Short term and dosage influences of palm based medium- and long-chain triacylglycerols on body fat and blood parameters in C57BL/6J mice
Yee-Ying
Lee
a,
Teck-Kim
Tang
a,
Nur Azwani
Ab Karim
b,
Noorjahan
Banu Mohamed Alitheen
c and
Oi-Ming
Lai
*ad
aInstitute of Bioscience, Universiti Putra Malaysia, 43400 Serdang, Malaysia
bSime Darby Research Sdn Bhd, R&D Carey Island-Upstream, Lot 2664 Jln Pulau Carey, 42960 Carey Island, Selangor, Malaysia
cDepartment of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 Serdang, Malaysia
dDepartment of Bioprocess Technology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 Serdang, Malaysia. E-mail: omlai@biotech.upm.edu.my; Fax: +603 8946 7510; Tel: +603 8946 7520
First published on 7th October 2013
Abstract
Structured lipid medium- and long-chain triacylglycerols (MLCT) are claimed to be able to manage obesity. The present study investigated the body fat influence of enzymatically interesterifed palm-based medium- and long-chain triacylglycerols (P-MLCT) on diet-induced obesity (DIO) C57BL/6J mice compared with commercial MLCT oil (C-MLCT) and a control, which was the non enzymatically modified palm kernel and palm oil blend (PKO–PO blend). It also investigated the low fat and high fat effects of P-MLCT. DIO C57BL/6J mice were fed ad libitum with low fat (7%) and high fat (30%) experimental diets for 8 weeks before being sacrificed to obtain blood serum for analysis. From the results, there is a trend that P-MLCT fed mice were found to have the lowest body weight, body weight gain, total fat pad accumulation (perirenal, retroperitoneal, epididymal and mesenteric), total triglyceride levels and efficiency in controlling blood glucose level, compared with C-MLCT and the PKO–PO blend in both low fat and high fat diets. Nevertheless, the PKO–PO blend and P-MLCT caused significantly (P < 0.05) higher total cholesterol levels compared to C-MLCT. P-MLCT present in low fat and high fat dosage were shown to be able to suppress body fat accumulation. This effect is more prominent with the low fat dosage.
Introduction
Obesity has transformed into a global epidemic. According to WHO, worldwide obesity has more than doubled since 1980, affecting both developed and developing countries with Mexico standing at the top of the list. Prevalent cases of childhood obesity are also catching up. Obesity is often correlated with comorbidities such as coronary heart disease, diabetes mellitus type 2, osteoarthritis and certain types of cancers, which further increase the chances of mortality and morbidity.1–3 A sedentary lifestyle, genetics, and diets rich in fat and carbohydrates are the major causes of obesity.In westernized countries, obesity has become an important health issue mainly due to diet, which is typically dense with fat. Structured lipids are one of the dietary approaches in managing obesity, along with physical exercise, bariatric surgery and medication. Enzyme catalysis or chemical modification are used to fabricate these new types of lipids that are nutritious and healthful in terms of lower calories, providing optimum levels of essential fatty acids, lower levels of trans fats, as well as specific absorption capabilities. Enzymatic modification is preferable for use in the modification of lipids due to its specificity and environmentally friendly property. Examples of this healthful lipid that are available on the market and incorporated in food products are: diacylglycerol (Kao Corp, Japan), Resetta™ (Nishhin Ollio Group Ltd., Japan), olestra (Procter & Gamble, United States), and SALATRIM (Nabisico, United States). Such modified lipids are considered as nutraceutical or functional foods.
The concept of MLCT (medium- and long-chain triacylglycerols) comes from medium-chain triacylglycerols (MCT), where both are considered anti-obesity functional oils mainly due to the presence of medium chain fatty acids (MCFA), which are more rapidly metabolized compared to long chain fatty acids (LCFA). MCFA are transported directly to the liver by the hepatic portal vein to undergo beta oxidation, giving a rapid source of energy, whereas LCFA are transported by the lymphatic system as chylomicrons that are prone to being deposited in the body as fat.4,5 MLCT are created mainly to deliver the long chain essential fatty acids such as monounsaturated fatty acids (MUFA) and/or polyunsaturated fatty acids (PUFA), to the body especially when placed at the sn2 position where MCT lack this functionality. Studies have shown that MLCT, when consumed, were able to reduce body weight gain and body fat accumulation, and increase the activity of the hepatic fatty acid oxidation enzyme, as well as improve blood lipid levels in both clinical and pre-clinical trials.6–11 Most of these studies focus on the commercially available MLCT made from interesterification of MCT with soft oil (Nisshin Ollio Group Ltd., Japan). No studies have been done so far to produce MLCT from other oil sources and to see their effect in pre-clinical and clinical trials.
The objective of the present study was to investigate the short term effects of enzymatically modified palm-based medium- and long-chain triacylglycerols (P-MLCT) on diet induced obese C57BL/6J mice in terms of the body fat accumulation, when present in either low fat and high fat diet conditions.
Results
Body weight, fat pad analysis, food intake and food efficiency
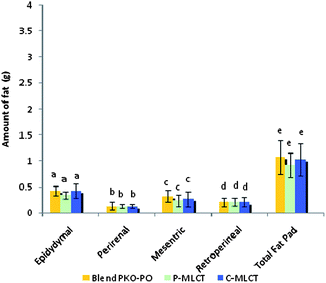
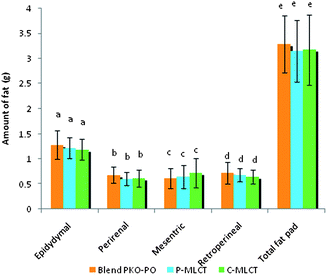
Tables 1 and 2 show the initial and final body weight, spleen and liver weight of mice fed with 7% fat (low fat) and 30% dietary fat (high fat), whereas Fig. 1 and 2 show the weight of four respective fat pads and the total fat pad accumulation of mice fed with 7% fat and 30% dietary fat, respectively. Mice fed with P-MLCT of either 7% fat or 30% fat have lower body weight, body weight gained, percentage of body weight gain and total fat pad accumulated compared to those fed with C-MLCT and PKO–PO blended oils at the end of the 8 weeks feeding trial, although there was statistically no significant difference (P > 0.05) among the treatments. Meanwhile, in terms of fat accumulation, P-MLCT and C-MLCT when compared with the PKO–PO blend of low fat and high fat feeding showed that P-MLCT is more efficient in suppressing the fat accumulation than C-MLCT. P-MLCT manage to lower 9.0% (7% P-MLCT) and 4.14% (30% P-MLCT) of body fat accumulation whereas C-MLCT showed to increase 1.08% (7% C-MLCT) and lower 2.9% (30% C-MLCT) of fat accumulation when both P-MLCT and C-MLCT were compared to the PKO–PO blend. Food efficiency in P-MLCT fed mice was the lowest compared to the PKO–PO blend and C-MLCT in both the low fat and high fat diet fed mice.| Parameters | Dietary fat (low fat group)a | ||
|---|---|---|---|
| PKO–PO blend | P-MLCT | C-MLCT | |
| a Values are mean ± SD, N = 12 mice per group. Mean values within a row with unlike subscript letters were significantly different. | |||
| Final body weight [g] | 28.07 ± 1.92a | 26.77 ± 2.57a | 27.75 ± 1.73a |
| Initial body weight [g] | 22.55 ± 1.91a | 22.12 ± 1.54a | 22.85 ± 1.41a |
| Weight gain [g] | 5.52 ± 1.78a | 4.65 ± 2.82a | 4.89 ± 2.43a |
| Percentage of weight gain [%] | 24.47 | 21.02 | 21.40 |
| Energy intake [kJ per cage per day] | 146.79c | 147.28c | 149.20c |
| Food efficiency [g per kJ per cage per day] | 0.037a | 0.032a | 0.033a |
| Feces lipid [g] | 1.88 ± 0.12a | 1.82 ± 0.31a | 1.75 ± 0.17a |
| Liver weight [g] | 0.84 ± 0.17a | 0.9 ± 0.12a | 0.89 ± 0.07a |
| Spleen weight [g] | 0.08 ± 0.02ab | 0.08 ± 0.02ab | 0.075 ± 0.01a |
| Parameters | Dietary fat (high fat group)a | ||
|---|---|---|---|
| PKO–PO blend | P-MLCT | C-MLCT | |
| a Values are mean ± SD, N = 12 mice per group. Mean values within a row with unlike subscript letters were significantly different. | |||
| Final body weight [g] | 37.00 ± 3.90b | 36.33 ± 2.63b | 37.39 ± 4.02b |
| Initial body weight [g] | 23.85 ± 1.87a | 23.94 ± 1.26a | 23.38 ± 1.76a |
| Weight gain [g] | 13.15 ± 4.08b | 12.39 ± 2.72b | 14.01 ± 4.64b |
| Percentage of weight gain [%] | 55.14 | 51.75 | 59.99 |
| Energy intake [kJ per cage per day] | 222.36b | 214.52b | 249.79a |
| Food efficiency [g per kJ per cage per day] | 0.059b | 0.058b | 0.056b |
| Feces lipid [g] | 5.13 ± 0.04b | 5.50 ± 0.23b | 5.22 ± 0.15b |
| Liver weight [g] | 1.24 ± 0.17b | 1.25 ± 0.13b | 1.41 ± 0.32b |
| Spleen weight [g] | 0.088 ± 0.01ab | 0.087 ± 0.01ab | 0.092 ± 0.01b |
Blood parameter
Tables 3 and 4 show the plasma glucose, triglyceride, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), HDL/LDL ratio, insulin and leptin concentrations in mice fed with experimental diets of 7% and 30% of dietary fat consisting of the PKO–PO blend, P-MLCT and C-MLCT. Total cholesterol and LDL levels were significantly (P < 0.05) higher in mice fed with the PKO–PO blend and P-MLCT compared to mice fed with C-MLCT in the low fat and high fat groups. C-MLCT fed mice had a higher HDL/LDL ratio compared to the P-MLCT and PKO–PO blend fed mice with the high fat diet as well as the low fat diet. Meanwhile, P-MLCT fed mice have the lowest triglyceride level in both the low fat and high fat groups and vice versa for C-MLCT. P-MLCT fed mice tend to have the lowest level of homeostatic model assessment insulin resistance (HOMA-IR) compared to the PKO–PO blend and C-MLCT fed mice with the low fat and high fat diets. There is a significantly lower leptin level in mice fed with 30% PKO–PO blend and 30% P-MLCT compared with 30% C-MLCT fed mice. Similarly, P-MLCT was shown to impart the lowest leptin level in low fat fed mice.| Parameters | Dietary fat (low fat group)a | ||
|---|---|---|---|
| PKO–PO blend | P-MLCT | C-MLCT | |
| a Values are mean ± SD, N = 12 mice per group. Mean values within a row with unlike subscript letters were significantly different. | |||
| Glucose [mg dL−1] | 164.10 ± 58.16a | 186.97 ± 67.41a | 191.61 ± 19.31a |
| Insulin [mg dL−1] | 62.58 ± 36.25bc | 45.41 ± 12.13bc | 44.95 ± 12.71c |
| HOMA-IR | 5.76 ± 2.74a | 5.06 ± 1.57a | 5.28 ± 1.26a |
| Triglyceride [mg dL−1] | 44.48 ± 88.80c | 46.51 ± 13.57bc | 60.67 ± 16.75ab |
| Leptin [pg mL−1] | 1818 ± 1594.97c | 1083 ± 756.48c | 1710 ± 1421.83c |
| Total cholesterol [mg dL−1] | 52.85 ± 20.57c | 70.89 ± 12.06b | 54.22 ± 10.56c |
| HDL [mg dL−1] | 49.67 ± 7.09b | 51.75 ± 9.92b | 50.41 ± 6.56b |
| LDL [mg dL−1] | 17.99 ± 6.05c | 28.71 ± 9.92bc | 21.22 ± 3.64c |
| HDL/LDL ratio | 3.30 ± 1.86a | 2.06 ± 1.12ab | 2.7 ± 0.72a |
| Parameters | Dietary fat (high fat group)a | ||
|---|---|---|---|
| PKO–PO blend | P-MLCT | C-MLCT | |
| a Values are mean ± SD, N = 12 mice per group. Mean values within a row with unlike subscript letters were significantly different. | |||
| Glucose [mg dL−1] | 278.10 ± 68.63b | 305.69 ± 51.87b | 281.69 ± 84.72b |
| Insulin [mg dL−1] | 109.08 ± 72.98a | 72.14 ± 28.47abc | 97.67 ± 66.77ab |
| HOMA-IR | 19.94 ± 21.67ab | 13.67 ± 5.76b | 20.26 ± 17.11ab |
| Triglyceride [mg dL−1] | 66.7 ± 12.94a | 60.14 ± 11.34ab | 62.92 ± 26.64a |
| Insulin [mg dL−1] | 109.08 ± 72.98a | 70.59 ± 16.45abc | 97.75 ± 48.26ab |
| Leptin [pg mL−1] | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 ± 8479.50b 722 ± 8479.50b |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 473 ± 9175.40b 473 ± 9175.40b |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043 ± 15023.28a 043 ± 15023.28a |
| Total cholesterol [mg dL−1] | 104.62 ± 11.64a | 105.12 ± 11.38a | 80.53 ± 16.86b |
| HDL [mg dL−1] | 60.9 ± 8.02a | 53.33 ± 9.12ab | 46.66 ± 6.32b |
| LDL [mg dL−1] | 65.45 ± 18.89a | 59.61 ± 7.92a | 37.59 ± 10.11b |
| HDL/LDL ratio | 1.12 ± 0.40b | 0.867 ± 0.20b | 2.35 ± 0.37ab |
Fat pad and liver fatty acid composition
Tables 5 and 6 show the fatty acid composition (FAC) of white adipose tissue (WAT) for mice fed with 7% and 30% dietary fat, respectively. In both low fat and high fat diets, the WAT for the PKO–PO blend and P-MLCT fed mice has a similar FAC with C18:1, C16:0 and C12:0 being the major fatty acids. Meanwhile, for C-MLCT fed mice, C18:1, C16:0 and C18:2 were the major fatty acids. All three types of diet also lead to the presence of new fatty acids (C14:1, C16:1, C20:1 and C21:0) that are different from the treatment oil FAC, with C16:1 being the main component, whereas C14:1, C20:1 and C21:0 are only present in minute amounts. Compared to the FAC of the respective experimental diets (Table 10) consumed by the mice, the low fat diet and high fat diet of the PKO–PO blend and P-MLCT cause an increase of around 50% for C18:1 and 40% for C16:0. In contrast, the C12 composition for both the PKO–PO blend and P-MLCT decreases by around 625% (low fat diet) and 320% (high fat diet).| FAC | Dietary fat (low fat group)a | ||
|---|---|---|---|
| PKO–PO Blend | P-MLCT | C-MLCT | |
| Percentage [%] | |||
| a Values represent means ± SD, with triplicate analysis. b TSAT = total saturated fatty acid, TMUFA = total monounsaturated fatty acid, TPUFA = total polyunsaturated fatty acid. | |||
| C12:0 | 6.34 ± 1.10 | 6.03 ± 0.29 | — |
| C14:0 | 5.47 ± 1.54 | 4.79 ± 0.14 | 1.27 ± 0.07 |
| C14:1 | 0.69 ± 0.45 | 0.36 ± 0.03 | — |
| C16:0 | 21.29 ± 3.20 | 19.16 ± 0.85 | 16.44 ± 0.30 |
| C16:1 | 11.74 ± 2.81 | 10.03 ± 0.74 | 5.99 ± 0.49 |
| C18:0 | 1.90 ± 0.41 | 1.70 ± 0.10 | 2.01 ± 0.25 |
| C18:1 | 43.63 ± 4.24 | 45.95 ± 0.63 | 54.49 ± 0.31 |
| C18:2 | 8.02 ± 4.91 | 10.67 ± 0.53 | 16.44 ± 0.59 |
| C20:1 | 0.45 ± 0.30 | 0.27 ± 0.04 | 2.20 ± 0.32 |
| C21:0 | 1.04 ± 0.09 | 1.05 ± 0.11 | 1.16 ± 0.09 |
| TSATb | 36.039 ± 6.344 | 32.731 ± 1.494 | 20.889 ± 0.704 |
| TMUFAb | 56.518 ± 7.797 | 56.604 ± 1.431 | 62.673 ± 1.129 |
| TPUFAb | 8.019 ± 4.911 | 10.665 ± 0.528 | 16.438 ± 0.593 |
| FAC | Dietary fat (high fat group)a | ||
|---|---|---|---|
| PKO–PO blend [%] | P-MLCT [%] | C-MLCT [%] | |
| a Values represent means ± SD, with triplicate analysis. b TSAT = total saturated fatty acid, TMUFA = total monounsaturated fatty acid, TPUFA = total polyunsaturated fatty acid. | |||
| C12:0 | 13.26 ± 0.48 | 12.86 ± 1.06 | — |
| C14:0 | 8.26 ± 0.09 | 8.12 ± 0.42 | 0.87 ± 0.08 |
| C14:1 | 0.66 ± 0.05 | 0.64 ± 0.07 | — |
| C16:0 | 18.70 ± 0.48 | 18.61 ± 0.52 | 11.66 ± 0.73 |
| C16:1 | 9.17 ± 0.31 | 8.80 ± 0.61 | 3.02 ± 0.55 |
| C18:0 | 1.24 ± 0.04 | 1.32 ± 0.18 | 1.60 ± 0.04 |
| C18:1 | 39.41 ± 0.72 | 40.30 ± 1.00 | 59.27 ± 0.50 |
| C18:2 | 8.83 ± 0.92 | 8.76 ± 0.70 | 18.25 ± 0.94 |
| C20:1 | 0.19 ± 0.04 | 0.23 ± 0.06 | 4.13 ± 0.27 |
| C21:0 | 0.51 ± 0.05 | 0.56 ± 0.03 | 0.87 ± 0.08 |
| TSATb | 41.97 ± 1.143 | 41.47 ± 2.208 | 15.00 ± 0.931 |
| TMUFAb | 49.44 ± 1.118 | 49.97 ± 1.727 | 66.49 ± 1.319 |
| TPUFAb | 8.83 ± 0.918 | 8.76 ± 0.697 | 18.25 ± 0.940 |
Tables 7 and 8 show the liver FAC for mice fed with 7% and 30% dietary fat. The major fatty acids present were C18:1 (∼40%), C16:0 (∼22%), C16:1 (∼10%), C18:2 (∼7%) with the PKO–PO blend and P-MLCT in both the low fat and high fat diets. Mice fed with C-MLCT had similar types and amounts of FAC as those with the PKO–PO blend and P-MLCT, but a lower amount of C16:1 (∼8%) and a higher amount of C18:2 (∼13%). The proportion of saturated and monounsaturated fatty acids was higher and that of polyunsaturated fatty acids was lower with the PKO–PO blend and P-MLCT compared to C-MLCT in both the low fat and high fat diets. As compared to fat pad FAC, liver FAC consisted of a variety of long chain saturated and polyunsaturated fatty acids such as C20:0, C21:0, C22:0, C20:3, C22:1, C23:0, C20:5 and C22:6.
| FAC | Dietary fat (low fat group)a | ||
|---|---|---|---|
| PKO–PO blend [%] | P-MLCT [%] | C-MLCT [%] | |
| a Values represent means ± SD, with triplicate analysis. b TSAT = total saturated fatty acid, TMUFA = total monounsaturated fatty acid, TPUFA = total polyunsaturated fatty acid. | |||
| C12:0 | 1.053 ± 0.28 | 0.907 ± 0.17 | 0.318 ± 0.38 |
| C14:0 | 2.292 ± 0.81 | 1.930 ± 0.66 | 1.303 ± 0.61 |
| C16:0 | 22.338 ± 0.58 | 22.818 ± 0.75 | 21.005 ± 1.07 |
| C16:1 | 10.693 ± 3.80 | 10.384 ± 1.89 | 8.225 ± 2.29 |
| C18:0 | 3.527 ± 0.78 | 3.461 ± 1.25 | 3.246 ± 0.18 |
| C18:1 | 40.259 ± 2.26 | 40.127 ± 2.50 | 39.207 ± 3.17 |
| C18:2 | 7.446 ± 1.56 | 7.523 ± 0.56 | 12.480 ± 3.01 |
| C20:0 | 0.520 ± 0.21 | 0.604 ± 0.24 | 0.747 ± 0.16 |
| C18:3 | — | — | 1.463 ± 1.05 |
| C21:0 | 0.815 ± 0.27 | 0.648 ± 0.08 | 0.593 ± 0.20 |
| C22:0 | 1.155 ± 0.07 | 1.171 ± 0.16 | 0.729 ± 0.35 |
| C20:3 | 0.551 ± 0.19 | 0.526 ± 0.23 | 0.466 ± 0.00 |
| C22:1 | 5.896 ± 1.58 | 6.123 ± 2.23 | 4.527 ± 0.71 |
| C23:0 | — | — | — |
| C20:5 | 0.850 ± 0.16 | 1.105 ± 0.84 | — |
| C22:6 | 2.605 ± 0.75 | 2.493 ± 0.91 | 4.833 ± 1.84 |
| TSATb | 31.18 ± 0.84 | 30.93 ± 0.60 | 26.95 ± 2.39 |
| TMUFAb | 56.85 ± 1.56 | 56.63 ± 2.16 | 51.96 ± 4.17 |
| TPUFAb | 23.76 ± 5.13 | 24.50 ± 6.49 | 27.78 ± 2.96 |
| PUFA/SAT | 0.77 ± 0.18 | 0.79 ± 0.22 | 1.04 ± 0.20 |
| 1.053 ± 0.28 | 0.907 ± 0.17 | 0.318 ± 0.38 | |
| FAC | Dietary fat (high fat group)a | ||
|---|---|---|---|
| PKO–PO blend [%] | P-MLCT [%] | C-MLCT [%] | |
| a Values represent means ± SD, with triplicate analysis. b TSAT = total saturated fatty acid, TMUFA = total monounsaturated fatty acid, TPUFA = total polyunsaturated fatty acid. | |||
| C12:0 | 2.361 ± 0.78 | 2.458 ± 1.27 | — |
| C14:0 | 3.475 ± 0.31 | 3.850 ± 0.81 | 0.643 ± 0.07 |
| C16:0 | 23.458 ± 0.87 | 22.625 ± 1.83 | 21.737 ± 2.89 |
| C16:1 | 8.657 ± 0.39 | 8.856 ± 1.09 | 2.098 ± 0.42 |
| C18:0 | 2.894 ± 0.22 | 2.797 ± 0.10 | 3.077 ± 0.77 |
| C18:1 | 42.158 ± 1.10 | 42.050 ± 4.28 | 46.158 ± 2.95 |
| C18:2 | 7.302 ± 0.66 | 7.434 ± 2.94 | 13.753 ± 2.73 |
| C20:0 | 0.345 ± 0.17 | 0.629 ± 0.13 | 0.521 ± 0.18 |
| C18:3 | — | — | 2.596 ± 0.58 |
| C21:0 | 0.932 ± 0.07 | 0.825 ± 0.44 | 1.044 ± 0.17 |
| C22:0 | 0.577 ± 0.08 | 0.610 ± 0.11 | |
| C20:3 | 0.601 ± 0.05 | 0.676 ± 0.19 | 0.704 ± 0.03 |
| C22:1 | 4.990 ± 0.48 | 4.883 ± 0.90 | 3.166 ± 0.96 |
| C23:0 | — | — | 0.788 ± 0.19 |
| C20:5 | 0.650 ± 0.09 | 0.676 ± 0.01 | — |
| C22:6 | 1.600 ± 0.16 | 1.170 ± 0.28 | 3.825 ± 0.89 |
| TSATb | 33.70 ± 1.72 | 33.16 ± 0.19 | 26.56 ± 2.28 |
| TMUFAb | 55.80 ± 1.20 | 55.79 ± 2.30 | 51.42 ± 2.42 |
| TPUFAb | 20.48 ± 1.29 | 20.35 ± 4.93 | 24.96 ± 5.74 |
| PUFA/SAT | 0.61 ± 0.06 | 0.61 ± 0.15 | 0.96 ± 0.31 |
| 2.361 ± 0.78 | 2.458 ± 1.27 | — | |
Discussion
The present study was carried out to examine the effect of enzymatically modified palm-based MLCT (P-MLCT) compared with a control, which was a non-enzymatically modified oil (PKO–PO blend), and commercial MLCT (C-MLCT) on the suppression of body fat accumulation for a short term of 8 weeks in 7% dietary fat (low fat) and 30% dietary fat (high fat) conditions. There was a trend that all of the three parameters (body weight, body weight gain and total fat accumulation) were found to be the lowest in P-MLCT fed mice, although these parameters were not statistically significant (P > 0.05). Consistent with our studies, most MLCT studies also showed no significant difference in terms of body weight gain for the mice subjects.9,10 Contradictory to our experiments, in terms of fat pad accumulation, some studies showed MLCT to have significantly (P < 0.05) reduced the fat pad accumulation. This is due to the fact that these studies compared MLCT (MCFA + LCFA) with LCT (LCFA), therefore significant differences can be seen between the groups in terms of the reduction in fat pad accumulation.6,11,12 In our studies, no LCT (LCFA) are included; instead both the control (PKO–PO blend) and