Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods
Nicolas
Bordenave
a,
Bruce R.
Hamaker
b and
Mario G.
Ferruzzi
b
aPepsiCo Global R&D, 617 W Main St., Barrington, IL 60010, USA. Tel: +1 847-304-2485
bDepartment of Food Science, Purdue University, 745 Agricultural Mall Dr., West Lafayette, IN 47906, USA. E-mail: mferruzz@purdue.edu; Fax: +1 765-494-7953; Tel: +1 765-494-0625
First published on 31st October 2013
Abstract
Many of the potential health benefits of flavonoids have been associated with their specific chemical and biological properties including their ability to interact and bind non-covalently to macronutrients in foods. While flavonoid–protein interactions and binding have been the subject of intensive study, significantly less is understood about non-covalent interactions with carbohydrates and lipids. These interactions with macronutrients are likely to impact both the flavonoid properties in foods, such as their radical scavenging activity, and the food or beverage matrix itself, including their taste, texture and other sensorial properties. Overall, non-covalent binding of flavonoids with macronutrients is primarily driven by van der Waals interactions. From the flavonoid perspective, these interactions are modulated by characteristics such as degree of polymerization, molecular flexibility, number of external hydroxyl groups, or number of terminal galloyl groups. From the macronutrient standpoint, electrostatic and ionic interactions are generally predominant with carbohydrates, while hydrophobic interactions are generally predominant with lipids and mainly limited to interactions with flavonols. All of these interactions are involved in flavonoid–protein interactions. While primarily associated with undesirable characteristics in foods and beverages, such as astringency, negative impact on macronutrient digestibility and hazing, more recent efforts have attempted to leverage these interactions to develop controlled delivery systems or strategies to enhance flavonoids bioavailability. This paper aims at reviewing the fundamental bases for non-covalent interactions, their occurrence in food and beverage systems and their impact on the physico-chemical, organoleptic and some nutritional properties of food.
1. Introduction
The association between increased fruit and vegetable consumption and protection against chronic and degenerative diseases1 has driven interest in phytochemical compounds and phytochemical extracts for applications in novel functional foods and beverages. Particularly, plant-derived polyphenolic flavonoids have received significant attention in recent years due to their reported biological activities and general abundance in the diet. The daily intake of proanthocyanidins was estimated to be 57.7 mg per person in the U.S. population in 2003, flavan-3-ol monomers, dimers and trimers accounting for 7.1, 11.2 and 7.8% of this total, respectively.2 During the same period, the daily intake of flavonoids (isoflavones, flavonols and flavones) was also estimated for the U.S. population to be around 20–34 mg per person.3,4 In a more recent study, the total daily intake of flavan-3-ols and proanthocyanidins has been estimated to be 454–125 and 455–101 mg per person, respectively, across Europe.5 Although being very variable, these levels are modest compared to macronutrient levels, but the potential health benefits associated with flavonoids have sparked interest in monitoring consumption, bioavailability and physiological activities, much like micronutrients. While interests were initially focused on the ability of these flavonoids to act as antioxidant and radical scavenging compounds, more recently their ability to impact inflammatory markers, cell signaling pathways and interact with specific biologically relevant proteins has been the subject of intensive investigation.6While being investigated extensively for these biological activities, perhaps the most recognized property of flavonoids in food systems is their ability to bind to proteins through both covalent and non-covalent interactions. Protein binding by flavonoids is often associated with the generation of characteristic sensory properties of foods and beverages including color, flavor and taste attributes, including bitterness and astringency.1,6–8 As a tactile component of flavor, astringency is believed to be a result of the direct interactions between specific flavonoid components and salivary proteins that result in the loss of solubility and loss of lubrication in the oral cavity.9 It is perhaps the most well documented example of how interactions between flavonoids and surrounding macronutrients may influence the “activity” of flavonoids. Thus, the complex macronutrient and micronutrient profile of foods may influence the nature and extent to which select flavonoids and proteins interact. However, significantly less is known regarding interactions between flavonoids and other macronutrients (carbohydrates and lipids).
From a mechanistic standpoint, non-covalent binding between flavonoids and macronutrients is the result of short range electrostatic and van der Waals forces. These interactions have wide and potentially significant implications, from biosynthesis, storage and transport in plants to bioavailability and mechanism of action of flavonoids in humans. While information on the biological consequences of flavonoid–protein interactions exists, this review focuses on interactions exclusively between flavonoids and main macronutrient classes (protein, carbohydrates and lipids) within raw and processed food and beverage matrices. We aim to synthesize the state of the science on the impact and uses of these plant-derived biologically active compounds on organoleptic and functional properties of foods and beverages.
2. Dietary flavonoids
Flavonoids are plant-derived polyphenolic compounds structurally characterized by a C6–C3–C6 backbone. The most common dietary flavonoid classes include: flavanols, also known as flavan-3-ols, (derived from 2-phenylchromen-4-one), flavones (derived from 3-phenylchromen-4-one), anthocyanidins (derived from 4-phenylchromen-4-one) and isoflavones (derived from 3-phenylchromen-4-one or 3-phenylchroman) (Fig. 1). These flavonoids are sub-classified according to their substitution patterns, conformations and oxidation states. Flavonoids are widely dispersed in the western diet but most commonly are found in fruits and fruit juices, vegetables, herbs, cocoa, tea, coffee and alcoholic beverages such as beer and wine (Table 1),2,10–13 where mainly flavonols, flavan-3-ols, flavanones, flavones, and isoflavones can be found. Di-, tri- or oligo-meric forms of flavanols referred to as proanthocyanidins are also common in food and beverages.3,10,14 It must be noted that in nature, flavonoids, with the exception of flavan-3-ols, are commonly found as glycosides, sugar substituted flavonoids. Substitution can occur through hydroxyl groups (O-glycosides) on rings A or B, or A-ring carbon atoms (C-glycosides), with up to four substituents per monomeric flavonoid.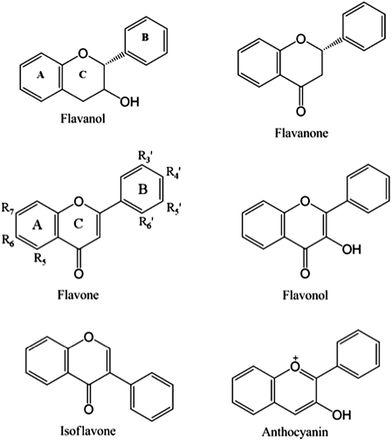 | ||
| Fig. 1 Main dietary flavonoid classes and backbone structures, with substitution nomenclature on the flavonol backbone. | ||
| Food description | Monomeric flavonoid class | Monomeric flavonoids | Mean (mg per 100 g edible portion) | Standard error |
|---|---|---|---|---|
| Dill weed, fresh (Anethum graveolens) | Flavonols | Isorhamnetin | 43.5 | 28.5 |
| Quercetin | 55.15 | 29.82 | ||
| Parsley, fresh (Petroselinum crispum) | Flavones | Apigenin | 215.46 | 36.08 |
| Flavonols | Myricetin | 14.84 | 6.76 | |
| Apples, fuji, raw, with skin | Flavan-3-ols | (−)-Epicatechin | 5.55 | 0.56 |
| Flavonols | Quercetin | 2.35 | 0.29 | |
| Grapefruit, raw, pink and red, all areas (Citrus paradisi) | Flavanones | Naringenin | 32.64 | 6.62 |
| Grapes, black (Vitis vinifera) | Flavan-3-ols | (−)-Epicatechin | 8.68 | 2.48 |
| (+)-Catechin | 10.14 | 2.91 | ||
| Grape seeds, raw | Flavan-3-ols | (−)-Epicatechin | 93.31 | 8.42 |
| (+)-Catechin | 74.63 | 5.78 | ||
| Oranges, raw, all commercial varieties (Citrus sinensis) | Flavanones | Hesperetin | 27.25 | 4.33 |
| Naringenin | 15.32 | 1.76 | ||
| Strawberries, raw (Fragaria X ananassa) | Anthocyanidins | Pelargonidin | 25.69 | 0.43 |
| Flavan-3-ols | (+)-Catechin | 3.11 | 0.19 | |
| Cabbage, red, raw (Brassica oleracea, Capitata Group) | Anthocyanidins | Cyanidin | 63.5 | 20.94 |
| Olive leaves, raw | Flavones | Apigenin | 2.84 | — |
| Luteolin | 27.7 | — | ||
| Flavonols | Quercetin | 6.24 | — | |
| Onions, red, raw | Flavonols | Quercetin | 31.77 | 1.73 |
| Celery, Chinese, raw | Flavones | Apigenin | 24.02 | — |
| Luteolin | 34.87 | — | ||
| Kale, raw (Brassica oleracea, Acephala Group) | Flavonols | Isorhamnetin | 23.6 | — |
| Kaempferol | 46.8 | 5.56 | ||
| Quercetin | 22.58 | 2.94 | ||
| Alcoholic beverage, beer, regular, all | Flavan-3-ols | (+)-Catechin | 2.07 | 0.84 |
| Alcoholic beverage, wine, table, red, Cabernet Franc | Anthocyanidins | Delphinidin | 5.81 | — |
| Malvidin | 44.09 | — | ||
| Petunidin | 6.99 | — | ||
| Flavan-3-ols | (−)-Epicatechin | 9.2 | — | |
| (+)-Catechin | 6.21 | — | ||
| Tea, green, large leaf, Quingmao, dry leaves | Flavan-3-ols | (−)-Epicatechin | 2300 | 300 |
| (−)-Epicatechin 3-gallate | 13620 | 340 | ||
| (−)-Epigallocatechin | 1600 | 200 | ||
| (−)-Epigallocatechin 3-gallate | 7380 | 220 | ||
| (+)-Catechin | 4320 | 120 | ||
| Cacao beans | Flavan-3-ols | (−)-Epicatechin | 99.18 | — |
| (−)-Epigallocatechin | 156.67 | — | ||
| (+)-Catechin | 88.45 | — | ||
| (+)-Gallocatechin | 8262 | — |
In fruits, flavan-3-ols, flavonols (quercetin in particular) and proanthocyanidins (polymeric flavan-3-ol forms) are the predominant forms. As a class, vegetables are considered a rich source of flavonols including quercetin and kaempferol.15 Beverages such as tea, wine and beer remain a significant source of flavonoids in the western diet. Tea is the most naturally concentrated flavonoid containing beverage in the world. In a typical tea infusion, catechins, monomeric flavan-3-ols, appear to be the most abundant flavonoid class,16 primarily as (−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin (EGC) and (−)-epicatechin gallate (ECG). Procyanidins, which are flavanol oligomers and a sub-class of proanthocyanidins, can make up to 40–50% of the flavonoid content in red wines and beer,10 which also contain monomeric flavonols, flavanols and various oxidized forms of these polyphenols.
In addition to the diversity of flavonoid forms in foods, once consumed, these molecules can be extensively modified both by mammalian and microbial systems. The process of digestion and xenobiotic metabolism in the intestine and liver convert many of the dietary flavonoid forms biological relevant conjugates including methylated, glucuronidated and sulfonated metabolites.17–19 Additionally, flavonoids unabsorbed in the gastric and small intestinal environment can be extensively metabolized by colonic microbiota in the large intestine to smaller molecular weight metabolites including 1,3-diphenylpropanes, γ-valerolactones, phenylalkyl carboxylic acids, benzoic acids, and other aromatic compounds.20–24 The combination of metabolites and dietary forms should be considered when evaluating biological actions of flavonoids, including their ability to interact with macromolecules.
While the focus of this review is on flavonoid–macronutrient non-covalent interactions, it is important to note that intra and inter-flavonoid interactions do occur. They play an important role in color properties of anthocyanin dietary sources through a process known as copigmentation. Indeed, most anthocyanin compounds are colored in their protonated forms, predominant at low pH, but under higher pH conditions the non-protonated colorless forms can exist. Nevertheless, even under these conditions, the colored forms are thermodynamically stabilized by vertical or T-shaped stacking of their planar π-rings, between flavonoids or between flavonoids and other molecules then called co-pigments.25 Glycosilation does not seem to sterically limit inter-flavonoid planar rings stacking.26 Some studies even suggest that this vertical π-stacking is favored by the higher ability of the flavonoids to participate in hydrogen-bonded networks,27 in which glycosylation as well as galloylation could play a role. These considerations highlight some of the molecular properties of flavonoids that will drive their interactions with macronutrients in foods and beverages, such as proteins.
3. Interactions of flavonoids with food proteins
Flavonoid–protein interactions have been well documented and both covalent and non-covalent interactions have been the subject of several reviews.1,28–31 Covalent binding of flavonoids and proteins typically results from the reaction between functional groups, such as proteins' amine and amide groups on the one hand, and quinones formed by flavonoid oxidation (chemically or enzymatically mediated) on the other hand. Oxidation of flavonoids in foods, fruits and vegetables in particular, can occur through exposure to high pH, heat and oxygen reactive species (as it happens during roasting of cocoa and coffee beans) or during post-harvest handling or processing as these processes facilitate the disruption of plant cellular materials and can, in some cases, release degradative enzymes such as polyphenol oxidases.32Regarding non-covalent binding with proteins and from the flavonoid perspective, the most commonly investigated forms include flavan-3-ols, flavonols and proanthocyanidins. Surprisingly, to the best of our knowledge, only limited work has been reported on the interactions between proteins and isoflavones from soy, the seeds of which contain a significant amount of economically important proteins and flavonoids.33
Non-covalent interactions or binding between flavonoids and proteins can either be chemically specific or non-specific. Specific binding is often reported for interactions between flavonoids and enzymes or proteins with a defined globular tertiary structure such as hemoglobin or milk immunoglobulins.34–36
From the broad class of flavonoids, flavan-3-ols from fruits, tea and cocoa have also demonstrated the ability to inhibit digestive enzymes in vitro, including α-amylase, pancreatic lipase and phospholipase A, through both competitive and non-competitive interactions.37–40 Generally, their inhibitory activity was found to increase with increase in flavan-3-ol polymerization.38–40 Flores et al. studied the inhibitory activity of blueberry phenolic extracts obtained with different solvents on α-amylase and α-glucosidase.41 In that study, all extracts showed high α-glucosidase inhibition (89 to 93% inhibition) and modest α-amylase inhibition (5 to 7% inhibition). Surprisingly, α-amylase inhibition could not be correlated with the total phenolic content or total monomeric anthocyanin content of the extract, suggesting that α-amylase inhibition by flavonoids may be more dependent on the structure of the flavonoids themselves rather than on their amount. Indeed, Lo Piparo et al. (2008) described the general structural features required for flavonoids to inhibit human salivary α-amylase through non-covalent binding in the catalytic site of the enzyme:42 the best inhibitors share a highly π-conjugated structure throughout rings A and C, thus providing great stability (with a conjugated carboxyl group and a double carbon bond on the C-ring as in flavonols and flavones, see Fig. 1), and hydroxyl groups in specific positions (positions R6 and R7 on the A ring, R4′ and R5′ on the B ring). This work shows the great potential of targeted flavonoids to be involved in specific non-covalent interaction with proteins.
Nevertheless, non-specific interactions between flavonoids and non-globular proteins are the most commonly reported in the literature. These include interactions with salivary proline-rich proteins (PRPs), as well as albumin-type proteins (bovine serum albumin BSA, ovalbumin and casein), as discussed further. BSA has been heavily utilized as a model of flavonoid–protein interactions because of its relevance to modeling biological interactions with salivary proteins and human serum albumin. Additionally, milk proteins such as β-casein and whey have been convenient models for food proteins, showing that their respective binding with flavonoids is driven by the same general characteristics.43–45
Several molecular characterization studies using NMR coupled with molecular modeling techniques and isothermal titration calorimetry have defined the broad characteristics of flavonoid–food protein interactions.46–49 From the literature, it appears that the main driving forces are van der Waals type interactions including: (a) hydrogen bonding (electrostatic interactions) driven by flavonoid hydroxyl groups, (b) London interactions between the non-polar polarizable aromatic rings of flavonoids and non-polar polarizable parts of protein side chains (Fig. 2) and (c) ionic interactions. The relative weight of these effects depends on the specific flavonoid–protein structures and the specific system investigated.50 In particular, the weight of London forces is positively correlated with the number of terminal galloyl groups on the polyphenol molecule.51 This suggests that flavonoids such as EGCG and ECG would, in fact, have relatively strong affinities for proteins. Moreover, although interactions between flavonoids and non-enzymatic proteins are generally non-specific, they can induce subtle conformational changes34,52,53 and possibly partial protein structures.46 These conformational changes require molecular flexibility from both protein chains as well as simple flavonoids or complex tannins.45,47,54 Such a feature has been observed in complex model systems such as β-lactoglobulin–gum acacia–quercetin systems studied by Aberkane and coworkers:55 conformational changes in β-lactoglobulin, namely the loss of β-sheets induced by the increased exposure of protein hydrophobic pockets involved in the non-covalent complexation with gum acacia, were increased in the presence of quercetin, thereby favoring the protein–polysaccharide interaction. As an effect, the formation of a flavonoid–protein complex leads to aggregation and eventual loss of solubility and precipitation of both proteins and flavonoids. However, in some rare instances, an increase in solubility of the flavonoid can be observed. For example, resveratrol has shown its solubility significantly increased upon 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding with β-lactoglobulin in alcoholic solutions53 (Fig. 3).
1 binding with β-lactoglobulin in alcoholic solutions53 (Fig. 3).
In depth investigation of using flavonoids and multigalloylated compounds, such as pentagalloyl glucose, with isothermal titration microcalorimetry has helped to better characterize “polyphenol”–BSA interaction chemistry and stoichiometry. These approaches have allowed for the assessment of relative weight of hydrogen bonding vs. London interactions, the impact of hydrogen-bonding forming groups or the impact of the number of unsaturated planar rings.54,56–60 Generally, it can be concluded that factors such as higher flexibility of the polyphenolic molecule, greater number of terminal galloyl groups, greater number of hydroxyl moieties and greater degree of polymerization (in the case of tannins) lead to an increase of binding constant with proteins. This non-specific binding can occur on multiple sites, leading to a “polyphenolic coating” of the protein, and eventually to the destabilization and precipitation of the complexes. Both London forces and hydrogen-bonding are involved in the binding phenomenon, similarly to π-stacking in co-pigmentation: peripheral hydrogen-bonding and structuration of water favor desolvation of the unsaturated planar rings and their eventual vertical or T-shaped stacking. In line with these general considerations, it has been reported that quercetin and its glycoside rutin bind more strongly to BSA than catechin and epicatechin,61 despite the bulky and hydrophilic rutinose group attached to the quercetin backbone. This supports the importance of London interactions: flavonols' non-polar groups as in quercetin and rutin are more polarizable than the non-polar groups in flavan-3-ols, hence leading to potentially stronger interactions between induced dipoles.
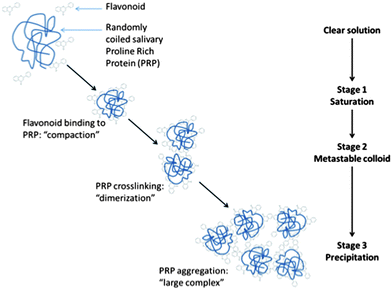
One important consequence of food protein–flavonoid nonspecific binding is that competitive binding should happen in the presence of other proteins. Hence, specific protein–polyphenol binding interactions are likely altered during formulation, processing and eventual consumption of food. Consumption provides the additional exposure to both salivary proteins and digestive enzymes. Indeed, the interaction between tannins or simpler flavonoids with salivary PRPs in the oral cavity is the primary driver62 for the oral astringency phenomenon. The complexation of flavonoids with salivary PRPs results in reduced lubrication and generation of the tactile sensation of astringency often described as dryness and shrinking.63 This phenomenon has been extensively investigated and has been the subject of reviews.6,28,64–66 Generally, oligomeric flavonoids, such as proanthocyanidins, and salivary PRP complexes67,68 aggregate and eventually precipitate. Monomeric flavonoid complexes with salivary proteins do not generally precipitate but still create astringency.63,69 In both cases, the main driving force is the London force between planar rings from the flavonoids and/or the galloyl groups with the pyrrolidine ring face of the proline residues.70,71 However, there are still contradictory studies, for example on the effect of pH on astringency: Charlton and coworkers72 found that enological tannins' affinity for salivary PRPs was not affected by pH between 3.8 and 6.0, while Obreque-Slier and coworkers73 reported the pH dependence of astringency with similar tannins and proteins, astringency being stronger at pH 3.5 compared to pH 7. Interestingly, the astringent response may serve to limit the availability of flavonoids for interaction with digestive enzymes. Naz et al. (2011) more recently demonstrated that the presence of freshly purified salivary PRPs can block the inhibition of lactase by EGCG.74 The interactions between flavonoids and food/salivary proteins through conditions present in the gut lumen must therefore be considered more closely in order to better understand the availability of individual flavonoids for reactivity in the gut. Additional studies in this area are needed to better understand not only reactivity but also bioavailability.
The presence of pre-existing flavonoid–protein complexes in foods and beverages is believed to directly affect subsequent interactions in the oral cavity, and as such modulate astringency and resulting overall sensorial properties of foods. A specific example of this is the attenuation of tea astringency by addition of milk.75,76 The binding of flavan-3-ols in tea beverages with milk derived caseins and whey proteins reduces the availability of flavanol-3-ols for subsequent interactions with salivary proteins thereby lowering the perception of oral astringency. The incidental question of the effect of flavan-3-ols–milk proteins binding on flavan-3-ols bioavailability from foods including tea, cocoa and other astringent foods has been the subject of intense scrutiny. Conflicting results appear in the literature about whether milk proteins may or may not hinder flavan-3-ols bioavailability and subsequent potential health benefits, in cocoa and tea products.77–82 While Egert et al. (2013) recently reported the co-ingestion of tea with proteins (milk, soy or caseinate) significantly reduced the bioavailability of galloyated tea catechins,83 investigation of the impact of milk proteins to impact flavan-3-ol bioavailability from cocoa and tea has shown conflicting results. However, generally, the addition of milk to tea or cocoa has had either no effect or modest effects on absorption or excretion of flavan-3-ols.84–88 However, flavan-3-ol mediated vasodilation was impaired in milk-tea beverages relative to tea alone suggesting that milk protein interactions may serve to limit the cardioprotective benefits of tea.78 The extent to which this outcome is due to protein interactions such as those observed by Egert et al. (2013) or may be related to instability of flavan-3-ols at elevated pH (>6) of milk based systems is not known. Considering oxidative products of monomeric flavan-3-ols that can include dimers and potentially other polymeric oxidized forms,89 it is probable that the loss of activity may be due to a combination of both oxidative breakdown and protein binding.
Flavonoid–protein interactions also play an important role in physico-chemical properties of foods and beverages. For example, these interactions have been associated with improving egg albumen foaming properties,90 degrading textural properties of cheese91 and skim milk gels.92 Of particular economic importance has been haze formation in fruit or plant-based beverages. Indeed, in a manner similar to oral astringency, what has been called tannin or polyphenol-assisted aggregation/precipitation of proteins93 can occur in such systems, in addition to other more extensive interactions that can develop and lead to precipitates, such as those involving oxalic acid with divalent metals. These hazing interactions occur naturally in flavonoid rich beverages such as apple juices,94 wine (red or white)95,96 and beer.97 Indeed, beverage processing from fruits or plants leads to the disruption of fruits' and plants' cell structures and to the presence of polyphenolic compounds and peptides or proteins in the same liquid phase. In the case of beer, haze active proteins are thought to be cereal storage proteins soluble in ethanol such as gliadins or hordeins,98 interacting with complex prenylated flavonoids derived from hops or other phenolics from the cereals themselves.99 Similar to observations with BSA and salivary PRPs, flavonoids may bind to the proteins, forming metastable aggregates that lead to haze formation visible as turbidity in the beverage. Formation of aggregates and haze is greater with increasing molecular weight of flavonoid polymers, with increasing number of terminal galloyl groups and with increasing proline content in the polypeptides.30 Temperature has contrasted effects on beverage hazing: heating a haze-prone beverage could first dissolve aggregates before heat initiates protein denaturation leading to increased exposure of their hydrophobic regions to soluble polyphenols and subsequent formation of greater and larger aggregates.100 Solutions developed to stabilize beverage haze often involve hydrolysis of the haze-active proteins by protease treatment, their removal (by adsorption on silica gel, for example) or removal of the flavonoid compounds by precipitation of haze-active polyphenols with extraneous proteins (gelatin) followed by filtration (clarification and fining processes).30 However, in the particular case of beer, proteins are needed to stabilize beer foam, and polyphenols from hops are an essential component of beer taste. Therefore, balancing stabilization efforts to generate the required visual clarity without overly impacting flavor and character of products requires a fundamental understanding and balanced approach to induce positive flavonoid–protein interactions without negative consequences.
Beyond perceived negative phenomena such as astringency or proteins precipitation, more recent research has focused on how these protein–polyphenol interactions can be used to stabilize, deliver and potentially enhance flavonoid antioxidant capacity. For example, flavonoids are generally prone to chemical transformations such as oxidative degradation at pH > 5 and elevated temperatures encountered during thermal processing of foods and beverages.101,102 Interactions with albumin103 or milk proteins104 have been shown to provide greater flavonoid stability over storage time, most likely due to trapping of polyphenolic molecules and reducing their availability for auto-oxidative degradative reactions. This stabilization effect can be leveraged for applications appropriate for use of proteins as carriers. For example, corn zein fibers have demonstrated the ability to stabilize monomeric catechins in food systems,105 and zein films have been developed for use for bioactive food packaging with various flavonoids.106 In this latter application, flavonoids improved the mechanical properties of zein films, helped them maintain their integrity upon hydration in liquid water and provided them antioxidant activity. Interestingly, catechin showed slower release in water than gallic acid, suggesting stronger interactions with zein, in line with the general drivers of these interactions. Intestinal stability and delivery of flavonoids has been investigated with BSA nanoparticles carrying quercetin (where quercetin antioxidant activity was preserved by protein encapsulation).107 In the case of zein-sodium caseinate microparticles carrying quercetin108 and kafirin microparticles loaded with catechin or tannins,109 the flavonoids and polyphenolic compounds had a direct influence on particles' shape and size, highlighting their impact on proteins conformation and inter-polymer associations. In addition to these effects on particles morphology, quercetin was stabilized and protected from degradation in zein particles and catechin and tannins showed controlled release from kafirin particles. Recently, lupin globulin proteins have been shown, under digestive conditions, to bind and gradually release the polyphenols they naturally contain (apigenin, in particular).110 Critical to this controlled delivery is that the protein–flavonoid interactions do not completely inhibit the digestibility of the protein itself. Another study demonstrated that the entrapment of EGCG in β-lactoglobulin nanoparticles allowed both the suppression of EGCG bitterness and astringency and the limited release of EGCG under gastric digestive conditions.111 These last applications may potentially be leveraged to enhance antioxidant capacities of some protein–flavonoid complexes compared to flavonoids alone. Indeed, while it has been reported that milk derived caseins and albumin mask the antioxidant activity of green and black tea flavonoids in beverage forms112 and that β-globulin-polyphenol (from tea, cocoa and coffee) complexes mask the antioxidant activity of the same polyphenolic extracts alone,113 other studies demonstrated that BSA and gelatin complexes with procyanidins114 or catechins115,116 exhibited higher antioxidant activity than the sum of their individual antioxidant activities. The mechanisms behind these effects are not well understood. Finally, after showing efficient sorption of cranberry and blueberry phenolic extracts in soybean flour,117 cranberry proanthocyanidins and anthocyanins were absorbed into soy, hemp, peanut and pea protein matrices:118 the absorbed extracts showed stability up to 15 weeks at 37 °C and interesting antimicrobial properties against S. aureus and E. coli. In that study, cranberry extracts showed different affinities for the proteins tested, with the soy protein isolate binding the least proanthocyanidins and anthocyanins, and hemp and roasted peanut proteins having the greatest affinity for these extracts. This last example is very representative of the recent efforts made to functionalize foods with polyphenol–protein combinations, taking advantage of their interactions.
It is clear from current literature that flavonoid–protein interactions not only play critical roles in food and beverages quality, but their effects extend beyond the food matrix to the mouth and GI tract impacting digestion and availability of flavonoids for absorption. While the consequences of most interactions reviewed so far (astringency, hazing, decrease of flavonoids bioavailability) are not typically desirable, a more fundamental understanding of their chemistry and biological impacts can be used to leverage positive aspects including controlled digestion to either enhance or limit macronutrient availability, stabilize flavonoids to food processing or digestive conditions and enhance biological delivery and bioactivity (bioavailability) of flavonoids through design of protein based controlled delivery systems.
4. Interactions of flavonoids with food carbohydrates
In food systems, carbohydrates can be broadly classified as either digestible or non-digestible. The former category can be further broken down to sugars, glucose oligomers (such as maltodextrins) and starch, while the latter category typically covers dietary fibers, a large category of polysaccharides with a vast chemical and structural diversity.119 In comparison to flavonoid–protein interactions, much less is known regarding the interaction between flavonoids and carbohydrates. However, given the polymeric character of most carbohydrates and their versatile physicochemical properties (regarding hydrophilic/hydrophobic character, for example), interactions driven by similar factors governing flavonoid–protein interactions are anticipated. As with proteins, the primary focus of this review will be on non-covalent interactions between flavonoids and polymeric carbohydrates.While these effects have not been extensively documented to date, some clear examples of non-covalent interactions between starch and specific flavonoids have been reported through observations of textural properties of starch pastes, though data are often contradictory and depend to a great extent on the type of starch and the type of polyphenolic compounds studied. For example, gelatinization and retrogradation of starch have been reported to be affected by the presence of green tea flavan-3-ols and/or quercetin, gelatinization being facilitated by the presence of quercetin and green tea flavan-3-ols, with peak viscosity of starch gelatinization being negatively correlated with the amount of flavonoids incorporated in starch (up to 15% w/w).120,121 Another study reported no effect of monomeric and oligomeric polyphenolic extracts from sorghum on starch peak viscosity and peak time as measured on a Rapid ViscoAnalyzer, whereas sorghum polymeric tannins contributed to an increase of these parameters in a dose-dependent manner.122 In the same study, monomeric and oligomeric polyphenolic extracts from sorghum increased setback and final viscosity of starch, whereas tannins had no effect on these parameters. In line with the effect on gelatinization, starch retrogradation may be slowed or inhibited by green tea flavan-3-ols.123,124 In these cases, flavonoids acted as plasticizers in starch similarly to what was reported in zein films in the previous section106 and like other hydroxyl-rich small molecules they act as typical plasticizers,125 establishing hydrogen bonds with amylose chains, thus preventing amylose double helices from packing into ordered structures and crystallites, thus retarding the rate of retrogradation. However, these latter observations may be taken with caution as Barros et al. (2012)122 observed that phenolic compounds from sorghum complexed with starch, beyond the simple role of a plasticizer or spacer: more precisely, it appeared that proanthocyanidins interacted more strongly with starch (and preferentially with amylose) than monomeric polyphenolic compounds (which appear to interact equally with amylose and amylopectin). The possibility of such complexes between linear glucan chains of starch and polyphenolic compounds is well supported by other studies reporting inclusion complexes between quercetin and cyclo-amylose126 or flavan-3-ols and cyclodextrins.127–129 Thus, V-type inclusion complexes of flavonoids with amylose can be suspected to form, thus impacting starch digestibility. Indeed, the inner cavities of single amylose helices, cyclodextrins and cycloamylose exhibit the same hydrophobic character. In this case, given the hydrophobicity of the amylose single helix's inner cavity,130 this association may be driven by London forces.
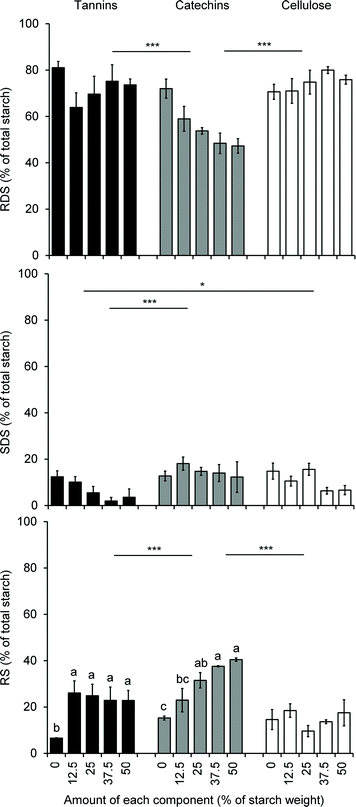
As discussed earlier, flavonoids, like many other polyphenol compounds, are known to alter starch digestion and post-prandial glucose response by interfering with gut digestive enzymes42 and glucose transporter at the intestinal brush border,131–135 but additionally, the structural changes in starch induced by the non-covalent interactions between flavonoids (polymeric or monomeric) described above are also likely to impact starch digestibility and nutritional properties. Here again, contradictory observations have been made. On the one hand, the starch structure was reported to be altered when co-processed with flavan-3-ols in a fashion consistent with enhancing the accessibility of amylose chains to amylase enzymes.136 On the other hand, sorghum phenolic extracts decreased the estimated glycemic index and the amount of resistant starch in porridges made of normal corn starch, high amylose corn starch and sorghum flours.137 Two other studies made interesting differences between the effects of monomeric and polymeric sorghum flavonoids on starch digestibility. The first showed that only sorghum tannins increased resistant starch levels in normal, waxy and high amylose corn starch, while lower molecular weight polyphenolic extracts had no impact.122 In contrast, the second study showed no impact of sorghum tannins on rapidly digestible, slowly digestible and resistant starch levels in native sorghum flours containing tannins at the level of 0 to 50 mg of catechin equivalents per gram of flour; however, sorghum tannins added to normal corn starch (up to 50% on starch weight basis) decreased the level of slowly digestible starch and increased the level of resistant starch in a non-dose dependent manner, while the same addition of catechins to normal corn starch decreased the level of rapidly digestible starch and increased the level of resistant starch in a dose dependent manner138 (Fig. 4). In summary and despite contradictory observations, it seems that proanthocyanidins and monomeric flavonoids impact starch digestibility through structural modifications of starch when present at high levels (well above 10% on starch weight basis). When present at naturally occurring levels in flours or starches, flavonoids may impact starch digestibility only through digestive enzymes inhibition as observed with flavonoids on α-amylase and amyloglucosidase139 in in vitro assays.
In this light, it is important to note that extension of in vitro enzyme inhibition assays must be taken in perspective considering the complexity of the starch structure as altered by processing and the presence of competing interactions with proteins (as described previously). Additional in vivo assessments are needed using both model and real food systems to better understand the physiological relevance of these effects in humans.
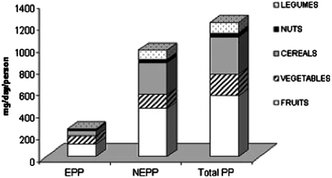
Interactions between flavonoids and non-starch polysaccharides, i.e. dietary fibers, in foods are also of interest. Indeed, in a flavonoid rich Mediterranean diet, a large portion of flavonoid intake is derived from forms that are not easily extractable from the food matrix, bound to dietary fibers in particular. Strictly considering flavonoids, the daily intake is provided equally from extractable and non-extractable forms (177 vs. 172 mg day−1) in the Mediterranean diet as proposed by Arranz and coworkers140 (Fig. 5). This role of “polyphenol carrier” by non-digestible carbohydrates has been described as an “essential physiological function” of fibers and may contribute, in part, to the positive health benefits associated with fiber-rich diets.141,142 At a molecular level, this statement is well supported by observations such as those made by Leong and Oey143 that loss of cellular structure through processing of fresh fruits and vegetables (heating and freezing) enhanced the release and extractability of polyphenols (anthocyanins in their study) as compared to the same fresh products. Similar results have been observed with tomatoes where high-intensity pulsed electric fields were applied to enhance the release and extractability of polyphenolic compounds relative to an unprocessed control.144 Additionally, two studies modeling plant cell walls using cellulose and pectin showed how such composite materials were indeed able to absorb and retain model phenolic acids145 and anthocyanins146via non-covalent interactions (ionic with pectin and hydrophobic with cellulose). It was also observed that the protein content of apple cell walls does not affect the non-covalent binding capacity of the cell walls themselves and that this capacity is rather driven by their pectin content, highlighting the importance of ionic interactions in flavonoid–polysaccharide interactions.147
Interactions with plant cell wall polysaccharides have potential effects on the manufacture of polyphenol and tannin-rich products such as wine and select fruit juices, including orange and apple juices that can have significant amounts of pectin in the native fruit. Tannins being a major factor of wine taste profile and quality, their extractability from grape berries and the limitations imposed by interactions with cell wall polysaccharides are crucial.148 In this perspective, hydrolysis of cell wall polysaccharides by enzymes at different stages of grape juice and wine-making has been studied and generally showed improvement of juice yield, wine color (due to anthocyanidins) intensity and stability, either via better extractability in the must149 or via hydrolysis of potential haze-active polysaccharides in the wine.150 Beyond the actual making of the wine, these interactions also impact the organoleptic properties of the finished product. Indeed, the soluble pectic polysaccharides contained in wine may interfere with salivary PRPs–tannins aggregation and subsequent perception of astringency151 often desired in wine characteristics. These observations have been supported by the rating of wine astringency after the addition of specific polysaccharides: while acidic polysaccharides such as rhamnogalacturonan II decreased astringency, other neutral polysaccharides (such as mannoproteins and arabinogalactan–proteins) had little to no effect.152–154 In line with this last observation, mannoproteins, produced by yeast fermentation in wine, seem to stabilize tannins155,156 in the finished product. Finally, it has been observed that sugars (glucose, fructose and sucrose) also improve stability of tannin–protein complexes in wine via an increase of their solubility, although the experiments carried out involved higher sugar concentrations than those traditionally observed in wine.157
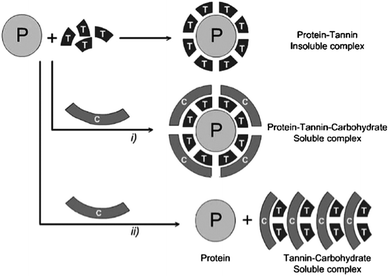
Generally, non-specific hydrogen bonding and ionic interactions seem to be the primary drivers for the association of non-starch polysaccharides with flavonoids. Gum arabic, cyclodextrins, pectins and other polygalacturonic acids, xanthan gum, dextrans, arabinogalactans and glucose have been assessed according to their ability to disrupt procyanidin–protein interactions.158–161 This ability obviously depends on the type of procyanidins and proteins studied. In these studies, ionic polysaccharides were better at preventing the formation of insoluble tannin–protein aggregates or at solubilizing them. This effect decreased as procyanidins' degree of polymerization increased. Two mechanisms were proposed to explain this observation. On the one hand, polysaccharides, such as pectins, seem to form a water-soluble protein–polyphenol–polysaccharide complex, thus stabilizing aggregates in solution or solubilizing them. On the other hand, polysaccharides such as gum arabic can compete directly with proteins for non-specific tannin-binding, and thus prevent the formation of aggregates. These mechanisms have been reviewed by Scollary et al. (2012)162 and are illustrated in Fig. 6. In this light, gum arabic is particularly interesting as it is mainly composed of a glucoronoarabinogalactan anionic fraction (70–90% of the gum in weight) and of a glycoproteic hydrophobic fraction (up to 10% in weight) where the polysaccharidic component is attached to the protein component via linkages to hydroxyproline units.163 The interaction of flavonoids with gum arabic potentially interferes with flavonoid–protein complexation. However, additional insight is needed to better understand this phenomenon and if and which specific fractions of gum arabic polymers might be involved in these interactions.
However, beyond their potential for interfering with or stabilizing flavonoid–protein aggregates, flavonoid–polysaccharide associations also lead to the formation of non-desirable insoluble aggregates in liquid phases. Similarly to gum arabic, ionic polysaccharides such as pectins or arabinogalactans can also bind to flavonoids directly and form complexes without the presence of proteins. Much like protein interactions, these associations may also lead to turbidity in, for example, orange albedo extracts164,165 or formation of nanovesicles in grape juice concentrates.166 Such interactions have also been reported to enhance haze formation created by polyphenols and proteins in fruit juices.100,167 When characterized, these interactions can be used advantageously in fining of wine proanthocyanidins, however they have been found to be less efficient than proteins (as used in beer processing). Still, apple and grape fibers from pomace have been recently reported to bind and precipitate wine tannins.168,169
In an attempt to leverage these binding properties, pectins in particular have been incorporated into bread doughs as a source of dietary fiber enrichment along with addition of fruit polyphenols.170–172 As expected, fruit polyphenols interacted with wheat proteins, starch and with added pectins, significantly modifying bread textural properties. Spectroscopic analyses provided evidence of the occurrence of hydrogen bonding, London forces and protein conformational changes, i.e. non-covalent interactions between polyphenolic compounds and bread macronutrients. However, the relatively low recovery of polyphenols from the finished bread and the textural property changes compared to the control bread suggest that polyphenolic compounds may have interacted with proteins mainly through covalent cross-linking during baking. Similarly, simple adsorption of green tea catechins on oat β-glucans has been studied, showing that adsorption was pH and temperature dependent and impacted antioxidant capacity of both β-glucans and flavan-3-ols.173,174 The potential for such adsorptive behavior is very interesting for the delivery of β-glucans and favan-3-ols simultaneously, given the physiological properties of these two compounds. However, these studies were conducted at pH 5–7 where flavan-3-ols are less stable and prone to auto-oxidative degradation,175 hence these results must be taken with caution until further additional experiments begin to better characterize this phenomenon. Nevertheless, flavonoid–β-glucan non-covalent interactions were observed elsewhere in an attempt to determine structural features of flavonoids that would favor these interactions.176,177 While this binding does not seem dependent on the β-glucan structure (no significant preferential binding for β-glucans from oat and barley), it seems dependent on the flavonoids core structure, with flavonols and flavones binding more with β-glucans than flavanones, isoflavones and flavan-3-ols. These characteristics are very similar to those observed by Lo Piparo et al. (2008)42 on specific binding of flavonoids with enzymes. Regardless of their core structure, it seems that binding of flavonoids to β-glucans is positively correlated with flavonoids hydroxylation with an optimum at three hydroxyl groups, before a decrease in binding with four or more hydroxyl groups. Flavonoids glycosylation generally limits binding to β-glucans, although exceptions occur (for myricetin and daidzein, in particular). In the case of catechins, galloylation seemed to increase the binding capacity to β-glucans. These observations suggest that flavonoid–β-glucan complexation is mainly driven by hydrogen bonding interactions.
Besides the study of these complexes, numerous efforts have been made to simply entrap or encapsulate flavonoids in polysaccharide particles. The use of polysaccharides for entrapment has been widely applied in the pharmaceutical field for stabilization, controlled or targeted delivery of bioactive compounds. Controlled release of flavonoids as bioactive compounds can also have applications in food safety and preservation or in functional foods for specific health benefits in the GI tract and systemically. A few examples of flavonoid-entrapment applications include: chitosan-entrapment of green tea polyphenols with demonstrated improved in vitro antitumoral action178 and improved in vivo wound healing activity;179 EGCG loaded nanoparticles made from gum arabic and maltodextrin with improved EGCG stability;180 quercetin incorporation into a guar gum-based matrix181 or anthocyanins entrapped in oxidized starch microgels182 for improved release profile; quercetin incorporation in pectin–hydroxypropylmethylcellulose tablets for specific delivery to the colon;183 quercetin complexation with pectin for enhanced bioavailability;184 resveratrol affinity with chitosan as well as methylcellulose in the making of edible films,185etc. Chitosan is well represented in these examples as it may indeed favor ionic interactions with flavonoids due to its naturally cationic character. Quercetin too has been entrapped in chitosan particles for inclusion in aqueous media and protection against degradation.186 Interestingly, the interaction between chitosan and quercetin has been reported to lead to a change in tautomeric configuration of quercetin.187 Indeed, the transition from the keto to the enolic tautomers leads to the zwitterionic form of the enolic tautomer which may be more favorable to ionic interactions with chitosan, according to former description of quercetin tautomers.188 However, the question of antimicrobial activity of chitosan–flavonoid complexes may be raised, as this non-covalent complexation is mediated by chitosan's cationic amine moieties, which are also in large part responsible for its antimicrobial properties: expected antimicrobial activity may thus be hindered by this complexation. To the best of our knowledge, this aspect has not been studied to date.
Similarly, the ultimate physiological consequences of such structural flavonoid–polysaccharide interactions may also have direct consequences on ultimate bioactivity of polyphenols. The extent to which these interactions alter availability for reaction and subsequent absorption in the gut merits further investigation. Utilization of both carbohydrates and flavonoids by intestinal microbiota may be altered by their interactions, both covalent and non-covalent. While the focus of this paper is on non-covalent interactions, Saura-Calixto et al.189 demonstrated that fermentation of non-extractable proanthocyanidins, likely covalently bound to cell wall fibers, was enhanced by the presence of dietary fiber. Similarly, cecal fermentation of apple polyphenols was found to be enhanced in rats treated simultaneously with apple pectin.190 These studies suggest that flavonoid–polysaccharide interactions may extend through digestion and impact availability of phenolics for interaction in the lower gut. Additional insight is needed to better understand the interrelationship between flavonoids, microbiota and substrates including carbohydrates as they relate to potential health benefits.
Finally, it is interesting to note that an isolated study showed that thermoreversible gels can be formed from tamarind seed xyloglucan by addition of EGCG,191 suggesting that EGCG plays the role of a non-covalent cross-linker in the three-dimensional polysaccharide network.
In summary, it is clear that a diverse array of interactions have been documented between flavonoids and carbohydrates. Flavonoids can interact with starch impacting both physico-chemical properties (gelatinization and retrogradation) as well as with its nutritional properties (digestibility and glucose transport). Like proteins, non-digestible polysaccharides also exhibit binding haze-active properties by themselves (mainly via ionic interactions). In the particular case of fruits' and vegetables' cell walls, polysaccharides can also bind directly to flavonoids which can be released by destructuration of the cell walls. Interestingly, while the potential to leverage specific carbohydrate interactions for stability and controlled delivery of flavonoids is underway, much less is known regarding the impact of natural interactions in commonly consumed fruits, vegetables and beverages. Considering the importance of carbohydrates and fruit fibers specifically as dietary sources of phenolics, polyphenolics and flavonoids specifically in processed foods, the potential of carbohydrate interactions in both fresh and processed foods merits further investigation.
5. Interactions of flavonoids with food lipids
Interactions of flavonoids with lipids in foods have received little attention, to date, with the notable exception of some specific interaction in fruit/vegetable oils. The most prominent oil source of phenolics is olive oil. Both olive pomace and its mill waste waters generated through oil processing contain appreciable quantities of various phenolic acids and polyphenolic compounds including select flavonoids as well as verbascoside, isoverbascoside and hydroxytyrosol in the order of several hundred ppm.192,193 However, only a small amount of a few flavones (apigenin, luteolin, and rutin) can be found in olive oil itself.194–197 This may be well explained by the partition coefficient of flavonoids between aqueous and lipid-based phases,198–200 or between octanol and water (Table 2): apigenin and rutin's partition coefficient is among the highest of common flavonoids. The advantage has been taken from this partition pattern with the study of oil-in-water emulsions stabilized by flavonoids. For example, tiliroside, rutin and naringin stabilized n-tetradecane-in-water emulsions, with average droplet sizes of 16, 6 and 5 μm, respectively200 (Fig. 7). The stability of the emulsions and their droplet sizes highlighted the ability of some flavonoids to be adsorbed at hydrophobic–hydrophilic interfaces as emulsifiers, which could potentially be used to enhance their bioavailability. Similarly, it has been shown that rutin is efficient at stabilizing sunflower oil-whey-water emulsions by partially replacing whey at the oil–water interface.201 It is critical to note that these examples were based on systems using flavonoid glycosides that exhibit amphiphilic character due to their hydrophilic sugar moiety and their more or less hydrophobic flavonoid component (particularly tiliroside and rutin, which are glycosides of flavonols kaempferol and quercetin).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/wat note log
Poct/wat note log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) for some flavanols, flavones, anthocyanidins and isoflavonoids. Values are means of data collected in the literature,227 stars indicate values computed with ALOGPS 2.1 software228,229
P) for some flavanols, flavones, anthocyanidins and isoflavonoids. Values are means of data collected in the literature,227 stars indicate values computed with ALOGPS 2.1 software228,229
| Common name | CAS # | Estimated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
|
|---|---|---|---|
| Flavanols | (+)-Catechin | 154-23-4 | 0.25 |
| (−)-Gallocatechin | 3371-27-5 | 0.6* | |
| (−)-Catechin 3-gallate | 130405-40-2 | 2.55* | |
| (−)-Epicatechin | 490-46-0 | 0.3 | |
| (−)-Epigallocatechin | 970-74-1 | 0.77* | |
| (−)-Epicatechin 3-gallate | 1257-08-5 | 2.55* | |
| (−)-Epigallocatechin 3-gallate | 989-51-5 | 1.08 | |
| Flavones | Luteolin | 491-70-3 | 2.37 |
| Apigenin | 520-36-5 | 2.23 | |
| Tangeretin | 481-53-8 | 2.85* | |
| Flavonols | Quercetin | 117-39-5 | 1.96 |
| Kaempferol | 520-18-3 | 2.3 | |
| Myricetin | 529-44-2 | 0.99* | |
| Fisetin | 528-48-3 | 1.73* | |
| Isorhamnetin | 480-19-3 | 1.69* | |
| Flavonones | Hesperetin | 520-33-2 | 1.99* |
| Naringenin | 480-41-1 | 2.26* | |
| Eriodictyol | 552-58-9 | 2.15 | |
| Flavanonol | Taxifolin | 480-18-2 | 1.18 |
| Anthocyanidins | Cyanidin chloride | 528-58-5 | 1.56* |
| Delphinidin chloride | 528-53-0 | 1.08* | |
| Pelargonidin chloride | 134-04-3 | 1.81* | |
| Isoflavones | Genistein | 446-72-0 | 3.04 |
| Daidzein | 486-66-8 | 2.71 | |
| Glycitein | 40957-83-3 | 1.97 | |
| Isoflavanes | (S)-Equol | 531-95-3 | 3.04* |
Nevertheless, solubility characteristics of olive flavonoids encouraged efforts to recover valuable flavonoids and other phenolic compounds from olive oil extraction wastes.202 In an attempt to convert vegetable oils into functional ingredients, flavonoids extracted from olive tree leaves have been used to enrich various vegetable oils, resulting in better stability of the oils towards oxidation.203 More generally, flavonoids from various sources are investigated in the perspective of using “natural antioxidants” for oils stabilization.204–208 For example, a thorough study209 investigated the effect of different flavonoids on palm oil auto-oxidation and showed that kaempferol, myricetin and quercetin (all of them flavonol compounds) exhibited higher antioxidative action than apigenin and luteolin (flavones), catechin (flavanol) and naringenin (flavanone). Similar observations were made on canola oil: myrecitin, quercetin, (−)-epicatechin, naringenin and naringin were the most efficient of all tested flavonoids at reducing the peroxide value of canola oil, all tested flavonoids providing equal or better antioxidant protection to the oil than BHA and BHT.210,211 This suggests again a relationship between the structure of flavonoids and their ability to bind with lipidic compounds. While promising, legal consideration of these additions as potential adulterants in oils must be carefully considered.
Dietary lipids have demonstrated the ability to enhance the acute absorption of select flavonoid aglycones such as quercetin.212 This suggests that flavonoids may be partially absorbed through lipophilic routes and associate with lipoprotein fractions such as HDL. This association with HDL has been shown by McAnlis et al. (1999) in lipoprotein fractions of human plasma, whereas no quercetin was found in LDL and VLDL fractions.213 However, the HDL fractions of plasma contained other proteins, this observation could be biased by other confounding protein–quercetin associations.
Aside from food systems, the molecular and mechanistic aspects of these interactions have been studied. Interestingly, interactions of flavan-3-ols and complex proanthocyanidins with cell-membrane lipid bilayers214,215 and liposome models216–218 have been demonstrated. Indeed, lipid bilayers have been reported to interact with various green tea catechins such as (−)-epigallocatechin gallate,219–221 (−)-epicatechin gallate222 and (+)-catechin,223 for example. By changing cellular membrane's physical properties such as permeability, these interactions are thought to partly explain the protecting effect of flavonoids against oxidation at the cellular level,224 and some of their antimicrobial properties.225 Similarly to what was observed in edible oils, non-polar flavonols exhibited the strongest interactions with liposomes,215 suggesting interaction mechanisms based on London interactions between non-polar polarizable groups as described for proteins. The polarizability of these non-polar groups is greater in isoflavones and flavonols than in flavanones and flavan-3-ols such as green tea catechins.135 Hence, it would be interesting to study the interactions of lipid bilayers with isoflavones and flavonols which can potentially lead to stronger interactions. It is interesting to note that non-polar flavonols showing the strongest interactions with lipid bilayers are also the major flavonoid compounds found in vegetable oils, especially in vegetable oils rich in mono and polyunsaturated fatty acids (with non-polar polarizable alkyl chains), such as olive oil. Although studies on lipid bilayers are not directly related to food or true biological systems, technological and nutritional applications directly derived from this knowledge can be foreseen. Applications such as polyphenol-loaded nano-scale liposomes have been studied, where tea flavonoids and vitamin E were entrapped in nano-scale liposomes and were prepared by reverse-phase evaporation.226 More directly related to the biological function is the ability of flavonoids and their subsequent metabolites to partition into specific lipoprotein particles and fatty tissues. This distribution may influence their ability to accumulate in specific tissues and drive biological processes consistent with modulation of disease risk. Therefore, additional insights into the interaction with lipid and impacts of lipid on stability, bioavailability/metabolism and function of flavonoids are needed.
6. Conclusions and future directions
Flavonoids are phytonutrients widely distributed in common foods and beverages including tea, cocoa, fruits, vegetables and their processed products. The potential for their positive effects on human health has led to increased interest in their incorporation into the diet and novel foods. In consideration of whole foods, interactions between flavonoids and macronutrients within food or beverage matrices are known to greatly impact their functionality and the properties of these matrices themselves. While significant research has focused on flavonoids–proteins non-covalent binding, it has been shown that similar interactions may also occur with carbohydrates and lipids. These interactions are the result of a combination of hydrogen bonding and van der Waals forces and thus vary depending upon the flavonoids chemical structure: higher numbers of hydrogen bonding capable moieties and higher numbers of non-polar polarizable aromatic rings favor these interactions. Still at the molecular level, non-covalent binding with polyphenols has shown to impact conformation of macromolecules involved in these complexes. The consequences of these interactions, including astringency or haze formation, are often seen as detrimental for the food and beverage systems. However, deep understanding of these phenomena provides opportunities for developing strategies for their inclusion in functional foods and nutraceuticals. Particularly, while thermodynamic aspects of these interactions have been extensively covered, it appears that controlling the kinetics of these interactions could open opportunities for enhanced food quality and properties. Additionally, flavonoid–macronutrient interactions have been studied from the perspective of enhancing stability and bioavailability of flavonoids with the hope of improving their effectiveness as health promoting agents. In this context, more studies are needed to better understand the role that these interactions may play in altering metabolism of flavonoids both by mammalian systems and by colonic microbiota. The potential of these interactions to modify the qualitative and quantitative profiles of biologically relevant flavonoid metabolites must be defined to better leverage strategies that optimize delivery of physiological health benefits in humans.However, it is important that strategies for delivering health promoting compounds do not compromise sensorial properties and quality attributes of the final food or beverage products. More knowledge on these interactions and a deep understanding of their driving forces should lead to a better understanding of formulation strategies and processing conditions for improved flavonoid delivery.
Disclaimer
Author N. Bordenave is an employee of PepsiCo Inc. The views expressed in this article are those of the author and do not necessarily reflect the position or policy of PepsiCo, Inc. Authors M.G. Ferruzzi and B. Hamaker have nothing to declare.Acknowledgements
The authors wish to thank Sidney E. Moser (Purdue University) for her insightful comments and suggestions.References
- A. Bennick, Crit. Rev. Oral Biol. Med., 2002, 13, 184–196 Search PubMed.
- L. Gu, M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz, S. Gebhardt and R. L. Prior, J. Nutr., 2004, 134, 613–617 CAS.
- G. R. Beecher, J. Nutr., 2003, 133, 3248S–3254S CAS.
- H. D. Sesso, J. M. Gaziano, S. Liu and J. E. Buring, Am. J. Clin. Nutr., 2003, 77, 1400–1408 CAS.
- V. Knaze, R. Zamora-Ros, L. Luján-Barroso, I. Romieu, A. Scalbert, N. Slimani, E. Riboli, C. T. M. Van Rossum, H. B. Bueno-De-Mesquita, A. Trichopoulou, V. Dilis, K. Tsiotas, G. Skeie, D. Engeset, J. Ramón Quirós, E. Molina, J. M. Huerta, F. Crowe, E. Wirfäl, U. Ericson, P. H. M. Peeters, R. Kaaks, B. Teucher, G. Johansson, I. Johansson, R. Tumino, H. Boeing, D. Drogan, P. Amiano, A. Mattiello, K. T. Khaw, R. Luben, V. Krogh, E. Ardanáz, C. Sacerdote, S. Salvini, K. Overvad, A. Tjønneland, A. Olsen, M. C. Boutron-Ruault, G. Fagherazzi, F. Perquier and C. A. González, Br. J. Nutr., 2012, 108, 1095–1108 CrossRef CAS PubMed.
- E. Jöbstl, J. O'Connell, J. P. A. Fairclough and M. P. Williamson, Biomacromolecules, 2004, 5, 942–949 CrossRef PubMed.
- M. A. Joslyn and J. L. Goldstein, Adv. Food Res., 1964, 13, 179–217 CAS.
- G. Luck, H. Liao, N. J. Murray, H. R. Grimmer, E. E. Warminski, M. P. Williamson, T. H. Lilley and E. Haslam, Phytochemistry, 1994, 37, 357–371 CrossRef CAS.
- E. C. Bate-Smith, Food, 1954, 23, 124–135 CAS.
- J. A. M. Kyle and G. G. Duthie, in Flavonoids: Chemistry, Biochemistry and Applications, ed. O. M. Andersen and K. R. Markham, CRC Press, Boca Raton, FL, 2006, pp. 219–262 Search PubMed.
- J. A. Ross and C. M. Kasum, Annu. Rev. Nutr., 2002, 22, 19–34 CrossRef CAS PubMed.
- U.S. Department of Agriculture, Agricultural Research Service, USDA Database for the Flavonoid Content of Selected Foods, Release 3.0, http://www.ars.usda.gov/nutrientdata/flav, accessed 2012 Search PubMed.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jiménez, Am. J. Clin. Nutr., 2004, 79, 727–747 CAS.
- A. Crozier, I. B. Jaganath and M. N. Clifford, Nat. Prod. Rep., 2009, 26, 1001–1043 RSC.
- M. M. Murphy, L. M. Barraj, D. Herman, X. Bi, R. Cheatham and R. K. Randolph, J. Acad. Nutr. Diet., 2012, 112, 222–229 CrossRef CAS PubMed.
- C. Lakenbrink, S. Lapczynski, B. Maiwald and U. H. Engelhardt, J. Agric. Food Chem., 2000, 48, 2848–2852 CrossRef CAS PubMed.
- C. Manach and J. L. Donovan, Free Radical Res., 2004, 38, 771–785 CrossRef CAS.
- A. P. Neilson and M. G. Ferruzzi, Annu. Rev. Food Sci. Technol., 2011, 2, 125–151 CrossRef CAS PubMed.
- J. P. E. Spencer, J. Nutr., 2003, 133, 3255S–3261S CAS.
- M. P. Gonthier, V. Cheynier, J. L. Donovan, C. Manach, C. Morand, I. Mila, C. Lapierre, C. Rémésy and A. Scalbert, J. Nutr., 2003, 133, 461–467 CAS.
- T. Kohri, F. Nanjo, M. Suzuki, R. Seto, N. Matsumoto, M. Yamakawa, H. Hojo, Y. Hara, D. Desai, S. Amin, C. Clifford Conaway and F. L. Chung, J. Agric. Food Chem., 2001, 49, 1042–1048 CrossRef CAS PubMed.
- Y. T. Lin, S. L. Hsiu, Y. C. Hou, H. Y. Chen and P. D. L. Chao, Biol. Pharm. Bull., 2003, 26, 747–751 CAS.
- M. Monagas, M. Urpi-Sarda, F. Sánchez-Patán, R. Llorach, I. Garrido, C. Gómez-Cordovés, C. Andres-Lacueva and B. Bartolomé, Food Funct., 2010, 1, 233–253 CAS.
- X. Tzounis, J. Vulevic, G. G. C. Kuhnle, T. George, J. Leonczak, G. R. Gibson, C. Kwik-Uribe and J. P. E. Spencer, Br. J. Nutr., 2008, 99, 782–792 CrossRef CAS PubMed.
- O. Dangles, C. Stoeckel, M. C. Wigand and R. Brouillard, Tetrahedron Lett., 1992, 33, 5227–5230 CrossRef CAS.
- S. Galland, N. Mora, M. Abert-Vian, N. Rakotomanomana and O. Dangles, J. Agric. Food Chem., 2007, 55, 7573–7579 CrossRef CAS PubMed.
- B. Berké and V. A. P. De Freitas, Food Chem., 2005, 90, 453–460 CrossRef PubMed.
- V. de Freitas and N. Mateus, Curr. Org. Chem., 2012, 16, 724–746 CrossRef CAS.
- A. Papadopoulou and R. A. Frazier, Trends Food Sci. Technol., 2004, 15, 186–190 CrossRef CAS PubMed.
- K. J. Siebert, J. Agric. Food Chem., 1999, 47, 353–362 CrossRef CAS PubMed.
- J. Xiao and G. Kai, Crit. Rev. Food Sci. Nutr., 2012, 52, 85–101 CrossRef CAS PubMed.
- S. Bittner, Amino Acids, 2006, 30, 205–224 CrossRef CAS PubMed.
- J. L. Rotundo and M. E. Westgate, Field Crop. Res., 2009, 110, 147–156 CrossRef PubMed.
- J. Xi and R. Guo, Int. J. Biol. Macromol., 2007, 40, 305–311 CrossRef CAS PubMed.
- J. Xiao, F. Mao, F. Yang, Y. Zhao, C. Zhang and K. Yamamoto, Mol. Nutr. Food Res., 2011, 55, 1637–1645 CAS.
- G. J. McDougall and D. Stewart, BioFactors, 2005, 23, 189–195 CrossRef CAS.
- S. C. Forester, Y. Gu and J. D. Lambert, Mol. Nutr. Food Res., 2012, 56, 1647–1654 CAS.
- Y. Gu, W. J. Hurst, D. A. Stuart and J. D. Lambert, J. Agric. Food Chem., 2011, 59, 5305–5311 CrossRef CAS PubMed.
- H. Sugiyama, Y. Akazome, T. Shoji, A. Yamaguchi, M. Yasue, T. Kanda and Y. Ohtake, J. Agric. Food Chem., 2007, 55, 5906 CrossRef CAS.
- H. Sugiyama, Y. Akazome, T. Shoji, A. Yamaguchi, M. Yasue, T. Kanda and Y. Ohtake, J. Agric. Food Chem., 2007, 55, 4604–4609 CrossRef CAS PubMed.
- F. P. Flores, R. K. Singh, W. L. Kerr, R. B. Pegg and F. Kong, J. Agric. Food Chem., 2013, 61, 4441–4447 CrossRef CAS PubMed.
- E. Lo Piparo, H. Scheib, N. Frei, G. Williamson, M. Grigorov and C. J. Chou, J. Med. Chem., 2008, 51, 3555–3561 CrossRef CAS PubMed.
- L. H. Riihimäki, M. J. Vainio, J. M. S. Heikura, K. H. Valkonen, V. T. Virtanen and P. M. Vuorela, J. Agric. Food Chem., 2008, 56, 7721–7729 CrossRef PubMed.
- R. Zorilla, L. Liang, G. Remondetto and M. Subirade, Dairy Sci. Technol., 2011, 91, 629–644 CrossRef CAS.
- V. Aguié-Béghin, P. Sausse, E. Meudec, V. Cheynier and R. Douillard, J. Agric. Food Chem., 2008, 56, 9600–9611 CrossRef PubMed.
- C. Pascal, F. Paté, V. Cheynier and M. A. Delsuc, Biopolymers, 2009, 91, 745–756 CrossRef CAS PubMed.
- T. Richard, D. Lefeuvre, A. Descendit, S. Quideau and J. P. Monti, Biochim. Biophys. Acta, Gen. Subj., 2006, 1760, 951–958 CrossRef CAS PubMed.
- C. Simon, K. Barathieu, M. Laguerre, J. M. Schmitter, E. Fouquet, I. Pianet and E. J. Dufourc, Biochemistry, 2003, 42, 10385–10395 CrossRef CAS PubMed.
- C. Le Bourvellec and C. M. G. C. Renard, Crit. Rev. Food Sci. Nutr., 2012, 52, 213–248 CrossRef CAS PubMed.
- A. E. Hagerman, M. E. Rice and N. T. Ritchard, J. Agric. Food Chem., 1998, 46, 2590–2595 CrossRef CAS.
- S. Soares, N. Mateus and V. De Freitas, J. Agric. Food Chem., 2007, 55, 6726–6735 CrossRef CAS PubMed.
- P. Bourassa, C. D. Kanakis, P. Tarantilis, M. G. Pollissiou and H. A. Tajmir-Riahi, J. Phys. Chem. B, 2010, 114, 3348–3354 CrossRef CAS PubMed.
- L. Liang, H. A. Tajmir-Riahi and M. Subirade, Biomacromolecules, 2008, 9, 50–56 CrossRef CAS PubMed.
- E. R. Deaville, R. J. Green, I. Mueller-Harvey, I. Willoughby and R. A. Frazier, J. Agric. Food Chem., 2007, 55, 4554–4561 CrossRef CAS PubMed.
- L. Aberkane, J. Jasniewski, C. Gaiani, R. Hussain, J. Scher and C. Sanchez, Food Hydrocolloids, 2012, 29, 9–20 CrossRef CAS PubMed.
- M. A. Dobreva, R. A. Frazier, I. Mueller-Harvey, L. A. Clifton, A. Gea and R. J. Green, Biomacromolecules, 2011, 12, 710–715 CrossRef CAS PubMed.
- R. A. Frazier, E. R. Deaville, R. J. Green, E. Stringano, I. Willoughby, J. Plant and I. Mueller-Harvey, J. Pharm. Biomed. Anal., 2010, 51, 490–495 CrossRef CAS PubMed.
- R. A. Frazier, A. Papadopoulou and R. J. Green, J. Pharm. Biomed. Anal., 2006, 41, 1602–1605 CrossRef CAS PubMed.
- R. A. Frazier, A. Papadopoulou, I. Mueller-Harvey, D. Kissoon and R. J. Green, J. Agric. Food Chem., 2003, 51, 5189–5195 CrossRef CAS PubMed.
- R. Ferrer-Gallego, R. Gonçalves, J. C. Rivas-Gonzalo, M. T. Escribano-Bailón and V. De Freitas, Food Chem., 2012, 135, 651–658 CrossRef CAS PubMed.
- A. Papadopoulou, R. J. Green and R. A. Frazier, J. Agric. Food Chem., 2005, 53, 158–163 CrossRef CAS PubMed.
- D. Rossetti, J. H. H. Bongaerts, E. Wantling, J. R. Stokes and A. M. Williamson, Food Hydrocolloids, 2009, 23, 1984–1992 CrossRef CAS PubMed.
- Astringency and Bitterness of Flavonoid Phenols, A. C. Noble, in Chemistry of Taste, ACS Symposium Series, ch. 15, vol. 825, pp. 192–201 Search PubMed.
- P. Bandyopadhyay, A. K. Ghosh and C. Ghosh, Food Funct., 2012, 3, 592–605 CAS.
- M. G. Ferruzzi, N. Bordenave and B. R. Hamaker, Physiol. Behav., 2012, 107, 591–597 CrossRef CAS PubMed.
- E. Halsam and T. H. Lilley, Crit. Rev. Food Sci. Nutr., 1988, 27, 1–40 CAS.
- P. Sarni-Manchado, V. Cheynier and M. Moutounet, J. Agric. Food Chem., 1999, 47, 42–47 CrossRef CAS PubMed.
- J. R. Bacon and M. J. C. Rhodes, J. Agric. Food Chem., 2000, 48, 838–843 CrossRef CAS PubMed.
- V. De Freitas and N. Mateus, J. Agric. Food Chem., 2001, 49, 940–945 CrossRef CAS PubMed.
- P. Sarni-Manchado and V. Cheynier, J. Mass Spectrom., 2002, 37, 609–616 CrossRef CAS PubMed.
- N. J. Murray, M. P. Williamson, T. H. Lilley and E. Haslam, Eur. J. Biochem., 1994, 219, 923–935 CrossRef CAS.
- A. J. Charlton, N. J. Baxter, M. L. Khan, A. J. G. Moir, E. Haslam, A. P. Davies and M. P. Williamson, J. Agric. Food Chem., 2002, 50, 1593–1601 CrossRef CAS PubMed.
- E. Obreque-Slier, Á. Peña-Neira and R. López-Solís, LWT–Food Sci. Technol., 2012, 45, 88–93 CrossRef CAS PubMed.
- S. Naz, R. Siddiqi, T. P. Dew and G. Williamson, J. Agric. Food Chem., 2011, 59, 2734–2738 CrossRef CAS PubMed.
- E. Jöbstl, J. R. Howse, J. P. A. Fairclough and M. P. Williamson, J. Agric. Food Chem., 2006, 54, 4077–4081 CrossRef PubMed.
- Y. Yan, J. Hu and P. Yao, Langmuir, 2009, 25, 397–402 CrossRef CAS PubMed.
- J. B. Keogh, J. McInerney and P. M. Clifton, J. Food Sci., 2007, 72, S230–S233 CrossRef CAS PubMed.
- M. Lorenz, N. Jochmann, A. Von Krosigk, P. Martus, G. Baumann, K. Stangl and V. Stangl, Eur. Heart J., 2007, 28, 219–223 CrossRef PubMed.
- M. Lorenz, K. Stangl and V. Stangl, Eur. Heart J., 2007, 28, 1398–1399 CrossRef PubMed.
- H. Schroeter, R. R. Holt, T. J. Orozco, H. H. Schmitzt and C. L. Keen, Nature, 2003, 426, 787–788 CrossRef CAS PubMed.
- M. Serafini and A. Crozier, Nature, 2003, 426, 788 CrossRef CAS.
- S. Stanner, Nutr. Bull., 2007, 32, 101–103 CrossRef.
- S. Egert, J. Tereszczuk, S. Wein, M. J. Müller, J. Frank, G. Rimbach and S. Wolffram, Eur. J. Nutr., 2013, 52, 281–288 CrossRef CAS PubMed.
- W. Mullen, G. Borges, J. L. Donovan, C. A. Edwards, M. Serafini, M. E. J. Lean and A. Crozier, Am. J. Clin. Nutr., 2009, 89, 1784–1791 CrossRef CAS PubMed.
- A. P. Neilson, J. C. George, E. M. Janle, R. D. Mattes, R. Ralf, N. V. Matusheski and M. G. Ferruzzi, J. Agric. Food Chem., 2009, 57, 9418–9426 CrossRef CAS PubMed.
- V. C. Reddy, G. V. V. Sagar, D. Sreeramulu, L. Venu and M. Raghunath, Ann. Nutr. Metab., 2005, 49, 189–195 CrossRef CAS PubMed.
- E. Roura, C. Andrés-Lacueva, R. Estruch, M. L. M. Bilbao, M. Izquierdo-Pulido and R. M. Lamuela-Raventós, Br. J. Nutr., 2008, 100, 846–851 CrossRef CAS PubMed.
- K. H. Van Het Hof, G. A. A. Kivits, J. A. Weststrate and L. B. M. Tijburg, Eur. J. Clin. Nutr., 1998, 52, 356–359 CAS.
- A. P. Neilson, A. S. Hopf, B. R. Cooper, M. A. Pereira, J. A. Bomser and M. G. Ferruzzi, J. Agric. Food Chem., 2007, 55, 8941–8949 CrossRef CAS PubMed.
- W. Wu, M. Clifford and N. K. Howell, J. Sci. Food Agric., 2007, 87, 1810–1819 CrossRef CAS.
- J. Han, M. Britten, D. St-Gelais, C. P. Champagne, P. Fustier, S. Salmieri and M. Lacroix, Food Res. Int., 2011, 44, 494–497 CrossRef CAS PubMed.
- C. Vega and M. K. Grover, J. Agric. Food Chem., 2011, 59, 6740–6747 CrossRef CAS PubMed.
- D. Zanchi, T. Narayanan, D. Hagenmuller, A. Baron, S. Guyot, B. Cabane and S. Bouhallab, Europhys. Lett., 2008, 82(5), 58001 CrossRef.
- T. Beveridge, Crit. Rev. Food Sci. Nutr., 1997, 37, 75–91 CrossRef CAS PubMed.
- M. Esteruelas, N. Kontoudakis, M. Gil, M. F. Fort, J. M. Canals and F. Zamora, Food Res. Int., 2011, 44, 77–83 CrossRef CAS PubMed.
- L. C. Wu and Y. W. Lu, J. Agric. Food Chem., 2004, 52, 3130–3135 CrossRef CAS PubMed.
- M. Miedl, M. A. Garcia and C. W. Bamforth, J. Agric. Food Chem., 2005, 53, 10161–10165 CrossRef CAS PubMed.
- K. Asano, K. Shinigawa and N. Hashimoto, J. Am. Soc. Brew. Chem., 1982, 40, 147–154 CAS.
- I. McMurrough, G. P. Hennigan and M. Y. Loughrey, J. Inst. Brew., 1983, 89, 15–23 CrossRef CAS.
- K. J. Siebert, A. Carrasco and P. Y. Lynn, J. Agric. Food Chem., 1996, 44, 1997–2005 CrossRef CAS.
- V. K. Ananingsih, A. Sharma and W. Zhou, Food Res. Int., 2013, 50(2), 469–479 CrossRef CAS PubMed.
- L. Bazinet, M. Araya-Farias, A. Doyen, D. Trudel and B. Têtu, Food Res. Int., 2010, 43, 1692–1701 CrossRef CAS PubMed.
- M. J. Bae, T. Ishii, K. Minoda, Y. Kawada, T. Ichikawa, T. Mori, M. Kamihira and T. Nakayama, Mol. Nutr. Food Res., 2009, 53, 709–715 CAS.
- T. F. Wegrzyn, J. M. Farr, D. C. Hunter, J. Au, M. W. Wohlers, M. A. Skinner, R. A. Stanley and D. Sun-Waterhouse, Food Chem., 2008, 109, 310–318 CrossRef CAS PubMed.
- Y. Li, L. T. Lim and Y. Kakuda, J. Food Sci., 2009, 74, C233–C240 CrossRef CAS PubMed.
- I. Arcan and A. Yemenicioǧlu, Food Res. Int., 2011, 44, 550–556 CrossRef CAS PubMed.
- R. Fang, R. Hao, X. Wu, Q. Li, X. Leng and H. Jing, J. Agric. Food Chem., 2011, 59, 6292–6298 CrossRef CAS PubMed.
- A. R. Patel, P. C. M. Heussen, J. Hazekamp, E. Drost and K. P. Velikov, Food Chem., 2012, 133, 423–429 CrossRef CAS PubMed.
- T. Janet, J. R. N. Taylor, P. S. Belton and M. Amanda, J. Agric. Food Chem., 2009, 57, 7523–7528 CrossRef PubMed.
- J. Czubinski, K. Dwiecki, A. Siger, P. Kachlicki, G. Neunert, E. Lampart-Szczapa and M. Nogala-Kalucka, J. Agric. Food Chem., 2012, 60, 1830–1836 CrossRef CAS PubMed.
- A. Shpigelman, Y. Cohen and Y. D. Livney, Food Hydrocolloids, 2012, 29, 57–67 CrossRef CAS PubMed.
- M. J. T. J. Arts, G. R. M. M. Haenen, L. C. Wilms, S. A. J. N. Beetstra, C. G. M. Heijnen, H. P. Voss and A. Bast, J. Agric. Food Chem., 2002, 50, 1184–1187 CrossRef CAS PubMed.
- M. Stojadinovic, J. Radosavljevic, J. Ognjenovic, J. Vesic, I. Prodic, D. Stanic-Vucinic and T. Cirkovic Velickovic, Food Chem., 2013, 136, 1263–1271 CrossRef CAS PubMed.
- K. M. Riedl and A. E. Hagerman, J. Agric. Food Chem., 2001, 49, 4917–4923 CrossRef CAS PubMed.
- M. P. Almajano, M. E. Delgado and M. H. Gordon, Food Chem., 2006, 101, 126–130 CrossRef PubMed.
- M. P. Almajano, M. E. Delgado and M. H. Gordon, Food Chem., 2007, 102, 1375–1382 CrossRef CAS PubMed.
- D. E. Roopchand, M. H. Grace, P. Kuhn, D. M. Cheng, N. Plundrich, A. Poulev, A. Howell, B. Fridlender, M. A. Lila and I. Raskin, Food Chem., 2012, 131, 1193–1200 CrossRef CAS PubMed.
- M. H. Grace, I. Guzman, D. E. Roopchand, K. Moskal, D. M. Cheng, N. Pogrebnyak, I. Raskin, A. Howell and M. A. Lila, J. Agric. Food Chem., 2013, 61(28), 6856–6864 CrossRef CAS PubMed.
- J. N. BeMiller, Carbohydrate chemistry for food scientists, AACC International, St. Paul, Minn., 2007 Search PubMed.
- Y. Wu, Q. Lin, Z. Chen and H. Xiao, Food Sci. Technol. Int., 2011, 17, 569–577 CrossRef CAS PubMed.
- L. Zhang, X. Yang, S. Li and W. Gao, LWT–Food Sci. Technol., 2011, 44, 787–792 CrossRef CAS PubMed.
- F. Barros, J. M. Awika and L. W. Rooney, J. Agric. Food Chem., 2012, 60, 11609–11617 CrossRef CAS PubMed.
- H. Xiao, Q. Lin, G. Q. Liu, Y. Wu, W. Tian, W. Wu and X. Fu, J. Med. Plants Res., 2011, 5, 4298–4303 CAS.
- H. Xiao, Q. Lin, G. Q. Liu, Y. Wu, W. Wu and X. Fu, Food Bioprocess Technol., 2011, 1–5 CAS.
- P. Veiga-Santos, L. M. Oliveira, M. P. Cereda and A. R. P. Scamparini, Food Chem., 2007, 103, 255–262 CrossRef CAS PubMed.
- S.-J. Rho, S. Mun and S. Long, Presented in part at the 2010 IFT Annual Meeting, Chicago, IL, 17–20 July 2010 Search PubMed.
- Y. Cai, S. H. Gaffney, T. H. Lilley, D. Magnolato, R. Martin, C. M. Spencer and E. Haslam, J. Chem. Soc., Perkin Trans. 2, 1990, 2197–2209 RSC.
- T. Ishizu, K. Kintsu and H. Yamamoto, J. Phys. Chem. B, 1999, 103, 8992–8997 CrossRef CAS.
- V. K. Smith, T. T. Ndou and I. M. Warner, J. Phys. Chem., 1994, 98, 8627–8631 CrossRef CAS.
- J. A. Putseys, L. Lamberts and J. A. Delcour, J. Cereal Sci., 2010, 51, 238–247 CrossRef CAS PubMed.
- K. Hanhineva, R. Törrönen, I. Bondia-Pons, J. Pekkinen, M. Kolehmainen, H. Mykkänen and K. Poutanen, Int. J. Mol. Sci., 2010, 11, 1365–1402 CrossRef CAS PubMed.
- T. Matsui, T. Tanaka, S. Tamura, A. Toshima, K. Tamaya, Y. Miyata, K. Tanaka and K. Matsumoto, J. Agric. Food Chem., 2007, 55, 99–105 CrossRef CAS PubMed.
- K. Nakahara, R. Izumi, T. Kodama, Y. Kiso and T. Tanaka, Phytother. Res., 1994, 8, 433–435 CrossRef.
- M. Shimizu, Y. Kobayashi, M. Suzuki, H. Satsu and Y. Miyamoto, BioFactors, 2000, 13, 61–65 CrossRef CAS.
- O. Dangles and C. Dufour, in Flavonoids: Chemistry, Biochemistry and Applications, ed. O. M. Andersen and K. R. Markham, CRC Press, Boca Raton, FL, 2006, pp. 443–469 Search PubMed.
- J. Liu, M. Wang, S. Peng and G. Zhang, J. Agric. Food Chem., 2011, 59, 4582–4588 CrossRef CAS PubMed.
- D. Lemlioglu-Austin, N. D. Turner, C. M. McDonough and L. W. Rooney, Molecules, 2012, 17, 11124–11138 CrossRef CAS PubMed.
- N. L. Mkandawire, R. C. Kaufman, S. R. Bean, C. L. Weller, D. S. Jackson and D. J. Rose, J. Agric. Food Chem., 2013, 61, 4448–4454 CrossRef CAS PubMed.
- Q. He, Y. Lv and K. Yao, Food Chem., 2006, 101, 1178–1182 CrossRef PubMed.
- S. Arranz, J. M. Silván and F. Saura-Calixto, Mol. Nutr. Food Res., 2010, 54, 1646–1658 CAS.
- F. Saura-Calixto, J. Agric. Food Chem., 2011, 59, 43–49 CrossRef CAS PubMed.
- H. Palafox-Carlos, J. F. Ayala-Zavala and G. A. González-Aguilar, J. Food Sci., 2011, 76, R6–R15 CrossRef CAS PubMed.
- S. Y. Leong and I. Oey, Food Chem., 2012, 133, 1577–1587 CrossRef CAS PubMed.
- A. Vallverdú-Queralt, G. Oms-Oliu, I. Odriozola-Serrano, R. M. Lamuela-Raventos, O. Martín-Belloso and P. Elez-Martínez, J. Agric. Food Chem., 2012, 60, 3126–3134 CrossRef PubMed.
- A. Padayachee, G. Netzel, M. Netzel, L. Day, D. Zabaras, D. Mikkelsen and M. J. Gidley, Food Chem., 2012, 135, 2287–2292 CrossRef CAS PubMed.
- A. Padayachee, G. Netzel, M. Netzel, L. Day, D. Zabaras, D. Mikkelsen and M. J. Gidley, Food Chem., 2012, 134, 155–161 CrossRef CAS PubMed.
- C. Le Bourvellec, A. A. Watrelot, C. Ginies, A. Imberty and C. M. G. C. Renard, J. Agric. Food Chem., 2012, 60, 9484–9494 CrossRef CAS PubMed.
- R. L. Hanlin, M. Hrmova, J. F. Harbertson and M. O. Downey, Aust. J. Grape Wine Res., 2010, 16, 173–188 CrossRef CAS.
- Z. Guadalupe, A. Palacios and B. Ayestarán, J. Agric. Food Chem., 2007, 55, 4854–4862 CrossRef CAS PubMed.
- M. A. Ducasse, R. M. Canal-Llauberes, M. de Lumley, P. Williams, J. M. Souquet, H. Fulcrand, T. Doco and V. Cheynier, Food Chem., 2010, 118, 369–376 CrossRef CAS PubMed.
- E. Carvalho, N. Mateus, B. Plet, I. Pianet, E. Dufourc and V. De Freitas, J. Agric. Food Chem., 2006, 54, 8936–8944 CrossRef CAS PubMed.
- S. Escot, M. Feuillat, L. Dulau and C. Charpentier, Aust. J. Grape Wine Res., 2001, 7, 153–159 CrossRef.
- S. Vidal, P. Courcoux, L. Francis, M. Kwiatkowski, R. Gawel, P. Williams, E. Waters and V. Cheynier, Food Quality and Preference, 2004, 15, 209–217 CrossRef.
- S. Vidal, L. Francis, P. Williams, M. Kwiatkowski, R. Gawel, V. Cheynier and E. Waters, Food Chem., 2004, 85, 519–525 CrossRef CAS.
- C. Poncet-Legrand, T. Doco, P. Williams and A. Vernhet, Am. J. Enol. Vitic., 2007, 58, 87–91 Search PubMed.
- A. Rodrigues, J. M. Ricardo-Da-Silva, C. Lucas and O. Laureano, Food Chem., 2012, 131, 907–914 CrossRef CAS PubMed.
- J. F. Harbertson, C. Yuan, M. S. Mireles, R. L. Hanlin and M. O. Downey, Food Chem., 2013, 138, 556–563 CrossRef CAS PubMed.
- E. Carvalho, M. J. Póvoas, N. Mateus and V. De Freitas, J. Sci. Food Agric., 2006, 86, 891–896 CrossRef CAS.
- V. De Freitas, E. Carvalho and N. Mateus, Food Chem., 2003, 81, 503–509 CrossRef CAS.
- N. Mateus, E. Carvalho, C. Luís and V. De Freitas, Anal. Chim. Acta, 2004, 513, 135–140 CrossRef CAS PubMed.
- S. I. Soares, R. M. Gonçalves, I. V. A. Fernandes, N. Mateus and V. De Freitas, J. Agric. Food Chem., 2009, 57, 4352–4358 CrossRef CAS PubMed.
- G. R. Scollary, G. Pásti, M. Kállay, J. Blackman and A. C. Clark, Trends Food Sci. Technol., 2012, 27, 25–36 CrossRef CAS PubMed.
- J. N. BeMiller, in Carbohydrate Chemistry for Food Scientists, ed. J. N. BeMiller, AACC International Press, St. Paul, MN, 2nd edn, 2007, pp. 313–320 Search PubMed.
- N. Ben-Shalom, I. Shomer and J. Kanner, LWT–Food Sci. Technol., 1984, 17, 125–128 CAS.
- N. Beń-Shalom and R. Pinto, LWT–Food Sci. Technol., 1986, 19, 158–160 Search PubMed.
- J. K. Jacob and G. Paliyath, J. Agric. Food Chem., 2008, 56, 1305–1315 CrossRef CAS PubMed.
- J. R. Whitaker, Enzyme Microb. Technol., 1984, 6, 341–349 CrossRef CAS.
- K. A. Bindon and P. A. Smith, Food Chem., 2013, 136, 917–928 CrossRef CAS PubMed.
- A. Cabello-Pasini, N. Victoria-Cota, V. Macias-Carranza, E. Hernandez-Garibay and R. Muñiz-Salazar, Am. J. Enol. Vitic., 2005, 56, 52–59 CAS.
- A. S. Sivam, D. Sun-Waterhouse, C. O. Perera and G. I. N. Waterhouse, Food Res. Int., 2011 Search PubMed.
- A. S. Sivam, D. Sun-Waterhouse, G. I. Waterhouse, S. Quek and C. O. Perera, J. Food Sci., 2011, 76, 97–107 CrossRef PubMed.
- D. Sun-Waterhouse, A. S. Sivam, J. Cooney, J. Zhou, C. O. Perera and G. I. N. Waterhouse, Food Res. Int., 2011, 44, 3047–3056 CrossRef CAS PubMed.
- Z. Wu, H. Li, J. Ming and G. Zhao, J. Agric. Food Chem., 2011, 59, 378–385 CrossRef CAS PubMed.
- Z. Wu, J. Ming, R. Gao, Y. Wang, Q. Liang, H. Yu and G. Zhao, J. Agric. Food Chem., 2011, 59, 10737–10746 CrossRef CAS PubMed.
- Q. Y. Zhu, A. Zhang, D. Tsang, Y. Huang and Z. Y. Chen, J. Agric. Food Chem., 1997, 45, 4624–4628 CrossRef CAS.
- Y. Wang, J. Liu, F. Chen and G. Zhao, J. Agric. Food Chem., 2013, 61, 4533–4538 CrossRef CAS PubMed.
- H. T. Simonsen, M. S. Nielsen, N. J. Christensen, U. Christensen, T. V. L. Cour, M. S. Motawia, B. P. M. Jespersen, S. B. Engelsen and B. L. Møller, J. Agric. Food Chem., 2009, 57, 2056–2064 CrossRef CAS PubMed.
- J. Liang, F. Li, Y. Fang, W. Yang, X. An, L. Zhao, Z. Xin, L. Cao and Q. Hu, Colloids Surf., B, 2011, 82, 297–301 CrossRef CAS PubMed.
- Y. Qin, H. W. Wang, T. Karuppanapandian and W. Kim, Chin. J. Traumatol., 2010, 13, 91–95 CAS.
- I. Peres, S. Rocha, J. Gomes, S. Morais, M. C. Pereira and M. Coelho, Carbohydr. Polym., 2011, 86, 147–153 CrossRef CAS PubMed.
- A. Singhal, H. Jain, V. Singhal, E. J. Elias and A. Showkat, Pharmacogn. Res., 2011, 3, 35–39 CrossRef CAS PubMed.
- Z. Wang, Y. Li, L. Chen, X. Xin and Q. Yuan, J. Agric. Food Chem., 2013, 61, 5880–5887 CrossRef CAS PubMed.
- L. M. Monteiro, A. E. Souza, E. A. S. Gianotto, M. M. F. Nery, J. C. Duarte, O. De Freitas, R. Casagrande and M. M. Baracat, Comprimidos matriciais preparados com hidroxipropilmetilcelulose e pectina contendo quercetina para liberação cólon-específica, 2007, 26, 179–184 CAS.
- 2010.
- C. Pastor, L. Sánchez-González, A. Chiralt, M. Cháfer and C. González-Martínez, Food Hydrocolloids, 2013, 30, 272–280 CrossRef CAS PubMed.
- Y. Zhang, Y. Yang, K. Tang, X. Hu and G. Zou, J. Appl. Polym. Sci., 2008, 107, 891–897 CrossRef CAS.
- Y. Wu, Q. Wu, Y. N. Wang and J. B. Ma, Acta Chim. Sin., 2003, 61, 614–618 CAS.
- G. J. Smith and K. R. Markham, J. Photochem. Photobiol., A, 1998, 118, 99–105 CrossRef CAS.
- F. Saura-Calixto, J. Pérez-Jiménez, S. Touriño, J. Serrano, E. Fuguet, J. L. Torres and I. Goñi, Mol. Nutr. Food Res., 2010, 54, 939–946 CAS.
- O. Aprikian, V. Duclos, S. Guyot, C. Besson, C. Manach, A. Bernalier, C. Morand, C. Rémésy and C. Demigné, J. Nutr., 2003, 133, 1860–1865 CAS.
- Y. Nitta, Y. Fang, M. Takemasa and K. Nishinari, Biomacromolecules, 2004, 5, 1206–1213 CrossRef CAS PubMed.
- A. El-Abbassi, H. Kiai and A. Hafidi, Food Chem., 2012, 132(1), 406–412 CrossRef CAS PubMed.
- M. Bouaziz, H. Jemai, W. Khabou and S. Sayadi, J. Sci. Food Agric., 2010, 90, 1750–1758 CrossRef CAS PubMed.
- D. Ocakoglu, F. Tokatli, B. Ozen and F. Korel, Food Chem., 2009, 113, 401–410 CrossRef CAS PubMed.
- M. Brenes, A. García, P. García, J. J. Rios and A. Garrido, J. Agric. Food Chem., 1999, 47, 3535–3540 CrossRef CAS PubMed.
- I. Rjiba, N. Gazzah, S. Dabbou and M. Hammami, J. Food Biochem., 2011, 35, 1413–1423 CrossRef CAS.
- S. Christophoridou, P. Dais, L. I. H. Tseng and M. Spraul, J. Agric. Food Chem., 2005, 53, 4667–4679 CrossRef CAS PubMed.
- J. A. Rothwell, A. J. Day and M. R. A. Morgan, J. Agric. Food Chem., 2005, 53, 4355–4360 CrossRef CAS PubMed.
- R. M. Han, Y. X. Tian, Y. Liu, C. H. Chen, X. C. Ai, J. P. Zhang and L. H. Skibsted, J. Agric. Food Chem., 2009, 57, 3780–3785 CrossRef CAS PubMed.
- Z. Luo, B. S. Murray, A. Yusoff, M. R. A. Morgan, M. J. W. Povey and A. J. Day, J. Agric. Food Chem., 2011, 59, 2636–2645 CrossRef CAS PubMed.
- L. Atarés, L. J. Marshall, M. Akhtar and B. S. Murray, Food Chem., 2012, 134, 1418–1424 CrossRef PubMed.
- B. Aliakbarian, A. A. Casazza and P. Perego, Food Chem., 2011, 128, 704–710 CrossRef CAS PubMed.
- V. Sánchez De Medina, F. Priego-Capote, C. Jiménez-Ot and M. D. Luque De Castro, J. Agric. Food Chem., 2011, 59, 11432–11441 CrossRef PubMed.
- S. Kiokias, T. Varzakas and V. Oreopoulou, Crit. Rev. Food Sci. Nutr., 2008, 48, 78–93 CrossRef CAS PubMed.
- D. Tsimogiannis and V. Oreopoulou, J. Am. Oil Chem. Soc., 2007, 84, 129–136 CrossRef CAS PubMed.
- S. A. Vekiari, V. Oreopoulou, C. Tzia and C. D. Thomopoulos, J. Am. Oil Chem. Soc., 1993, 70, 483–487 CrossRef CAS.
- R. Malheiro, S. Casal, H. Lamas, A. Bento and J. A. Pereira, Food Res. Int., 2012, 48, 148–154 CrossRef CAS PubMed.
- R. Malheiro, N. Rodrigues, G. Manzke, A. Bento, J. A. Pereira and S. Casal, Ind. Crops Prod., 2013, 44, 37–43 CrossRef CAS PubMed.
- N. P. Das and T. A. Pereira, J. Am. Oil Chem. Soc., 1990, 67, 255–258 CrossRef CAS.
- F. Shahidi and U. Wanasundara, Dev. Food Sci., 1995, 37, 469–479 Search PubMed.
- U. N. Wanasundara and F. Shahidi, Food Chem., 1994, 50, 393–396 CrossRef CAS.
- Y. Guo, E. Mah, C. G. Davis, T. Jalili, M. G. Ferruzzi, O. K. Chun and R. S. Bruno, Mol. Nutr. Food Res., 2013, 57, 896–905 CAS.
- G. T. McAnlis, J. McEneny, J. Pearce and I. S. Young, Eur. J. Clin. Nutr., 1999, 53, 92–96 CAS.
- T. Nakayama, T. Hashimoto, K. Kajiya and S. Kumazawa, BioFactors, 2000, 13, 147–151 CrossRef CAS.
- X. Yu, S. Chu, A. E. Hagerman and G. A. Lorigan, J. Agric. Food Chem., 2011, 59, 6783–6789 CrossRef CAS PubMed.
- J. Y. Fang, T. L. Hwang, Y. L. Huang and C. L. Fang, Int. J. Pharm., 2006, 310, 131–138 CrossRef CAS PubMed.
- S. V. Verstraeten, C. L. Keen, H. H. Schmitz, C. G. Fraga and P. I. Oteiza, Free Radicals Biol. Med., 2003, 34, 84–92 CrossRef CAS.
- T. Hashimoto, S. Kumazawa, F. Nanjo, Y. Hara and T. Nakayama, Biosci., Biotechnol., Biochem., 1999, 63, 2252–2255 CrossRef CAS.
- K. Kajiya, S. Kumazawa, A. Naito and T. Nakayama, Magn. Reson. Chem., 2008, 46, 174–177 CrossRef CAS PubMed.
- S. Kumazawa, K. Kajiya, A. Naito, H. Saitô, S. Tuzi, M. Tanio, M. Suzuki, F. Nanjo, E. Suzuki and T. Nakayama, Biosci., Biotechnol., Biochem., 2004, 68, 1743–1747 CrossRef CAS.
- Y. Sun, W. C. Hung, F. Y. Chen, C. C. Lee and H. W. Huang, Biophys. J., 2009, 96, 1026–1035 CrossRef CAS PubMed.
- Y. Uekusa, M. Kamihira-Ishijima, O. Sugimoto, T. Ishii, S. Kumazawa, K. Nakamura, K. I. Tanji, A. Naito and T. Nakayama, Biochim. Biophys. Acta, Biomembr., 2011, 1808, 1654–1660 CrossRef CAS PubMed.
- H. Yoshioka, H. Haga, M. Kubota and Y. Sakai, Biosci., Biotechnol., Biochem., 2006, 70, 395–400 CrossRef CAS.
- P. I. Oteiza, A. G. Erlejman, S. V. Verstraeten, C. L. Keen and C. G. Fraga, Clin. Dev. Immunol., 2005, 12, 19–25 CrossRef CAS.
- K. Kajiya, H. Hojo, M. Suzuki, F. Nanjo, S. Kumazawa and T. Nakayama, J. Agric. Food Chem., 2004, 52, 1514–1519 CrossRef CAS PubMed.
- O. H. Ma, Y. Z. Kuang, X. Z. Hao and N. Gu, J. Nanosci. Nanotechnol., 2009, 9, 1379–1383 CrossRef PubMed.
- Sangster Research Laboratories, LOGKOW – Sangster Research Laboratories, http://logkow.cisti.nrc.ca/logkow/index.jsp, 2012.
- VCCLAB and V. C. C. Laboratory, http://www.vcclab.org, 2012.
- I. V. Tetko, J. Gasteiger, R. Todeschini, A. Mauri, D. Livingstone, P. Ertl, V. A. Palyulin, E. V. Radchenko, N. S. Zefirov, A. S. Makarenko, V. Y. Tanchuk and V. V. Prokopenko, J. Comput.-Aided Mol. Des., 2005, 19, 453–463 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |