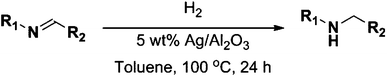Open Access Article
Open Access ArticleSilver nanoparticles supported on alumina–a highly efficient and selective nanocatalyst for imine reduction†
Raju
Poreddy
,
Eduardo J.
García-Suárez
,
Anders
Riisager
and
Søren
Kegnæs
*
Centre for Catalysis and Sustainable Chemistry, Department of Chemistry, Building 207, Technical University of Denmark, 2800 Kgs, Lyngby, Denmark. E-mail: skk@kemi.dtu.dk; Tel: (+45) 45252402
First published on 28th November 2013
Abstract
Silver nanoparticles supported on alumina were prepared and tested in the catalytic reduction of various imines to primary and secondary amines and were shown to be exceptionally active and chemoselective. Furthermore, the catalytic activity of the prepared nanocatalyst was also tested in the synthesis of secondary amines from primary amines in a tandem reaction protocol (oxidation–imination–reduction) using air and molecular hydrogen as oxidizing and reducing agents, respectively. The reported synthesis is performed under mild reaction conditions, which complies with the demands of modern organic synthesis. Due to the mild reaction conditions and high conversion as well as high selectivity, we consider that the utilization of silver nanoparticles supported on alumina represents an attractive and environmentally friendly alternative to the current synthesis of N-alkyl amines.
Introduction
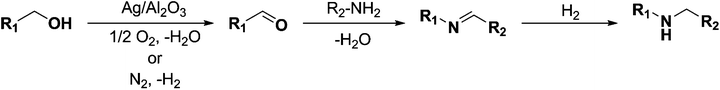
In recent years amines and imines have received much attention due to their wide usability as synthetic intermediates in the production of pharmaceuticals, agrochemicals, fine chemicals, bio-active compounds, and dye chemicals.1–6 In particular, N-alkyl amines are important pharmacophores in numerous bio-active compounds due to their physiological activities, and are also widely used in the area of drug discovery.7N-Alkyl amines are prepared by the reaction of primary amines with organic halides,8 reductive alkylation9 or hydroamination and hydroaminomethylation reactions.10 Despite the widespread interest, these known methods for the formation of secondary amines are often associated with problems such as high cost of the starting materials, harsh reaction conditions, poor yields, and/or low chemical selectivity, along with inevitable environmental issues.11 On the other hand, imines are being synthesized by acid–base promoted condensation of primary amines and carbonyl compounds,12 oxidative coupling of secondary amines,13 hydroamination of alkynes and other methods.14 Within these methods, the homogeneous transition metal-catalyzed oxidative dehydrogenation of alcohols to carbonyl compounds that can form an imine by condensation with an amine is an attractive synthetic approach due to high atom efficiency.15–19 Nevertheless, these homogeneous catalysts suffer from drawbacks such as difficulty in recovery and reuse of expensive metals, necessity of special handling of metal complexes, and the required use of co-catalysts such as a base promoter and a stabilizing ligand. Hence, the application of efficient, easily recoverable heterogeneous catalysts which are relatively inexpensive, would clearly be advantageous. Taking up these challenges a few heterogeneous catalysts based on, for instance, molecular sieves, amorphous silica–alumina, gallosilicates, boron–aluminum, and iron phosphate have been reported in the 1990s.20 More recently, Shimizu and co-workers reported that besides the catalytic activity of silver clusters, alumina with acid–base surface sites has a cooperative effect on oxidation of alcohols under oxidant-free conditions.21 Very recently, Jaenicke and co-workers reported that alumina entrapped Ag nanoparticles can catalyze the N-alkylation of amines with alcohols using the borrowing-hydrogen methodology.22 However, the essential use of a co-catalyst or a base promoter (e.g. Cs2CO3, K3PO4) to make the dehydrogenation feasible results in low atom efficiency, high metal waste/by-product production and increased difficulty in purification. Accordingly, development of new highly efficient low-cost heterogeneous catalysts that can circumvent these drawbacks – making the whole process greener – is required.In this work we report that alumina-supported silver nanoparticles can efficiently catalyze the reduction of imines to their corresponding amines using hydrogen as the reducing agent (Scheme 1). Furthermore, the same catalyst was able to catalyze the two-step synthesis of N-alkylated amines without adding any co-catalyst at low temperatures (100 °C) (Scheme 2).
An Ag/Al2O3 (SBET of Al2O3 = 256 m2 g−1) catalyst was prepared by incipient wetness impregnation of the solid support with an aqueous solution of silver nitrate resulting in 5 wt% content of silver on the support. The prepared nanocatalyst was fully characterized by means of X-ray powder diffraction (XRPD; ESI Fig. S1†), scanning electron microscopy (SEM; ESI Fig. S1†), energy dispersive X-ray (EDX; ESI Fig. S2†) spectroscopy and transmission electron microscopy (TEM; ESI Fig. S1†) and Temperature Programmed Desorption (TPD-NH3, Fig. S4†) and Temperature Programmed Reduction (TPR-H2, Fig. S5†).
Fig. 1 shows a representative TEM image of the silver nanoparticles supported on alumina. Well distributed silver nanoparticles throughout the support material were obtained with an average diameter in the range of 6.3 ± 1.4 nm.
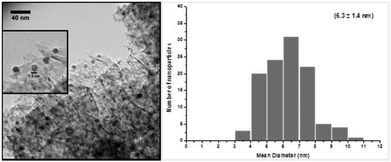 | ||
| Fig. 1 Representative TEM image of the 5 wt% silver–alumina nanocatalyst (magnified image inset) and corresponding histogram with mean particle diameter and standard deviation. | ||
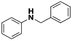
Firstly, the catalytic activity of the prepared nanocatalyst was tested in the reduction of N-benzylidineaniline using formier gas (10 bar, 10% H2/N2 mixture) as a reducing agent at different temperatures in order to optimize the reaction temperature. As shown in Table 1, the conversion of imine to the corresponding amine increased from 35 to 99% when the temperature was increased from 80 to 120 °C in a 24 hour reaction. Since there was only a minor difference in catalyst performance between 100 and 120 °C the lowest reaction temperature is always preferred, and it was selected for further experiments. Furthermore, a control experiment using only the support in the role of a catalyst to discard its influence in the catalytic activity was performed in the absence of silver. As expected no conversion was achieved in our reaction conditions (entry 4, Table 1).
The effect of hydrogen pressure in the N-benzylidineaniline reduction was subsequently studied using pure hydrogen instead of formier gas (10% H2/N2 mixture); the results are shown in Fig. 2. The conversion increased from 49 to 99% by increasing the hydrogen pressure from 1 to 4 bars, whereas no significant changes were observed when the pressure was increased further to 6 bars. Thus 4 bars were considered to be an appropriate pressure for the reaction.
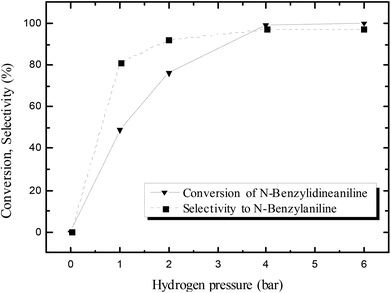 | ||
| Fig. 2 Effect of hydrogen pressure on the activity of the catalyst in N-benzylidineaniline reduction after 24 hours at 100 °C. | ||
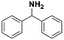
The selectivity towards N-benzylaniline increased as well with hydrogen pressure, and a maximum selectivity and yield of 97% were reached with 4 or 6 bars of hydrogen. At lower pressures of hydrogen (1 or 2 bars) the reduction of N-benzylidineaniline was significantly slower, thus allowing the generation of side products formed by, e.g. retro-condensation to benzaldehyde and aniline followed by the reduction of benzaldehyde to benzyl alcohol (Scheme 3; ESI Fig. S3†), thus decreasing the selectivity.
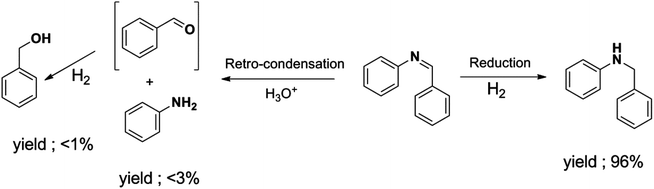 | ||
| Scheme 3 Retro-condensation of imine to its amine and transient formation of aldehyde which is reduced to its corresponding alcohol. | ||
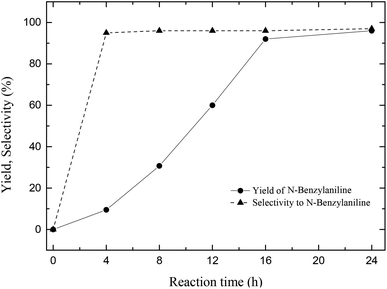
After optimization of two important parameters in the catalytic reaction such as reaction temperature (100 °C) and hydrogen pressure (4 bars) the objective was shifted to study the reaction progress as a function of time. Even though 96% of the N-benzylidineaniline is converted to its reduction product in about 16 h (Fig. 3), the reaction was run for 24 h to get sheer conversion of the substrate with 96% selectivity.
Once the reaction parameters were optimized, the prepared nanocatalyst was tested with different substrates in order to explore the expediency. Accordingly, structurally diverse aromatic (activated) as well as one sample of aliphatic (non-activated) imines were examined towards the formation of the corresponding amines. The results are shown in Table 2.
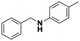
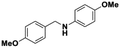
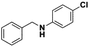
All the examined substrates were converted to their corresponding amines with good to excellent conversion and with a remarkably high selectivity (>99%), thus demonstrating the versatility of the prepared nanocatalyst in the reduction of both the activated and non-activated imines to their corresponding amines with no substantial difference in performance after 24 h of reaction. However, the conversion of (E)-N,1-bis(4-methoxyphenyl)methanimine (entry 4) was significantly lower under the given reaction conditions. The decrease in conversion or slow rate of reaction may be attributed to the steric hindrance, or electronic effects caused by substituents on the substrates (entries 4, 5, and 6).
Retro-condensation of the imines to their corresponding amines and aldehydes with subsequent reduction to the alcohols was also here noticed by GC analysis (ESI, Fig. S3†). This minor side reaction seemed unavoidable and could possibly be facilitated by trace amounts of moisture present within the particles of the catalyst.
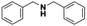
The important features of heterogeneous catalysts are their recovery from the reaction medium and reuse. The prepared nanocatalyst could easily be separated from the reaction mixture by simple filtration and it was reused in the hydrogenation reaction of N-benzylidineaniline (Table 2, entry 2). Upon second use, the catalytic performance remained unchanged with yields of 96–95%, showing the apparent stability of the prepared catalyst in our reaction conditions. Furthermore, in order to prove that there was no leaching occurring during the reaction, the filtered solution was tested in the catalytic reaction without exhibiting any activity under the same reaction conditions. This clearly supported the heterogeneity of the reaction. In addition, the absence of silver leaching was also confirmed by ICP-AES analysis of the filtered solution. These findings clearly ruled out the contribution of leached silver in the imine reduction reaction.
The highly selective reduction of imines to their corresponding amines by the silver nanoparticles enticed us to further examine the synthesis of secondary amines via a two-step (1) oxidative dehydrogenation-condensation and (2) reduction reaction (Scheme 4).
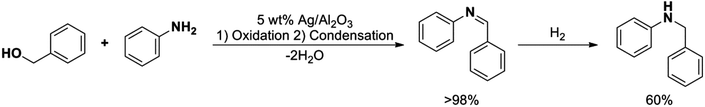 | ||
| Scheme 4 Synthesis of N-benzylaniline via oxidation/condensation/reduction sequence using supported silver nanoparticles as a heterogeneous catalyst. | ||
The overall reaction yield was found to be 59% with a selectivity of 99% towards the desired product. In the first step, involving oxidation of the alcohol to the aldehyde and further condensation with the aniline, the conversion and selectivity were higher than 98% and 99%, respectively. On the other hand, only a modest 60% conversion with 99% selectivity was attained in the second step. We speculate that the low conversion reached in the reduction step could be explained in terms of irreversible chemisorption of the hydride ion on the surface active sites of the silver clusters, as well as due to the formation of Ag2O during the oxidation step. The low conversion could be also attributed to the agglomeration of the nanoparticles since it is already well-known and reported in the literature that the increase in the size of the nanoparticles decreases the catalytic activity due to the lower surface area of the nanoparticles.
Our results are comparable with previous studies in which silver catalysts were used for dehydrogenation or in the formation of amines. For example, Shimizu et al.21 employed 24 hours reaction time for dehydrogenation of alcohols while Liu et al.22 employed 19 hours for the formation of amines; however, they used higher reaction temperatures (120 °C) in comparison with 100 °C reported in the current work. Furthermore, in both the previous studies they used additives that were avoided under our reaction conditions.
In summary, the prepared nanocatalyst was found to be highly efficient, yielding excellent conversion and selectivity towards the reduction of both aromatic and aliphatic imines under relatively mild reaction conditions. The versatility of the nanocatalyst is demonstrated by its application in the synthesis of secondary amines via a tandem reaction, where high selectivity (up to 98%) towards the targeted product was reached. Since water is the only formed by-product and no extra added bases are required under our reaction conditions, the prepared nanocatalyst is supposed to be an attractive alternative green route to the previously reported method in the synthesis of secondary amines. Further investigations are on going in order to improve the recyclability of the catalytic system.
Acknowledgements
We gratefully acknowledge the support of the Danish Council for Independent Research, grant no. 10-093717 and grant no. 12-127580.References
- S. A. Lawrence, Amines: Synthesis Properties and Applications, Cambridge University, Cambridge, 2004 Search PubMed.
- (a) K. P. C. Vollhardt and N. E. Schore, Organic Chemistry: Structure and Function, W.H. Freeman, New York, 3rd edn, 1999, p. 936 Search PubMed; (b) A. A. Núñez Magro, G. R. Eastham and D. J. Cole-Hamilton, Chem. Commun., 2007, 3154 RSC.
- B. R. Brown, The Organic Chemistry of Aliphatic Nitrogen Compounds, Oxford University, New York, 1994 Search PubMed.
- S. K. Klitgaard, K. Egeblad, U. V. Mentzel, A. G. Popov, T. Jensen, E. Taarning, I. S. Nielsen and C. H. Christensen, Green Chem., 2008, 10, 419 RSC.
- S. Kegnæs, J. Mielby, U. V. Mentzel, C. H. Christensen and A. Riisager, Green Chem., 2010, 12, 1437 RSC.
- S. Kegnæs, J. Mielby, U. V. Mentzel, T. Jensen, P. Fristrup and A. Riisager, Chem. Commun., 2012, 48, 2427 RSC.
- S. S. Insaf and D. T. Witiak, Synthesis, 1999, 435 CrossRef CAS PubMed.
- W. J. Kyung, H. Y. Cheol and R. N. Salvatore, Tetrahedron, 2001, 57, 7785 CrossRef.
- (a) J. F. Hartwig, Synlett, 2006, 1283 CrossRef CAS PubMed; (b) S. L. Buchwald, C. Mauger, G. Mignani and U. Scholz, Adv. Synth. Catal., 2006, 348, 23 CrossRef CAS; (c) O. Navarro, N. Marion, J. Mei and S. P. Nolan, Chem.–Eur. J., 2006, 12, 5142 CrossRef CAS PubMed.
- (a) T. E. Muller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795 CrossRef PubMed; (b) M. Ahmed, A. M. Seayad, R. Jackstell and M. Beller, J. Am. Chem. Soc., 2003, 125, 10311 CrossRef CAS PubMed; (c) X. Q. Shen and S. L. Buchwald, Angew. Chem., Int. Ed., 2010, 49, 564 CrossRef CAS PubMed; (d) K. D. Hesp, S. Tobisch and M. Stradiotto, J. Am. Chem. Soc., 2010, 132, 413 CrossRef CAS PubMed.
- M. S. Gibson, in The Chemistry of the Amino Group, ed. S. Patai, Interscience, New York, 1968, p. 37 Search PubMed.
- P. Fontaine, A. Chiaroni, G. Masson and J. Zhu, Org. Lett., 2008, 10, 1509 CrossRef CAS PubMed.
- (a) L. Blackburn and R. J. K. Taylor, Org. Lett., 2001, 3, 1637 CrossRef CAS PubMed; (b) A. L. Korich and T. S. Hughes, Synlett, 2007, 2602 CAS; (c) L. Paquin, J. Hamelin and F. Texier-Boullet, Synthesis, 2006, 1652 CAS.
- (a) R. W. Layer, Chem. Rev., 1963, 63, 489 CrossRef CAS; (b) M. T. Robak, M. A. Herbage and J. A. Ellman, Chem. Rev., 2010, 110, 3600 CrossRef CAS PubMed; (c) X. F. Wu, C. Vovard-Le Bray, L. Bechki and C. Darcel, Tetrahedron, 2009, 65, 7380 CrossRef CAS PubMed.
- (a) M. Haniti, S. A. Hamid and J. M. J. Williams, Chem. Commun., 2007, 725 Search PubMed; (b) M. Haniti, S. A. Hamid and J. M. J. Williams, Tetrahedron Lett., 2007, 48, 8263 CrossRef PubMed; (c) K. Fujita, Z. Li, N. Ozeki and R. Yamaguchi, Tetrahedron Lett., 2003, 44, 2687 CrossRef CAS; (d) A. Prades, R. Corberan, M. Poyatos and E. Peris, Chem.–Eur. J., 2008, 14, 11474 CrossRef CAS PubMed.
- (a) G. Guillena, D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2007, 46, 2358 CrossRef CAS PubMed; (b) F. Shi, M. K. Tse, X. Cui, D. Gördes, D. Michalik, K. Thurow, Y. Deng and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 5912 CrossRef CAS PubMed.
- Y. Q. Wang, S. M. Lu and Y. G. Zhou, J. Org. Chem., 2007, 72, 3729 CrossRef CAS PubMed.
- J. Krupka and J. Patera, Appl. Catal., A: Gen., 2007, 330, 96 CrossRef CAS PubMed.
- X. Y. Zhou, M. Bao and Y. G. Zhou, Adv. Synth. Catal., 2011, 353, 84 CrossRef CAS.
- P. G. Pappas and J. B. Melville, US Patent 1992, 5,159,115 Search PubMed.
- K.-I. Shimizu, K. Sugino, K. Sawabe and A. Satsuma, Chem.–Eur. J, 2009, 15, 2341 CrossRef CAS PubMed.
- H. Liu, G.-K. Chuah and S. Jaenicke, J. Catal., 2012, 292, 130 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3dt52499j |
| This journal is © The Royal Society of Chemistry 2014 |