treatment groups (P-MLCT and C-MLCT) contain MCFA and LCFA. Thus, it is unlikely that significant differences in the body weight and fat pad accumulation will be observed over a short time period.The body fat suppression effect of P-MLCT may be due to the amount of MCFA present in the triacylglycerol molecule (by comparing the PKO–PO blend and P-MLCT with C-MLCT). The molecular structures of MCFA (C8–C12), which are shorter, smaller, and more hydrophilic in nature compared to LCFA,13,14 caused MCFA to have different absorption, transportation and metabolism features. They left the intestinal mucosa rapidly into the portal venous system and were absorbed by the liver. Inside the liver cells, MCFA are carnitine independent, so they can pass through the mitochondria cells more easily to undergo beta oxidation. The rapid oxidation of MCFA causes them to have only a small tendency to deposit as body fat.4 In contrast, LCFA have the tendency to re-synthesize into new triglycerides and to be transported as chylomicrons. Table 9 shows the FAC of both the control and treatment oils. P-MLCT and the PKO–PO blend have more MCFA, 47% (from C8, C10, C12), compared to C-MLCT which have only 8% MCFA (from C8 and C10), suggesting that the amount of MCFA is the vital factor contributing to the weight reducing effect of P-MLCT and the PKO–PO blend compared to C-MLCT. An interesting finding was found in this study, which showed the ability of C12 fatty acids to lower the weight gained.
| Ingredients | Dietary group [w/w%] | |||||
|---|---|---|---|---|---|---|
| Low fat | High fat | |||||
| PKO–PO blend | P-MLCT | C-MLCT | PKO–PO blend | P-MLCT | C-MLCT | |
| a TBHQ = tert-Butylhydroquinone | ||||||
| Casein | 20 | 20 | 20 | 25.29 | 25.29 | 25.29 |
| L-Cysteine | 0.3 | 0.3 | 0.3 | 0.38 | 0.38 | 0.38 |
| Corn starch | 39.75 | 39.75 | 39.75 | 2.66 | 2.66 | 2.66 |
| Maltodextrin 10 | 13.2 | 13.2 | 13.2 | 16.69 | 16.69 | 16.69 |
| Sucrose | 10 | 10 | 10 | 12.65 | 12.65 | 12.65 |
| Cellulose BW 200 | 5 | 5 | 5 | 6.32 | 6.32 | 6.32 |
| PKO–PO blend | 7 | — | — | 30 | — | — |
| P-MLCT | — | 7 | — | — | 30 | — |
| C-MLCT | — | — | 7 | — | — | 30 |
| TBHQa | 0.0014 | 0.0014 | 0.0014 | 0.0018 | 0.0018 | 0.0018 |
| Mineral mix | 3.5 | 3.5 | 3.5 | 4.43 | 4.43 | 4.43 |
| Vitamin mix | 1 | 1 | 1 | 1.27 | 1.27 | 1.27 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.32 | 0.32 | 0.32 |
| Energy [kJ per 100 g] | 1662.6 | 1662.6 | 1662.6 | 2307.3 | 2307.3 | 2307.3 |
Structural differences (by comparing P-MLCT with the PKO–PO blend) of the triacylglycerol might have also contributed to the weight and body fat reducing effect of P-MLCT. Unlike the PKO–PO blend, P-MLCT are not a physical mixture, instead they are an interesterified oil. PO consists mainly of LCFA, most of the sn1,2,3 positions of it are occupied by LCFA. When PO is interesterified with PKO using 1,3-specific Lipozyme TLIM lipase, MCFA from PKO will be interesterified in the 1,3 positions of the PO replacing the LCFA positions. This reaction increased the amount of MLCT, and lowered the amount of LCT. As such, a physical mixture of a PKO–PO blend will have more LCT compared to P-MLCT. The LCT can result in increasing chylomicron circulation, which eventually causes fat accumulation. In addition, the dosage of fat might also have an influence on the weight gained and body fat accumulation. The present result demonstrated that the weight reducing property of P-MLCT was more prominent in the low fat diet compared with the high fat diet, in which less fat was accumulated in the low fat diet fed mice (Fig. 1). Matsuo8et al. showed that rats fed with 5% and 10% of MLCT have significantly lower body weight gain compared with 15% and 20% of MLCT due to the reduction in epididymal, perirenal, and mesenteric fat pads. In the present study, the high fat diet masked the weight reducing effect of enzymatically modified MLCT (P-MLCT), but not in the low fat diet.
HOMA-IR is a method used to quantify insulin resistance. Higher HOMA-IR values show that the subjects are prone to development of diabetes mellitus. Consuming P-MLCT was found to increase insulin sensitivity towards glucose, where it confers the lowest HOMA-IR values in both low fat and high fat diets. Studies found that insulin secretion by the pancreatic beta cell was not only governed by the glucose level but the pool of unbound FFA.15 Fatty acid chain length (positively) and degree of saturation (negatively) also influence the insulinotropic potency. C8, C18:2, C18:1 and C18 enhanced glucose stimulated insulin secretion (GSIS) by 3.4, 5.3, 9.4 and 21-fold, respectively.16,17 As such, mice fed with C-MLCT had higher insulin levels compared to those with the PKO–PO blend and P-MLCT due to higher amounts of LCFA in C-MLCT oil. We can also infer that the 1,3 positions of the P-MLCT consist more of MCFA compared with the PKO–PO blend in which, upon digestion at these positions by the pancreatic and linguinal enzyme lipase, the FA will be released into the blood stream as free fatty acid (FFA) and this subsequently reduced GSIS. Besides, the weight reducing ability of P-MLCT might have also given rise to the insulin sensitivity in P-MLCT fed mice. Weight losses caused by a reduction in total fat pads are likely to increase insulin sensitivity as less stress is exerted on the beta cells of the islets of Langerhans. This is in agreement with the findings showing that a reduction in the body weight of overweight individuals leads to a marked reduction in developing diabetes.18,19 This is consistent with the study showing that intake of commercial MLCT was able to ameliorate insulin resistance in rats.20
As shown in Table 4, P-MLCT and PKO–PO blend fed mice tend to have a lower HDL/LDL ratio compared with C-MLCT fed mice, suggesting the tendency of P-MLCT and the PKO–PO blend to increase the cholesterol level. This may be due to the presence of C12, C14 and C16, which are hypercholesteromic. C14 was found to be the most potent saturated hypercholesteromic fatty acid followed by C16 and C12 in causing an increase in total and LDL cholesterol.21Table 4 shows that P-MLCT fed mice had lower triglyceride levels compared to C-MLCT and the PKO–PO blend fed mice. This may be due to the structural differences and dietary fatty acids (medium chain and long chain composition) present. As mentioned above, enzymatic interesterification causes more MCFA to occupy the sn1,3 positions of P-MLCT than the PKO–PO blend. As such, when pancreatic lipases react, more MCFA will be released into the liver to undergo beta oxidation. Unlike long chain fatty acids, there will be fewer chances for the free fatty acids of MCFA to be re-synthesised in the body into triglyceride which are transported as chylomicrons. Studies have also shown that fasting increases lipolysis, whereas a higher insulin level and overfeeding reduces lipolysis.22 This is inconsistent with our studies in which P-MLCT fed mice had lower insulin levels which induced lipolysis.
De novo fatty acid synthesis in the body is carried out by the desaturase enzymes, which include Δ9 desaturase, Δ6 desaturase, Δ5 desaturase, and Δ4 desaturase, whereby these enzymes are involved in chain desaturation and elongation to produce essential fatty acids in the body. Δ9 desaturase is responsible for converting palmitic acid and stearic acid to oleic acid (MUFA) as it showed to have preferences for converting saturated fatty acid (SFA) into unsaturated fatty acid. In contrast, the synthesis of unsaturated fatty acids such as eicosapentaenoic , docosahexaenoic acids (from linolenic acid) and arachidonic acid (from linoleic acid) involve the enzymes Δ6 desaturase, Δ5 desaturase, and Δ4 desaturase. De novo synthesis of fatty acids can be affected by the nutrients supplied, which affect the activity of the desaturase enzymes.23–25Tables 5–8 show the FAC of WAT and livers of the mice fed with 7% and 30% dietary fat, respectively. Consistent with the present study, the types of diet fed to mice influences the FAC in the liver and white adipose tissue (WAT). Structured lipids did not have any effect on the adipose tissue FAC, which can be seen from the results of the PKO–PO blend and P-MLCT fed mice that have an almost similar FAC in WAT (Tables 5 and 6) and in the liver (Tables 7 and 8). Nevertheless, when both the PKO–PO blend and P-MLCT were compared with C-MLCT, there were differences in the FAC of both livers and WAT, showing that FAC in the diet has more influence compared with FA structural differences. Although both the PKO–PO blend and P-MLCT diets have around ∼77% SFA and ∼23% MUFA + PUFA in them, the adipose tissue accumulated has a different FAC compared with the diet where SFA and MUFA + PUFA is around ∼40% and ∼60%, respectively, showing an improvement in the FAC. Both the P-MLCT and PKO–PO blend diets have the tendency to synthesize MUFA + PUFA (C14:1, C16:1 and C20:1) although initially the consumed diet contained more SFA. There were more new types of PUFA fatty acids in the liver compared with the adipose tissue showing that chain elongation and desaturation actions may be carried out in the liver and the fatty acids are then transported around the body where they accumulate. C18:3, being the most effective substrate for microsomal chain elongation, is mostly converted to C22:6. This is consistent with our study indicating that C-MLCT having more C18:3 give rise to a higher amount of C22:6 in the liver than P-MLCT and the PKO–PO blend.
Experimental
Materials
Refined palm oil and palm kernel oil (Sime Darby Research Sdn Bhd, Banting, Malaysia), Lipozyme TLIM (Novozymes, Bagsvaerd, Denmark), DIO C57BL/6J mice (Jackson Laboratory, MA, USA), D12492 standard DIO diet and custom-made diet (Research Diets Inc., New Jersey, USA) were obtained. All chemical and reagents used were of analytical or high performance liquid chromatography (HPLC) grade. Commercial MLCT were purchased from an available market.Synthesis of palm-based MLCT (P-MLCT)
Enzymatic transesterification of palm oil (PO) and palm kernel oil (PKO) was carried out in the presence of 5% (w/w) of Lipozyme TLIM lipase (Novozyme, Bagsvaerd, Denmark), substrate ratio of PKO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PO (90
PO (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/w), 50 °C, 7.26 h and which produced approximately 60% of MLCT in a 10 kg stirred tank batch reactor with stirring speed of 350 rpm.26 A physical refiner was used to remove free fatty acids from the enzymatically modified P-MLCT.
10 w/w), 50 °C, 7.26 h and which produced approximately 60% of MLCT in a 10 kg stirred tank batch reactor with stirring speed of 350 rpm.26 A physical refiner was used to remove free fatty acids from the enzymatically modified P-MLCT.
Animal and diets
72 C57BL/6J diet induced obesity (DIO) male mice of 6 weeks old were obtained from Jackson Laboratory (Bar Harbor, Maine USA). All the mice were housed in cages of four, in a room with controlled temperature (24–26 °C), humidity (40% to 60%) and lighting (12 h light and 12 h dark). During the acclimatization period, the mice were fed the commercial standard DIO diet D12492 (Research Diets Inc., New Brunswick, New Jersey, USA) and water ad libitium for 1 week. The 72 mice were randomly divided into six groups. Each group of mice was fed by free access to an experimental diet and water for 8 weeks. Experimental diets were based on modified AIN 93G, which is custom made by Research Diets Inc.The feeding trial was divided into two groups, in which the first group of mice was fed with the low fat diet, whereas the second group of mice was fed with the high fat diet, in order to determine the effects of a normal diet (low fat) and a high fat diet upon the consumption of P-MLCT. The analysis for both the experiments was similar. Experiment 1: The modified diets were such that the soybean oil was replaced with a PKO–PO blend, P-MLCT, and C-MLCT with each contributing 7% kcal, representing a low fat diet. Experiment 2: The modified diets were such that the soybean oil was replaced with a PKO–PO blend, P-MLCT, and C-MLCT with each contributing 30% kcal fat, representing a high fat diet. The compositions of the experimental diets are listed in Table 9. Table 10 shows the fatty acid composition (FAC) of the respective treatment oils.
| PKO–PO blend [%] | P-MLCT [%] | C-MLCT [%] | |
|---|---|---|---|
| a Values represent means ± SD, with triplicate analysis. TSAT = total saturated fatty acid, TMUFA = total monounsaturated fatty acid, TPUFA = total polyunsaturated fatty acid. | |||
| Acylglycerol | |||
| FFA, DAG, MAG | 1.20 ± 0.37 | 3.931 ± 0.59 | 2.29 ± 0.42 |
| LCT Type TAG | 57.85 ± 0.43 | 37.33 ± 0.22 | 52.97 ± 0.38 |
| MLCT Type TAG | 40.95 ± 1.75 | 58.74 ± 1.22 | 44.74 ± 1.31 |
| FAC | |||
| C8 | 2.618 ± 0.06 | 2.278 ± 0.17 | 6.73 ± 0.03 |
| C10 | 2.811 ± 0.02 | 2.653 ± 0.01 | 2.565 ± 0.01 |
| C12 | 42.92 ± 0.05 | 41.49 ± 0.03 | — |
| C14 | 15.01 ± 0.03 | 14.89 ± 0.03 | — |
| C16 | 11.75 ± 0.04 | 12.40 ± 0.06 | 3.595 ± 0.01 |
| C18 | 2.348 ± 0.01 | 2.412 ± 0.03 | 1.475 ± 0.01 |
| C18:1 | 18.85 ± 0.03 | 19.72 ± 0.10 | 56.22 ± 0.06 |
| C18:2 | 3.608 ± 0.02 | 3.758 ± 0.01 | 18.36 ± 0.03 |
| C18:2t | — | — | 0.725 ± 0.01 |
| C18:3 | — | — | 8.79 ± 0.03 |
| C20:0 | — | — | 0.455 ± 0.01 |
| C20:1 | — | — | 1.085 ± 0.01 |
| SAT | 77.457 | 76.123 | 14.82 ± 0.06 |
| MUFA + PUFA | 22.458 | 23.478 | 85.91 ± 0.13 |
Body weight and feed intake were measured on a weekly basis. Energy values from the macronutrient composition were calculated using the values of 16.7, 16.7 and 39.6 kJ g−1 for carbohydrate, protein and TAG oil, respectively. Fat pads consisting of retroperitoneal (WAT), epididymal (WAT), perirenal (WAT) and mesenteric (WAT) were dissected out and weighed. At the end of the experiment, the mice were deprived of food for 12 h prior to being scarified under light anesthesia of ketamine/xylazine. Blood serums were collected and kept at −80 °C prior to analysis. All mice were treated in accordance with the Animal Care and Use Committee, Faculty of Veterinary Medicine, Universiti Putra Malaysia.
Analytical measurements
Serum blood profiles such as serum total cholesterol, high density lipoprotein, low density lipoprotein (Biovision, California, USA), total triglyceride, total glucose (Cayman Chemical, Michigan, USA), hormone insulin (Mercodia, Upssala, Sweden), hormone leptin (Spi Bio Bertin Pharma, France) were tested using enzymatic and ELISA kits according to the manufacturers' instructions. Homeostatic model assessment insulin resistance (HOMA-IR) was calculated based on the following formula:| HOMA-IR = [glucose (mmol L−1) × insulin (mU L−1)/22.5] |
White adipose tissue (WAT) and liver fatty acid composition (FAC)
Extraction of lipids from the liver and WAT tissues (retroperitoneal, epididymal, perirenal and mesenteric) were carried out according to the previously reported method.27 The lipids extracted from both the tissues were then derivatized into fatty acid methyl esters (FAME) according to the reported method28. Gas chromatography was used to determine the FAC.Statistical analysis
All data is presented as mean ± SD. Data analyses were carried out using Statistical Analysis System (SAS 9.2, SAS Institute, Cary, NC, USA) to determine the analysis of variance (ANOVA) and significant differences between the treatments.Significant differences were determined using the least significant different t-test. Significant differences were defined as P < 0.05 level.
Conclusion
In conclusion, the types of fatty acid, structural differences of the triacylglycerol molecules and the amount of fat consumed closely affect the fat accumulation in the body as well as the blood parameters. The present study demonstrated that enzymatically interesterified P-MLCT, either when present in low fat or high fat composition, have the ability to suppress body fat accumulation and total triglyceride levels, as well as improve the blood glucose level compared to C-MLCT and a PKO–PO blend. However, P-MLCT were found to increase the blood cholesterol level. Long term studies on animal models are still needed to see the effect more clearly.Acknowledgements
Work reported in this paper was supported by Sime Darby Research Sdn Bhd.Notes and references
- E. S. Ford, B. A. Bowman and A. H. Mokdad,
et al.
, JAMA, J. Am. Med. Assoc., 2003, 289, 76–79 CrossRef
.
- J. R. Sowers, Am. J. Med., 2003, 115(8, Supplement 1), 37–41 CrossRef PubMed
.
- P. Von Hafe, F. Pina, A. Pérez, M. Tavares and H. Barros, Obes. Res., 2004, 12, 1930–1935 CrossRef PubMed
.
- T. Aoyama, N. Nosaka and M. Kasai, J. Med. Invest., 2007, 54, 385–388 CrossRef
.
- A. A. Papamandjaris, D. E. Macdougall and P. J. H. Jones, Life Sci., 1998, 62, 1203–1215 CrossRef CAS
.
- M. Kasai, N. Nosaka, H. Maki, S. Negishi, T. Aoyama, M. Nakamura, Y. Suzuki, H. Tsuji, H. Uto, M. Okazaki and K. Kondo, Asia Pac. J. Clin. Nutr., 2003, 12, 151–160 CAS
.
- T. Matsuo, M. Matsuo, M. Kasai and H. Takeuchi, Asia Pac. J. Clin. Nutr., 2001, 10, 46–50 CrossRef CAS
.
- T. Matsuo and H. Takeuchi, Br. J. Nutr., 2004, 91, 219–225 CrossRef CAS PubMed
.
- H. Shinohara, H. Shimada, O. Noguchi, F. Kubota and T. Aoyama, J. Oleo Sci., 2002, 51, 621–626 CrossRef CAS
.
- H. Shinohara, J. Wu, M. Kasai and T. Aoyama, Biosci., Biotechnol., Biochem., 2006, 70, 2919–2926 CrossRef CAS
.
- Y. Zhang, Y. Liu, J. Wang, R. Zhang, H. Jing, X. Yu, Y. Zhang, Q. Xu, J. Zhang, Z. Zheng, N. Nosaka, C. Arai, M. Kasai, T. Aoyama, J. Wu and C. Xue, Lipids, 2010, 45, 501–510 CrossRef CAS PubMed
.
- J.-I. Nagata, M. Kasai, S. Watanabe, I. Ikeda and M. Saito, Biosci., Biotechnol., Biochem., 2003, 67, 1937–1943 CrossRef CAS
.
- B. Marten, M. Pfeuffer and J. Schrezenmeir, Int. Dairy J., 2006, 16, 1374–1382 CrossRef CAS PubMed
.
- J. H. Bragdon and A. Karmen, J. Lipid Res., 1960, 1, 167–170 CAS
.
- V. Esser, D. T. Stein, B. E. Stevenson, K. E. Lane, J. H. Whiteside, M. B. Daniels, S. Chen and J. Denis McGarry, J. Clin. Invest., 1997, 97, 2728–2735 Search PubMed
.
- W. B. G. Stephen, R. Crespin, III and D. Steinberg, J. Clin. Nutr., 1973, 52, 1979–1984 Search PubMed
.
- B. E. S. Daniel, T. Stein, M. W. Chester, M. Basit, M. B. Daniels, S. D. Turley and J. Denis McGarry, Am. J. Clin. Nutr., 1997, 100, 398–403 Search PubMed
.
- D. F. Williamson, T. J. Thompson, M. Thun, D. Flanders, E. Pamuk and T. Byers, Diabetes Care, 2000, 23, 1499–1504 CrossRef CAS
.
- R. R. Wing, E. Venditti, J. M. Jakicic, B. A. Polley and W. Lang, Diabetes Care, 1998, 21, 350–359 CrossRef CAS
.
- S. Terada, S. Yamamoto, S. Sekine and T. Aoyama, Nutrition, 2012, 28, 92–97 CrossRef CAS PubMed
.
- P. L. Z. Martijti, B. Katan and R. P. Mensink, Am. J. Clin. Nutr., 1994, 60, 1017S–1022S Search PubMed
.
- C. Morimoto, T. Tsujita and H. Okuda, J. Lipid Res., 1998, 39, 957–962 CAS
.
- J. Blond, G. Durand and J. Bézard, J. Am. Oil Chem. Soc., 1998, 75, 269–274 CrossRef CAS
.
- J.-M. Cao, J. Blond, P. Juaneda, G. Durand and J. Bézard, Lipids, 1995, 30, 825–832 CrossRef CAS
.
- J. M. Ntambi, J. Lipid Res., 1999, 40, 1549–1558 CAS
.
- Y.-Y. Lee, T.-K. Tang, E.-T. Phuah, N. A. Ab Karim, S. M. M. Alwi and O.-M. Lai, J. Food Sci., 2013, 1–12 Search PubMed
.
- J. Folch, A simple method of isolation and purification of total lipids from animal tissues, J. Biol. Chem., 1957, 226, 497–509 CAS
.
- J. V. O'Fallon, J. R. Busboom, M. L. Nelson and C. T. Gaskins, J. Anim. Sci., 2007, 85, 1511–1521 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |