (Table 8).
| Entry | Conversiona [%] | Selectivitya [%] | ||
|---|---|---|---|---|
| 2 [%] | 3/4 [%] | 5/6 [%] | ||
| Conditions: 200 mg (1.47 mmol) 1, 3.0 mL acetic acid, 0.5 eq. sodium acetate, 20 mol% HQ, 5.0 mol% Fe(Pc), 5.0 mol% Pd(OAc)2′, 60 °C, 24 h.a Conversion and selectivity were determined by GC-MS using tetradecane as internal standard.b Without sodium acetate.c 20 mol% DMSO. | ||||
| 1 | 97b | 11 | 32 | 57 |
| 2 | 89c | 9 | 36 | 55 |
The obtained results show that the absence of sodium acetate has no significant effect on conversion or selectivity. If DMSO was added, a slightly lower conversion and improved selectivity was observed, whereas the expected (see results above) selective formation of 2 was not achieved. Moreover, if an equivalent amount of DMSO was used, only a very low conversion was obtained after 24 hours. Thus, DMSO might prevent the reoxidation of the oxidant by the used ETM.
With respect to the established catalytic acetoxylation processes, it would be very interesting to apply both acetoxylation procedures sequentially in order to efficiently functionalize the exo- and endocyclic double bond of 1 to obtain the respective diacetoxylated products. Therefore, the mono-acetoxylated products of 1 were used in further investigations. First the mixture of enantiomers (3, 4, 5 and 6) obtained by the aerobic tandem catalytic system was used for the acetoxylation process using DMSO and stoichiometric amounts of BQ. After 48 hours of reaction, no conversion was observed. Therefore, as a second approach, the reverse strategy was tested (Fig. 4). Herein, the product mixture obtained from the DMSO/BQ system was used in the aerobic tandem catalytic acetoxylation procedure. Indeed, the diacetoxylated products 7 and 8 as well as 9 and 10 were formed as respective enantiomers, although only a moderate conversion of 45% was obtained after a reaction time of 5 days. The presented sequential catalytic acetoxylation process resulted in a product selectivity of 7 and 8 (72%) as well as 9 and 10 (28%).
The presented sequential diacetoxylation process is interesting although the reaction conditions still need to be optimized. However, this is the first catalytic two step procedure to obtain the diacetoxylated products directly from limonene; usually, longer reaction sequences are reported.23Inter alia, with such a diacetoxylation protocol, precursors for polycondensation reactions can be obtained. Simple saponification leads to the corresponding diol compounds. Moreover, these products as well as the mono-acetoxylated limonene are valuable precursors for transition metal catalyzed allylic substitution reactions.24,25
Conclusion
The successful synthesis of regioselectively acetoxylated limonene derivatives via two efficient catalytic systems is described. The redox couple system of palladium(II) acetate/BQ using DMSO and acetic acid as solvents enabled the acetoxylation of limonene at the exocyclic double bond. The catalyst loading, amount of oxidant, amount of solvent and temperature was systematically optimized with regard to a maximum conversion and product selectivity. Using the optimized reaction conditions, good conversions as well as remarkable selectivity were obtained. Additionally, some other polar and aprotic solvents were investigated, which showed a different selectivity to the aforementioned catalytic system using DMSO. On the other hand by utilizing an aerobic tandem catalytic system of palladium(II) acetate/hydroquinone/iron(II) phthalocyanine in acetic as solvent, very good conversions and selectivities were obtained. Herein, the substrate-selective catalyst highly promoted the acetoxylation of limonene at the endocyclic double bond. Furthermore, contrary to the redox coupled system, the use of an ETM allowed for use of only catalytic amounts oxidant (HQ). Thus, this catalytic system revealed to be highly efficient and more environmentally benign due to the reduction the amount of toxic BQ, which is further supported by a reduction of the E-factor by 1.4 (meaning 1.4 kg less waste are produced per kg of product). Finally, both catalytic procedures were combined in a sequential acetoxylation that facilitated the synthesis of diacetoxylated products and provide access to various limonene derivatives.References
- M. A. R. Meier, J. O. Metzger and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1788–1802 RSC.
- U. Biermann, U. Bornscheuer, M. A. R. Meier, J. O. Metzger and H. J. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 3854–3871 CrossRef CAS PubMed.
- A. Jering, J. Günther, A. Raschka, M. Carus, S. Piotrowski and L. Scholz, ETC/SCP report 1/ 2010 Search PubMed.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- F. R. Marín, C. Soler-Rivas, O. Benavente-García, J. Castillo and J. A. Pérez-Alvarez, Food Chem., 2007, 100, 736–741 CrossRef PubMed.
- E. A. Nonino, Perfum. Flavor., 1997, 22, 53–58 Search PubMed.
- E. Breitmaier, in Terpenes: flavors, fragrances, pharmaca, pheromones, GmbH & Co. KGaA, Wiley-VCH Verlag, 2006, pp. 119–159 Search PubMed.
- I. C. Sun, H. K. Wang, Y. Kashiwada, J. K. Shen, L. M. Cosentino, C. H. Chen, L. M. Yang and K. H. Lee, J. Med. Chem., 1998, 41, 4648–4657 CrossRef CAS PubMed.
- R. H. Cichewicz and S. A. Kouzi, Med. Res. Rev., 2004, 24, 90–114 CrossRef CAS PubMed.
- A. J. D. Silvestre and A. Gandini, Monomers, Polymers and Composites from Renewable Resources, ed. M. N. Belgacem and A. Gandini, Elsevier, Oxford, 2008 Search PubMed.
- C. Wilson and P. Shaw, J. Org. Chem., 1973, 38, 1684 CrossRef CAS.
- M. J. da Silva, P. Robles-Dutenhefner, L. Menini and E. V. Gusevskaya, J. Mol. Catal. A: Chem., 2003, 201, 71–77 CrossRef CAS.
- E. Gusevskaya and J. A. Gonsalves, J. Mol. Catal. A: Chem., 1997, 121, 131–137 CrossRef CAS.
- L. S. Hegedus, G. F. Allen, J. J. Bozell and E. L. Waterman, J. Am. Chem. Soc., 1978, 100, 5800 CrossRef CAS.
- L. El Firdoussi, A. Baqqa, S. Allaoud, B. A. Allal, A. Karim, Y. Castanet and A. Mortreux, J. Mol. Catal. A: Chem., 1998, 135, 11–22 CrossRef CAS.
- J. Piera and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2008, 47, 3506–3523 CrossRef CAS PubMed.
- J. E. Bäckvall, R. E. Nordberg and D. Wilhelm, J. Am. Chem. Soc., 1985, 107, 6892 CrossRef.
- M. S. Chen, N. Prabagaran, N. A. Labenz and M. C. White, J. Am. Chem. Soc., 2005, 127, 6970–6971 CrossRef CAS PubMed.
- M. von Czapiewski, O. Kreye, H. Mutlu and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2013, 115, 76–85 CrossRef CAS.
- C. Le, K. Kunchithapatham, W. H. Henderson, C. T. Check and J. P. Stambuli, Chem. – Eur. J., 2013, 00, 0 Search PubMed.
- J. E. Bäckvall, R. B. Hopkins, H. Grennberg, M. Mader and A. K. Awasthi, J. Am. Chem. Soc., 1990, 112, 5160 CrossRef.
- J. E. Bäckvall, A. K. Awasthi and Z. D. Renko, J. Am. Chem. Soc., 1987, 109, 4750 CrossRef.
- B. Ravindranath and P. Srinivas, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1985, 24, 163–165 Search PubMed.
- B. M. Trost, Angew. Chem., Int. Ed. Engl., 1989, 28, 1173–1192 CrossRef.
- C. G. Frost, J. Howatth and J. M. J. Williams, Tetrahedron: Asymmetry, 1992, 3, 1089 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |