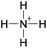
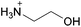
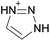
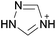
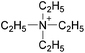
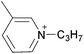
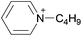
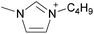
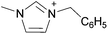
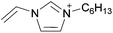
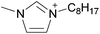
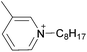
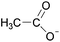
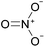
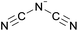
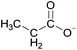
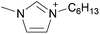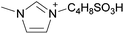Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mixing ionic liquids – “simple mixtures” or “double salts”?
Gregory
Chatel
,
Jorge F. B.
Pereira
,
Varun
Debbeti
,
Hui
Wang
and
Robin D.
Rogers
*
Center for Green Manufacturing and Department of Chemistry, The University of Alabama, Tuscaloosa, AL 35487, USA. E-mail: RDRogers@ua.edu; Tel: +1 205-348-4323
First published on 21st January 2014
Abstract
In the third issue of the then new journal Green Chemistry (2001, 3, 156–164), we published our first paper describing the physical properties of a few hydrophilic and hydrophobic ionic liquids (ILs) representing one of the first such studies to be published. To help celebrate the 15th anniversary of the Journal, we revisit the ‘design’ aspect of ILs by reviewing the growing area of what most are calling ‘ionic liquid mixtures’. In 2001, designing IL properties meant essentially independent variation or synthesis of the cations and anions and determining what physical or chemical properties these liquid salts possessed. Recently, however, the mixing of ILs has been proposed and investigated as a way to add increased scope to the accessible properties of IL media. In this review, we question whether the same thinking and approach used for organic solvent mixtures should be applied to ILs simply because of the way they were made. Unlike organic solvent mixtures, IL compositions of varied ions, do not retain their individual nature, need not be made by simple mixing of two-ion salts, and preferential interactions of a given cation for a given anion are possible in these 3-ion, 4-ion, or higher order liquids. When two ILs are mixed together, one can't identify which ion is from which IL, and the chemistry is simply not based on the identity of the individual ILs, but on the ions comprising them and the interactions of each individual ion, independently of the counterion. Thus, we ask if it would not be better to consider these as unique ion combinations whose solvent properties are derived from the specific choice and abundance of each ion in the system. Through this review of the available literature, we support the concept of Double Salt Ionic Liquids (DSILs) and discuss the interactions involved in these systems, by examining their physicochemical properties and the novel applications they offer.
1. Introduction
During the 15 years of existence of the journal Green Chemistry, the number of communications, patents, and publications reporting on ionic liquids (ILs) in different areas of chemistry increased considerably.1,2 These salts with low (typically less than 100 °C) or no melting points attracted a lot of attention from the Green Chemistry community as a result of some unique properties (observed in some examples) often unavailable with traditional solvents, including negligible vapor pressure, high chemical and electrochemical stabilities, etc.3–6 However, at the beginning of the 2000s, any ‘eco-friendly’ nature of ILs was still questioned, primarily because there simply was little to no data concerning the physical, chemical, and toxicological properties of any ILs (much less ILs ‘in general’) and thus it was not evident to the chemical community that ILs would be consistent with the twelve principles of Green Chemistry.7It was in this context that in 2001 we published our first paper relating the characterization and comparison of a few hydrophilic and hydrophobic ILs,8 providing a benchmark and sometimes target for the many scientists and engineers entering the IL field wishing to study and understand IL properties. Fifteen years later, numerous ‘green’ uses and applications of ILs have been proposed or demonstrated, but the knowledge needed to understand and predict the properties of ILs still requires a lot of study.9,10
Given our inability to predict which cation/anion combinations would lead to IL behavior or to predict what the resulting physical properties of these simple salts would be, the vast majority of publications discussing ILs have been simple one cation, one anion salts (in this review abbreviated as two-ion ILs). This is in spite of Seddon's oft quoted claim that there could be 1018 or more ILs if one considers binary, ternary, and higher order mixtures.11,12 It is telling to note that the early IL community understood such binary, ternary, etc. mixtures to be unique ILs, not simple mixtures of two or more two-ion ILs which would have been analogous to mixtures of organic solvents.13,14
It is in this context that IL–IL mixtures, initially based on the combination of several ILs, have recently received particular attention.15,16 Interestingly, in following almost the same path as the original studies of ILs, the primary focus on the study of mixtures of ILs has been on the resulting physical properties. In 2012, Welton's group reviewed the literature reported in this area and discussed the ideality and non-ideality of IL–IL mixtures, focusing on property changes and new opportunities for their applications.16 The physical properties such as density and viscosity were found to follow their corresponding mixing laws, respectively linear mixing of molar volumes and linear mixing on the logarithmic scale, while properties dependent upon specific chemical associations, such as phase transitions were not as easy to predict, and often non-ideal.16 Additional studies have since been published, however, despite instances of non-ideal behavior, these are still viewed as simple mixtures of two compounds.17
When ILs are mixed together, the ‘solutions’ obtained are quite different from mixtures of molecular solvents. A mixture refers to the physical combination of two or more substances in which the identities of the individual components are retained. Take an ethanol–water mixture for example. Ethanol's properties are considered to be derived from the structure of the ethanol molecule. A chemist would consider the properties of an ethanol–water mixture from the standpoint of how ethanol, because of its molecular structure, should interact with a water molecule.
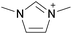
By definition, an ionic liquid is a salt, which is defined as both its cation and its anion, as neither one can be isolated without the other. A chemist would not consider 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl, to be the same compound as 1-butyl-3-methylimidazolium bis((trifluoromethane)sulfonyl)imide, [C4C1im][NTf2], since changing one of the ions changes the compound. When these two ILs are mixed together, the ionic associations found in the individual ILs are lost and one can't identify which ion is from which ionic liquid or the unique interactions of the individual ILs. Indeed, if instead of two ILs with a common ion, two ILs with different monovalent ions (e.g., [A][B] and [C][D]) are mixed, the resulting mixture could be considered a combination of four different salts, namely [A][B], [C][D], [A][D], and [B][C]. With such complex mixtures, one might be tempted to try and predict the properties of the resulting solutions based upon all possible two-ion ILs that might form, but on what basis would this approach be founded?
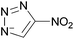
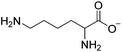
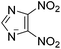
Situations also exist when solutions of multiple ions are not prepared by simply mixing different salts, but rather are the product of chemical reactions. For example, in the reaction depicted in Scheme 1a, [C1C1im][4-NO2-tri]0.64[4,5-diNO2-im]0.36 was synthesized independently via a one-pot reaction, although it could also be prepared as a mixture of two salts.18 Similarly, the statistical mixture of symmetric and asymmetric dialkylimidazolium acetates depicted in Scheme 1b19 and the chloride analog were also prepared using a one-pot synthetic process with appropriate starting materials in the desired ratios.20
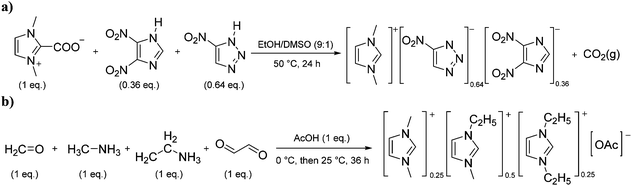 | ||
| Scheme 1 One-pot synthesis of (a) [C1C1im][4-NO2-tri]0.64[4,5-diNO2-im]0.3618 and (b) [C1C1im]0.25[C2C1im]0.5[C2C2im]0.25[OAc].19 | ||
The chemistry is simply not based on the identity of the individual ILs, but on the ions comprising them and the interactions of each individual ion which take place independently of the original counterion. However, and this is confusing at times, some of the physical properties obtained by mixing two ILs (especially when they are liquid at room temperature) can be predicted from the properties of the two ILs mixed. This does not change the fact that the overall properties of this ‘mixture’ are derived from the individual ions, not necessarily derived from the one possible ion pair which was used to prepare the ‘solution’. For example, Pereiro et al.21 demonstrated that by mixing either of the ILs 1-ethyl-3-methylimidazolium ethylsulfate, [C2C1im][EtSO4], or 1-ethyl-3-methylimidazolium acetate, [C2C1im][OAc], with the inorganic salt ammonium thiocyanate, [NH4][SCN], new liquids comprised of multiple ions, with higher ionicity could be formed. In predicting the properties of these mixtures should one then compare the density and viscosity of crystalline higher melting [NH4][SCN] with those of room temperature liquids [C2C1im][EtSO4] or [C2C1im][OAc] or would one simply declare this particular mixture of ions a solution and decide another methodology was needed?
There is a well-known concept which classifies ionic compounds comprised of more than two types of ions as double salts, and these are known to possibly exhibit unexpected associations that yield non-ideal properties.22 Double salts are thus defined as salts containing more than one cation or anion and presenting different physicochemical properties than of its component single salts.23 When the ions composing a double salt crystallize together the resulting double salt is considered one pure substance, rather than two separate salts,24 even though these can be melted and the ion combinations separated into simpler salts. Additionally, many properties of double salts, such as solubility, are dependent on the unique chemical associations between the ions, and altering the ionic ratios can induce different chemical interactions leading to new properties.25
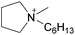
By analogy, we suggest pure liquid ionic compositions containing more than one cation or anion, should be viewed as ‘Double Salt Ionic Liquids’ (DSILs) rather than as simple mixtures of ILs or solutions of a salt in an IL. For the purposes of this review we will thus define DSILs as salts composed of more than two types of ions, liquid at low temperature (<373 K); where each unique combination of ion types and ion ratios constitutes a unique DSIL leading to properties specific to that combination. We consider this conceptual approach as essential to providing the framework needed to change the emphasis from what the ion properties and interactions are in two-ion ILs, to the properties unique to the presence of several ions, and thus allow a fuller study and understanding of the new ionic associations in DSILs. Such DSILs are composed of several ions and can show unique melting points and glass transition temperatures, often forming eutectic regions, disruptions in glass transitions, or even acting as a single set of ions.26,27 For example, the DSIL prepared by the equimolar mixing of N-propyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ([C3C1pyr][NTf2]) with N-butyl-N-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ([C4C1pyr][NTf2]) exhibited only a single melting transition (Tm = −42 °C) as was also found for the two IL parents albeit at higher temperatures (10 °C for [C3C1pyr][NTf2] and −7 °C for [C4C1pyr][NTf2]).27 Other papers (discussed herein) have reported unique ionic interactions occurring in DSILs with ions, solutes, or solvents.15,16,28 These studies indicate that some DSILs often exhibit properties not readily apparent from those of the IL parents and we will address these further in the discussion below.
As initially highlighted in this introduction due to their ionic composition, two-ion ILs are already considered as unconventional solvents (compared to molecular solvents),10,29 but when several different ions are introduced in different ratios all such interactions could be changed. Thus, in this review, we will look at examples from the literature and the reported physicochemical properties in light of the DSIL concept and see how this insight might be used to design new low temperature liquid salts composed of several ions. (Note: we have not considered in this review ILs based on chloroaluminate anions which are known to react and enter into complex equilibria forming new types of ions as a function of composition.30)
We must also call attention to issues with nomenclature which currently emphasize the two-ion ILs from which the multi-ion combinations are made rather than the resulting unique DSIL.16 As a combination of ions, DSILs can be composed of one cation with several anions, one anion with several cations, or even several cations and anions. By convention, we have decided to group cations together and anions together, and to order them in ascending order of the ions’ charge and molecular weights. Since all the systems studied to date and reviewed here contain singly charged cations and anions, the majority of the abbreviations suggested in Table 1 represent these DSILs. Here, we have fixed the total cation and anion charge to be +1 and −1 which will facilitate the study of the entire range of possible compositions; however, we do understand that representing only one example from each system might better be represented with integers (e.g., [C1]0.5[C2]0.5[A1]0.5[A2]0.5 could easily be represented as [C1][C2][A1][A2]). When higher charged ions are present, the lower charge should take priority followed by the molecular weight (see Table 1 for an example of ascending charges and ascending molecular weights). For ready comparison with the systems with monovalent anions, we also suggest that the total cation and anion charges in these formulas be fixed to +1 and −1. This systematic nomenclature allows the consistent representation of any DSIL based on composition in terms of the ions and their abundance.
| Number of ions | Monovalent ion composition | Abbreviationa,b | Name | Example |
|---|---|---|---|---|
| a Increasing molar mass of ions in the following order: [C1] < [C2] < … and [A1] < [A2] < … b x and y represent the mole ratio of the corresponding ion in the DSIL. c To avoid any confusion, the term “two-ion ILs” will be used throughout this review to define ILs that are not DSILs. d These salts have not yet been reported and are only used here as nomenclature examples. Note that the total cation charge has been constrained to be +1 for comparison with the majority of DSIL examples with monovalent ions. | ||||
| 2 | 1 Cation, 1 Anion | [C][A] | Ionic liquid (IL) or “Two-ion IL”c | [C2C1im][OAc] |
| 3 | 1 Cation, 2 Anions | [C][A1]x[A2](1−x) | Double Salt Ionic Liquid (DSIL) | [C2C1im][N(CN)2]x[BF4](1−x) |
| 2 Cations, 1 Anion | [C1]x[C2](1−x)[A] | [C2C1im]x[C3C1pyr](1−x)[NTf2] | ||
| 4 | 2 Cations, 2 Anions | [C1]x[C2](1−x)[A1]y[A2](1−y) | [C2C1im]x[C4C2im](1−x)[EtSO4]y[NTf2](1−y) | |
| 4 | 1 Cation, 3 Anions | [C1][A1]x[A2]y[A3](1−x−y) | [C4C1im][Cl]x[Br]y[MeSO4](1−x−y) | |
| 4 | 3 Cations, 1 Anion | [C1]x[C2]y[C3](1−x−y)[A] | [C1C1im]x[C2C1im]y[C2C2im](1−x−y)[OAc] | |
| DSILs containing multivalent ions | ||||
| 4 | 2 Cations, 2 Anions |
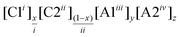
|
DSIL | [C4C1im]0.3[C6C1im]0.7[Cl]0.6[SO4]0.2d [C2C1im]0.2[Co(H2O)]0.4[Cl]0.8[SO4]0.1d |
In this review, we will first discuss the physical properties of DSILs in order to determine the most important ionic interactions in these salts. We then explore the reported thermal and enthalpic properties and discuss the importance of DSIL solvent properties useful for new applications. Throughout the review, we discuss the relationship of the reported data to the concept of double salt ionic liquids and how the unique interactions involved in these systems might be responsible for specific properties. We hope that by understanding such complex interactions and noting where our understanding is limited and new data is needed, that DSILs might help the field of ionic liquids live up to its reputation as producing designer solvents and materials.
2. Physical properties of DSILs
Of the 102 different DSIL systems reported to date, the most common IL ion combinations are based on three ions with 42% comprising a unique cation and 42% comprising a unique anion (Fig. 1a). DSILs composed of four or more ions represent only 16% of the studied DSILs. It is important to note that in these early studies, relatively commonly used, singly charged IL ions were studied. For example, about 55% of the cations studied were imidazolium derivatives and the most reported anions were [NTf2]− (60%), [BF4]− (21%), [PF6]− (16%), and halides (8%). The most studied properties (Fig. 1b) included viscosity, density, and molar excess volume (about one study in three), followed by simulations and modeling studies and enthalpy and conductivity measurements. It is clear now, as it was in 2001, that physical properties have been the subject of most interest.8 While we do hope that systematic characterization of the physicochemical properties of DSILs will continue, since this should lead to an ability to ultimately model and predict which ion combinations will provide which properties, we will show through this review that the unique chemical and solvent properties of DSILs could lead to the development of new applications.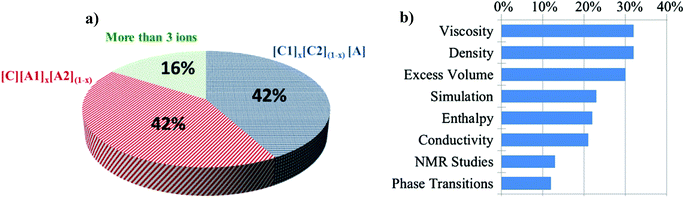 | ||
| Fig. 1 (a) Distribution of DSIL types reported (% of systems studied) and (b) most studied DSIL topics (% of systems where the given property was described). | ||
Interestingly, the water content in the publications noted above was indicated in less than half of the reported studies (ca. 45%), and the purity (e.g., residual halide ions, organic solvent traces, etc.) of the ILs used for preparing DSILs is almost never indicated, sometimes leading us to question the reliability of the data. The importance of water content and other impurities cannot be understated, especially since impurities have considerable effect on an IL's physical properties (e.g., viscosity, conductivity, and diffusivity) has been a key consideration in the field for over 15 years.31,32 For example, the role of water in hydrogen bonding and solvating IL ions has been known for some time,33 and water can certainly affect the reactivity of some solutes dissolved in ILs and modify the IL's solvent properties.34 Indeed our Green Chemistry physical properties paper compared and contrasted the properties of dried vs. wet ILs.8 Some lessons, however, seem destined to be relearned and such things as water content are not yet sufficiently and rigorously reported in the studies of DSIL physicochemical properties.
Below, we first review common physical properties of DSILs such as viscosity, density, excess molar volume, and conductivity, reporting the water content when it was indicated. We note in the tables where water content is not provided with N/A and a footnote ‘data not provided’ suggesting some caution in the use of this data. We compare the properties of these systems as others have done by calculating the deviation from the average obtained by molar abundance averaging (unless otherwise indicated) of the same property measured for the IL parents. When the data corresponding to the desired ratios were not directly available in the literature, we estimated the values from any provided data or plots.
2.1. Viscosity
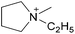
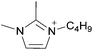
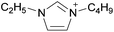
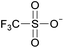
Coulombic interactions are considered to be the major source of attraction in ILs comprised of a single type of cation and anion, depending on the ion charges, the interionic distances, and the coordination number,35 but van der Waals interactions between alkyl chains, π–π stacking interactions, and hydrogen bonding between polar groups are also present.36,37 Based on Fourier Transform InfraRed (FTIR) spectroscopic studies and some ab initio calculations, Fumino et al. showed that strong and directional hydrogen bonds formed between cations and anions can destroy the charge symmetry. Hydrogen bonds can introduce defects into the Coulombic network of ILs that may increase the dynamics of the cations and anions, resulting in reduced viscosities.38–40 Thus, they proposed that the physical properties of some imidazolium-based ILs can be tuned by adjusting the ratio between hydrogen bonding energies and Coulombic interactions to lead to lower viscosities. However, it is also important to emphasize that hydrogen bonding is a weaker, albeit more directional, electrostatic force than Coulombic interactions.38 Based on standard ab initio molecular orbital theory calculations, Izgorodina and MacFarlane estimated that the Coulombic attractions dominate the energetics of imidazolium-based ion pairs and contribute between 86 to 88% of the overall attractive energy.41In the case of DSILs, hydrogen bonding seems to have a negligible effect on the viscosity (see for example the use of methylsulfate and ethylsulfate anions in Table 2, entries 2 and 3). Hydrogen bonding between the cation and anion that actually does affect the viscosity of two-ion ILs might become even less important compared to Coulombic interactions, when more than two ions are present. However, there are very few studies on the viscosity of the type of DSILs needed to truly study this reported in the literature. For example, the viscosity of [C2C1im]x[C2C1C1im](1−x)[NTf2] as a function of mole ratio would be interesting to compare to [C2C1im][NTf2] and [C2C1C1im][NTf2] since the two-ion ILs themselves have very different viscosities of 34 mPa s and 88 mPa s, respectively.42 That is, in the early studies of DSILs, we would suggest using ions and two-ion ILs that have very different properties, such that any differences found for the DSILs are more easily recognizable.
| Entry | DSIL | Deviation from the mixing averagea (mPa s) | H2O content (ppm) | Temperature (K) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Three-ion DSILs: | |||||||||
| Deviations less than 5 mPa sb | |||||||||
| Cation | Anion 1 | Anion 2 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
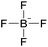
| 1 | [C2C1im] | [N(CN)2]x | [BF4](1−x) | −1.3 | −3.3 | −2.1 | <100 | 298.15 | 17 |
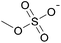
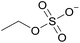
| 2 | [C2C1im] | [EtSO4]x | [NTf2](1−x) | +2.9 | +1.3 | +3.7 | <1000 | 298.15 | 46 |
| 3 | [C4C1im] | [MeSO4]x | [BF4](1−x) | −3.4 | −3.0 | −5.1 | N/Ac | 298.15 | 47 |
| Deviations greater than 5 mPa sb | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cation | Anion 1 | Anion 2 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
| 8 | [C4C1im] | [BF4]x | [PF6](1−x) | −37.1 | −33.1 | −25.0 | N/Ac | 298.15 | 47 |
| 9 | [C4C1im] | [BF4]x | [PF6](1−x) | +6.4 | −12.8 | −15.7 | <50 | 298.15 | 49 |
| 10 | [C6C1im] | [BF4]x | [PF6](1−x) | +10.4 | −3.8 | −12.4 | <50 | 298.15 | 49 |
| 11 | [C3C1pyr] | [N(CN)2]x | [NTf2](1−x) | −2.3 | −4.5 | −12.4 | <310 | 293.15 | 26 |
| 12 | [C4C1pyr] | [NTf2]x | [N(S2O4C5F12)](1−x) | −37.5 | −65.0 | −57.5 | <2 | 293.15 | 50 |
| 13 | [C4C1pyr] | [NTf2]x | [N(S2O4C5F12)](1−x) | N/Ac | −22.0 | N/Ac | <20 | 303.00 | 51 |
| 14 | [C4C1pyr] | [NTf2]x | [N(SO2C2F5)2](1−x) | N/Ac | +31.8 | N/Ac | <20 | 303.00 | 51 |
| 15 | [C4C1pyr] | [N(SO2C2F5)2]x | [N(S2O4C5F12)](1−x) | N/Ac | −28.5 | N/Ac | <20 | 303.00 | 51 |
| 16 | [C4py] | [BF4]x | [NTf2](1−x) | −8.9 | −13.6 | −15.0 | <10 | 303.15 | 52 |
| Four-ion DSILs: | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cation 1 | Cation 2 | Anion 1 | Anion 2 | x = y = 0.5 | |||||
| a Deviation of the viscosity (Δη, mPa s), compared to ideal mixing average of the two IL parents calculated using eqn (1): Δη = η − ∑χi × ηi where η is the viscosity of the DSIL, χi and ηi are the mole fraction and viscosity of the IL parents (i). b To highlight differences an arbitrarily chosen limit of |Δη| ≤ 5 mPa s compared to the ideal mixing average of the two IL parents was considered. c Data not provided. d The comparison with the ideal mixing average value is not possible since the [NH4][SCN] salt parent is a solid at 298.15 K (Tm = 389 K).21,53 | |||||||||
| 20 | [C2C1im]x | [C4C2im](1−x) | [EtSO4]y | [NTf2](1−y) | −56.0 | <1000 | 298.15 | 46 | |
| 21 | [NH4]x | [C2C1im](1−x) | [SCN]y | [EtSO4](1−y) | —d | <1200 | 298.15 | 21 | |
| 22 | [NH4]x | [C2C1im](1−x) | [SCN]y | [OAc](1−y) | —d | <1200 | 298.15 | 21 | |
Although several mixing laws have been proposed in the literature to fit the DSIL data, such as the Katti and Chaudhri law (based on molar volumes),43 the Grunberg and Nissan law (logarithmic law involving only the viscosity),44 or the Bingham law (developed by analogy to electric resistors in parallel circuits),45 good agreement between the experimental viscosity data and any of these seems rather limited.16,17 For this reason, and in order to facilitate the comparison across all of the available data, we calculated the deviation of viscosity for each system compared to the linear mixing average of the IL parents (Table 2), in which we have calculated the deviations of the viscosity compared to the ideal mixing average of the two IL parents calculated using the linear eqn (1):
| η = ∑χi × ηi | (1) |
In order to provide an initial global comparison of all the published data, we highlight below the less ideal behavior compared to this law by considering an arbitrary line boundary (i.e., a deviation of 5 mPa s). Of course, this limit is arbitrary and may be better assessed after the study of additional systems.
Table 2 indicates that the most important deviations are found when the ion sizes are quite different. The presence of bulky cations such as [P6,6,6,14]+ (Table 2, entry 19), cations with longer alkyl chains such as [C4C1im]+ and [C6C1im]+ (Table 2, entries 8–10, 17, and 18), or bulky anions such as [N(S2O4C5F12)]− and [N(SO2C2F5)2]− (Table 2, entries 12–15) led to greater deviations of the viscosity from the ideal mixing average. Navia et al. also concluded that the largest deviations in viscosity were obtained for DSILs with larger dissimilarities in the structures of their ions.47
Based on our analysis on the data obtained to date (Table 2), steric effects seem to have a considerable contribution to the organization of the ions causing the largest deviations in viscosity; while hydrogen bonding and dissymmetry factors seem to have negligible effects on the viscosity of DSILs, compared to the IL parents. Due to the presence of more than two ions, the viscosity seems to be even more controlled by Coulombic interactions than in two-ion ILs.
Interestingly, the viscosity of a DSIL composed of 4 ions, such as [C2C1im]x[C4C2im](1−x)[EtSO4]y[NTf2](1−y) showed an important deviation (>20 mPa s) compared to the average based on the ideal mixing of [C2C1im][NTf2] and [C4C2im][EtSO4] (Table 2, entry 20).46 The maximum deviation of the viscosities from those assuming a linear mixing law was 60 mPa s (40% deviation) achieved when x was between 0.5 and 0.6 (y = 0.4–0.5). The authors attributed this deviation from ideality to the dissimilarity of the structures of the ions (particularly in terms of the lengths of alkyl substituent chains on the cations). Non-linear changes were expected and observed for the two other four-ion systems, [NH4]x[C2C1im](1−x)[SCN]y[EtSO4](1−y) and [NH4]x[C2C1im](1−x)[ SCN]y[OAc](1−y), with increasing [NH4]− and [SCN]− concentrations (Table 2, entries 21 and 22). However, the [NH4][SCN] salt parent is a crystalline solid,21,53 thus it is not possible to measure the average deviation.
2.2. Density and excess molar volume
The general trends in density with ionic composition are less evident than for viscosity and the number of reported examples is low. As a general rule, the densities of two-ion ILs tend to increase with increasing molecular weight of the anions and decrease with increasing alkyl chain length.54 In the literature, densities of DSILs are often reported as having good correlation with a linear mixing law.16Table 3 confirms this for DSILs composed of three or four ions where globally only a very small deviation from linear averaging (mainly less than 0.01 g cm−3 and almost equivalent to the measurement errors) is observed. These differences do not provide unique information, however, since density is defined by the ratio between the mass and volume, ideal or nonideal behavior should be assessed by considering excess molar volumes.| Entry | DSIL | Deviation from the linear averagea (g cm−3) | Excess molar volumeb (cm3 mol−1) | H2O content (ppm) | Temp. (K) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Three-ion DSILs: | ||||||||||||
Deviations less than 0.20 cm3 mol−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
||||||||||||
| Cation | Anion 1 | Anion 2 | x = 0.25 | x = 0.50 | x = 0.75 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
| 1 | [C2C1im] | [N(CN)2]x | [BF4](1−x) | −0.002 | −0.005 | −0.002 | +0.04 | −0.02 | +0.04 | <100 | 298.15 | 17 |
| 2 | [C2C1im] | [Cl]x | [SCN](1−x) | +0.001 | −0.001 | −0.001 | —e | −0.15 | —e | N/Ac | 295.00 | 56 |
| 3 | [C2C1im] | [MeSO4]x | [BF4](1−x) | +0.003 | +0.004 | +0.003 | −0.05 | −0.06 | −0.04 | N/Ac | 298.15 | 57 |
| 4 | [C4C1im] | [BF4]x | [PF6](1−x) | +0.004 | +0.004 | +0.004 | +0.04 | +0.07 | +0.06 | N/Ac | 298.15 | 57 |
| 5 | [C4C1im] | [BF4]x | [PF6](1−x) | N/Ad | N/Ad | N/Ad | +0.04 | +0.12 | +0.09 | <70 | 298.15 | 58 |
| 6 | [C4C1im] | [PF6]x | [NTf2](1−x) | N/Ad | N/Ad | N/Ad | +0.13 | +0.12 | +0.09 | <70 | 298.15 | 58 |
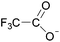
| 7 | [C5C1im] | [CF3COO]x | [PF6](1−x) | N/Ad | 0.000 | N/Ad | −0.1 | −0.1 | 0.1 | <100 | 293.00 | 59 |
| 8 | [C4C1pyr] | [NTf2]x | [N(S2O4C5F12)](1−x) | +0.006 | +0.007 | +0.012 | −0.06 | −0.03 | 0.19 | <2 | 293.15 | 50 |
| Cation 1 | Cation 2 | Anion | x = 0.25 | x = 0.50 | x = 0.75 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | [C1C1im]x | [C4C1im](1−x) | [NTf2] | −0.005 | −0.005 | −0.005 | −0.09 | −0.13 | −0.05 | <700 | 298.15 | 60 |
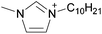
| 10 | [C2C1im]x | [C8C1im](1−x) | [NTf2] | N/Ad | N/Ad | N/Ad | +0.13 | +0.12 | +0.11 | <70 | 298.15 | 58 |
| 11 | [C4C1im]x | [C8C1im](1−x) | [NTf2] | N/Ad | N/Ad | N/Ad | +0.13 | +0.12 | +0.11 | <70 | 298.15 | 58 |
| 12 | [C4C1im]x | [C8C1im](1−x) | [NTf2] | −0.004 | −0.005 | −0.004 | −0.10 | +0.18 | −0.08 | <700 | 298.15 | 60 |
| 13 | [C6C1im]x | [C10C1im](1−x) | [NTf2] | N/Ad | N/Ad | N/Ad | +0.07 | +0.11 | +0.08 | <70 | 298.15 | 58 |
| 14 | [C8C1im]x | [C10C1im](1−x) | [NTf2] | N/Ad | N/Ad | N/Ad | —e | +0.08 | —e | <70 | 298.15 | 58 |
| 15 | [C2C1im]x | [C3C1pyr](1−x) | [NTf2] | +0.001 | +0.002 | +0.001 | +0.15 | 0.00 | −0.010 | <10 | 293.15 | 26 |
| 16 | [C3C1pyr]x | [C6C1pyr](1−x) | [NTf2] | −0.004 | −0.006 | −0.004 | 0.0 | 0.0 | 0.0 | <10 | 293.15 | 26 |
| 17 | [C3C1pyr]x | [C5C1pyr](1−x) | [NTf2] | −0.004 | −0.002 | −0.007 | −0.02 | +0.01 | −0.06 | <50 | 303.15 | 48 |
| 18 | [C3C1pyr]x | [C4C1pyr](1−x) | [NTf2] | +0.008 | +0.002 | +0.026 | −0.02 | +0.07 | +0.14 | <10 | 293.15 | 26 |
| 19 | [C2C1im]x | [C6C1im](1−x) | [BF4] | −0.009 | −0.012 | −0.008 | −0.03 | −0.13 | −0.09 | N/Ac | 298.15 | 57 |
| 20 | [C4C1im]x | [C6C1im](1−x) | [BF4] | −0.003 | −0.002 | −0.001 | −0.03 | −0.05 | −0.05 | N/Ac | 298.15 | 57 |
Deviations greater than 0.20 cm3 mol−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cation | Anion 1 | Anion 2 | x = 0.25 | x = 0.50 | x = 0.75 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
| 21 | [C2C1im] | [EtSO4]x | [NTf2](1−x) | +0.012 | +0.014 | +0.012 | +0.47 | +0.76 | +0.75 | <1000 | 298.15 | 46 |
| 22 | [C4C1im] | [BF4]x | [NTf2](1−x) | N/Ad | N/Ad | N/Ad | +0.26 | +0.28 | +0.17 | <70 | 298.15 | 58 |
| 23 | [C5C1im] | [Br]x | [NTf2](1−x) | N/Ad | +0.173 | N/Ad | +0.9 | +0.5 | +0.5 | <10 | 293.00 | 59 |
| 24 | [C3C1pyr] | [N(CN)2]x | [NTf2](1−x) | +0.020 | +0.030 | +0.035 | +0.48 | +0.21 | −0.18 | <310 | 293.15 | 26 |
| 25 | [C4py] | [BF4]x | [NTf2](1−x) | +0.014 | +0.024 | +0.011 | +0.24 | +0.39 | +0.42 | <10 | 303.15 | 52 |
| Cation 1 | Cation 2 | Anion | x = 0.25 | x = 0.50 | x = 0.75 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | [C2C1im]x | [C4C1im](1−x) | [NTf2] | +0.032 | +0.012 | +0.050 | +1.2 | +1.7 | +1.2 | <700 | 298.15 | 60 |
| 27 | [C2C1im]x | [C10C1im](1−x) | [NTf2] | N/Ad | N/Ad | N/Ad | +0.17 | +0.24 | +0.19 | <70 | 298.15 | 58 |
| 28 | [C4C1im]x | [C5C1im](1−x) | [NTf2] | +0.027 | +0.006 | +0.050 | −0.98 | −1.4 | −0.96 | <700 | 298.15 | 60 |
| 29 | [C4C1im]x | [C6C1im](1−x) | [NTf2] | +0.031 | +0.001 | −0.032 | −0.15 | −0.22 | −0.14 | <700 | 298.15 | 60 |
| 30 | [C4C1im]x | [C10C1im](1−x) | [NTf2] | −0.007 | −0.011 | −0.009 | −0.13 | −0.24 | −0.14 | <700 | 298.15 | 60 |
| 31 | [C3C1pyr]x | [C4C1pyr](1−x) | [NTf2] | +0.008 | +0.002 | +0.026 | −0.02 | +0.07 | +0.14 | <10 | 293.15 | 26 |
| 32 | [C3C1pyr]x | [P6,6,6,14](1−x) | [NTf2] | −0.044 | −0.082 | N/Ad | +0.41 | +0.80 | N/Ac | <10 | 293.15 | 26 |
| Four-ion DSILs: | Excess molar volumeb (cm3 mol−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cation 1 | Cation 2 | Anion 1 | Anion 2 | x = y = 0.5 | x = y = 0.5 | ||||
| a Deviation of the density (Δρ, g cm−3), compared to the linear average of the two IL parents calculated as Δρ = ρ − ∑χi × ρi where ρ is the density of the DSIL, χi and ρi are the mole fraction and density of the IL parents (i). b Excess molar volumes (VE, cm3 mol−1) calculated according to eqn (2). c To highlight differences an arbitrarily chosen limit of |Δρ| ≤ 0.20 cm3 mol−1 was considered. d Data not provided. e Not sufficient data for calculations. f Comparison is not possible since the [NH4][SCN] salt parent is a solid at 298.15 K (Tm = 389 K).53 | |||||||||
| 33 | [C2C1im]x | [C4C2im](1−x) | [EtSO4]y | [NTf2](1−y) | +0.002 | +0.75 | <1000 | 298.15 | 46 |
| 34 | [NH4]x | [C2C1im](1−x) | [SCN]y | [EtSO4](1−y) | —f | —f | <1200 | 298.15 | 21 |
| 35 | [NH4]x | [C2C1im](1−x) | [SCN]y | [OAc](1−y) | —f | —f | <1200 | 298.15 | 21 |
By definition, excess molar volume, noted VE, is the difference in the measured molar volume of a mixture from the expected molar volume.55 The expected molar volume is determined from the sample density and the weighted average of the molar volume of each parent compound at a specific temperature (indicated in Table 3) and at ambient pressure. VE is determined by eqn (2):
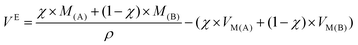 | (2) |
The molar excess volumes for DSILs allows one to discuss the volumetric aspect of the salts, where a positive VE is obtained when the volume is larger than expected compared to the mixing of two two-ion ILs, and a negative VE is obtained when the volume is smaller than expected. Table 3 summarizes the excess molar volumes for several DSILs at x = 0.25, 0.50, and 0.75. In order to highlight the differences, we consider an arbitrary line boundary (deviation of 0.20 cm3 mol−1). Of course, this limit is arbitrary and may be better assessed after the study of additional systems in the literature.
By analysis of Table 3, significant excess molar volumes are observed and several trends can be observed, showing the importance of considering this parameter. The molecular weight and especially the volume occupied by each ion appear to play a key role in the explanation of any deviation (Table 3, entries 10–13, and 27, 30, 32). As a general observation, the greater the differences in the sizes of the different ions composing a DSIL, the greater the positive excess molar volumes. While these trends may be contradicted by future studies, it is crucial that additional studies be designed to with ions of widely different properties to facilitate understanding the results.
As the long-range Coulomb forces between the charges lead to highly ordered ionic lattices,61 the differences in the sizes and compositions of the ions in the DSILs might explain the deviations observed. These are essentially steric effects, where the differences in sizes can interfere with organization of the ions and thus could result in major deviations where Coulombic effects dominate, for example excess volume, viscosity, etc.
Castejón and Lashock reported the same conclusions from molecular dynamic simulation studies based on three-ion and four-ion DSIL systems composed of 1,2,3-triazolium and 1,2,4-triazolium cations with perchlorate and nitrate anions.62 Based on these data, they found that DSILs with anions and cations of comparable molar volumes appear to form ‘regular solutions’ and obey the ideal cross-square rules of mixing. The only deviations observed in their work were noted for DSILs having differences in molar volumes sufficient to upset the configurational entropy of mixing.
The data to date support the idea that the structure and physical properties of DSILs depend directly on the final ion composition, not the composition of the two-ion ILs used to make them. Several papers reported the hypothesis that three-dimensional organization characterized by the presence of different domains in DSILs is important. For example, D'Anna et al. reported the analysis of three-dimensional organization of [BzC4im]x[Bz(F5)C4im](1−x)[NTf2], and [BzC4im][BF4]x[NTf2](1−x) by a combination of resonance light scattering (RLS), and 1H and 19F Nuclear Magnetic Resonance (NMR) spectroscopy ([BzC4im]+: 1-benzyl-3-methylimidazolium and [BzC4im]+: 1-(2,3,4,5,6-pentafluorobenzyl)-3-butylimidazolium).63
RLS measurements give information on the presence of aggregates of molecules containing chromophores and the RLS intensity can be correlated to the size of aggregates.64 In general, more significant variations in RLS intensity were detected for [BzC4im][BF4]x[NTf2](1−x) than for [BzC4im]x[Bz(F5)C4im](1−x)[NTf2], indicating a more important reorganization in the three-dimensional network in the presence of two different anions.63
1H and 19F NMR measurements indicated significant chemical shifts compared to the IL parents.63 No changes were detected in the chemical shift of H-2 of the imidazolium ring for [BzC4im]x[Bz(F5)C4im](1−x)[NTf2], but significant upfield shifts were observed for the aliphatic protons (−0.18 ppm) of the butyl chain as x increased. All signals in the 19F NMR spectra shifted downfield with increasing [BzC4im]+ abundance. The authors ascribed these trends to the interaction between the butyl chain of an imidazolium cation and the aromatic ring (imidazolium, phenyl or/and pentafluorophenyl) of a cation, suggesting that the cations are organized in parallel planes, as previously reported for aromatic two-ion ILs.65 Contrasting results were obtained for [BzC4im][BF4]x[NTf2](1−x), where the most significant downfield shifts were detected for the H-2 proton of the imidazolium ring (+0.9 ppm) and the benzylic protons (+0.2 ppm) with increasing [BF4]− abundance, while aliphatic proton signals shifted upfield. At the same time, the 19F NMR signals for the [NTf2]− anions exhibited downfield shifts reflecting the decrease in interactions with the cations as the abundance of [BF4]− increased. These results seem to indicate that [BF4]− outcompetes [NTf2]− for cation interactions because of its greater basicity.66
All of this spectroscopic data suggests very specific changes in the ion–ion interactions which are clearly dependent on the nature of the various ions being combined. For the two-anion-based DSILs, these results are attributed to arrangements favoring interactions between the imidazolium and phenyl rings, the imidazolium and pentafluorophenyl rings, and the phenyl and pentafluorophenyl rings. In the last case, a quadrupole–quadrupole interaction should be operative, as a consequence of the reverse charge distribution in the interacting aromatic rings67,68 and as a result, this system should be stabilized by π–π, π–quadrupole, and C–H–π interactions.
For the two-cation-based DSILs, the more significant shifts are attributed to the increase in cation–anion interactions with the increase in anion basicity. These types of spectroscopic studies are essential to better understand the interactions between ions and the early published results support the concept that the behavior of these salts are closer to double salts where each ion can maximize its most favorable interactions to a variety of counter ions, a situation not found in mixtures of solvents.
2.3. Conductivity
Table 4 presents the deviations from the mixing average in ionic conductivity for several reported DSILs compared to the IL parents. Even if it is difficult to conclude what is ‘ideal’ in terms of ionic conductivity, MacFarlane et al. explained the observed deviations by either a larger number of charge carriers or facilitated movement of the charge carriers.26 They argued that decreased clustering of the ions leading to reduced correlated ion movement and more independent charge carriers could explain the increased conductivity.| Entry | DSIL | Deviation from the mixing averagea (mS cm−1) | H2O content (ppm) | Temperature (K) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Three-ion DSILs: | |||||||||
| Cation | Anion 1 | Anion 2 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
| 1 | [C2C1im] | [N(CN)2]x | [BF4](1−x) | +0.48 | +0.75 | +0.66 | <100 | 298.15 | 17 |
| 2 | [C2C1im] | [CF3SO3]x | [NTf2](1−x) | N/Ab | −0.10 | N/Ab | <1000 | rt | 69 |
| 3 | [C3C1pyr] | [N(CN)2]x | [NTf2](1−x) | −1.0 | −0.5 | 0.0 | <310 | 293.15 | 26 |
| 4 | [C4C1pyr] | [NTf2]x | [N(S2O4C5F12)](1−x) | −0.2 | −0.3 | −0.3 | <2 | 293.15 | 50 |
| Cation 1 | Cation 2 | Anion | x = 0.25 | x = 0.50 | x = 0.75 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | [C2C1im]x | [C3C1pyr](1−x) | [NTf2] | +0.60 | +0.70 | +0.30 | <10 | 293.15 | 26 |
| 6 | [C3C1pyr]x | [C4C1pyr](1−x) | [NTf2] | −0.44 | +0.33 | −0.11 | <10 | 293.15 | 26 |
| 7 | [C3C1pyr]x | [C6C1pyr](1−x) | [NTf2] | −0.55 | +0.30 | −0.10 | <10 | 293.15 | 26 |
| 8 | [C3C1pyr]x | [P6,6,6,14](1−x) | [NTf2] | −1.5 | −1.5 | N/Ab | <10 | 293.15 | 26 |
| 9 | [C4C1pyr]x | [C5C1pyr](1−x) | [NTf2] | 0 | 0 | 0 | <50 | 303.15 | 48 |
| Four-ion DSILs: | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cation 1 | Cation 2 | Anion 1 | Anion 2 | x = y = 0.5 | ||||
| a Deviation of the ionic conductivity (Δσ, mS cm−1) compared to ideal mixing average of the two IL parents calculated by the equation Δσ = σ − ∑χi × σi where σ is the ionic conductivity of the DSIL, χi and σi are the mole fraction and ionic conductivity of the IL parents (i). b Data not provided. c Only molar conductivities are provided here (ΔΛm = +3.8 S cm2 mol−1 when x = 0.5). | ||||||||
| 10 | [C2C1im]x | [C3C1im](1−x) | [CF3SO3]y | [NTf2](1−y) | —c | N/Ab | 363.15 | 70 |
| 11 | [C2C1im]x | [C3C1im](1−x) | [Br]y | [BF4](1−y) | −3.2 | <450 | 298.00 | 71 |
Unfortunately, very few data on the ionic conductivity of DSILs are available at the moment, and it is difficult to develop any trends with this data. However, some experiments have confirmed a higher ionic conductivity for some three-ion DSILs (Table 4, entries 1 and 5–7) and four-ion DSILs (Table 4, entry 10) compared to either of the two-ion IL parents, which suggests unique properties are accessible by the DSIL approach compared to any series of two-ion ILs. DSILs have to be regarded as media entirely consisting of ions, and the thermodynamic properties can be described solely by ion–ion interactions, contrary to solvent–solvent mixtures or IL–solvent mixtures that can be described as ion–solvent, ion–ion, and solvent–solvent interactions.71 Thus, the ion–ion interactions may be modified according to the nature of the ions and their abundance in the DSIL. The stronger attractions or steric hindrance/bulk of some ions might make the other ions more mobile and therefore, more highly conductive.
We believe that this type of ionic conductivity data is essential to better understand the interactions in DSILs. In addition, since many electrochemical applications based on ILs need improvement in ionic conductivity, DSILs might find applications here if the reasons for enhanced conductivities can be determined and therefore predicted.72
2.4. Walden plots
An interesting tool, a Walden plot combines temperature dependent viscosity and conductivity to provide insight into IL behavior.73–75 Walden plot data for the DSILs [NH4]x[C2C1im](1−x)[SCN]y[OAc](1−y) was reported by Pereiro et al. (Fig. 2)21 who described the maximum deviation from ideality (i.e., the 0.01 M KCl line, where the system is fully dissociated into ions of equal mobility)75 at 298.15 K when x = 0.33 and y = 0.66, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio of the cations [C2C1im]+/[NH4]+. All of the tested DSILs showed deviations from the ideal electrolyte line less than that of the IL and salt parents, indicating that the ionicity of DSILs might be higher than the two-ion ILs (Fig. 2). It is important to note that one of the salts used to prepare these DSILs, namely [NH4][SCN], melts at 116 °C (389 K)53 and is not defined as an IL. The formation of a DSIL by the combination of a low or non-melting IL and a high-melting crystalline salt supports the use of the ‘DSIL’ concept, and this will be discussed in detail later in this review (see section 6.2.3).
2 mole ratio of the cations [C2C1im]+/[NH4]+. All of the tested DSILs showed deviations from the ideal electrolyte line less than that of the IL and salt parents, indicating that the ionicity of DSILs might be higher than the two-ion ILs (Fig. 2). It is important to note that one of the salts used to prepare these DSILs, namely [NH4][SCN], melts at 116 °C (389 K)53 and is not defined as an IL. The formation of a DSIL by the combination of a low or non-melting IL and a high-melting crystalline salt supports the use of the ‘DSIL’ concept, and this will be discussed in detail later in this review (see section 6.2.3).
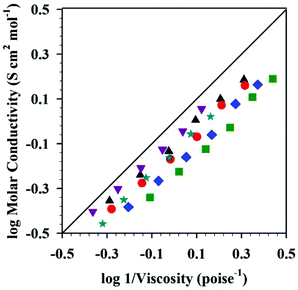 | ||
Fig. 2 Walden plots for [C2C1im][OAc] ( ), [NH4]0.1[C2C1im]0.9[SCN]0.1[OAc]0.9 ( ), [NH4]0.1[C2C1im]0.9[SCN]0.1[OAc]0.9 ( ), [NH4]0.2[C2C1im]0.8[SCN]0.2[OAc]0.8 ( ), [NH4]0.2[C2C1im]0.8[SCN]0.2[OAc]0.8 ( ), [NH4]0.3[C2C1im]0.7[SCN]0.3[OAc]0.7 (▲), [NH4]0.4[C2C1im]0.6[SCN]0.4[OAc]0.6 ( ), [NH4]0.3[C2C1im]0.7[SCN]0.3[OAc]0.7 (▲), [NH4]0.4[C2C1im]0.6[SCN]0.4[OAc]0.6 ( ), [NH4]0.6[C2C1im]0.3[SCN]0.6[OAc]0.3 ( ), [NH4]0.6[C2C1im]0.3[SCN]0.6[OAc]0.3 ( ), and the 0.01 M KCl(aq) line (___) at 298.15 K. (Drawn from the data from Fig. 1 and S7 in ref. 21 as provided by the authors with permission of the Royal Society of Chemistry.) ), and the 0.01 M KCl(aq) line (___) at 298.15 K. (Drawn from the data from Fig. 1 and S7 in ref. 21 as provided by the authors with permission of the Royal Society of Chemistry.) | ||
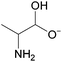
The authors further utilized 1H NMR spectroscopic data (Fig. 3) to better understand the specific interactions occurring in these DSILs that led to the interesting behavior observed.21 Compared to [C2C1im][OAc], the ring protons of the imidazolium cation (H-2, H-4, and H-5) shifted upfield (with H-2 exhibiting the greatest shifts) with increasing [NH4]+ and [SCN]− concentrations with a corresponding downfield shift for those of the [OAc]− methyl group indicating a decrease in electron density in the [OAc]− anion and increase of electron density on the imidazolium ring. The [NH4]+ proton signals shifted downfield indicating new interactions with the acetate anions. Interestingly, the 13C NMR data showed a downfield shift for C-2 of imidazolium ring, but almost no change for C-4 and C-5. Current interpretations of this NMR data for four-ion DSILs is limited, however, these results do seem to indicate a complex interplay between structural effects and competing Coulombic interactions between the ions in the studied DSILs.
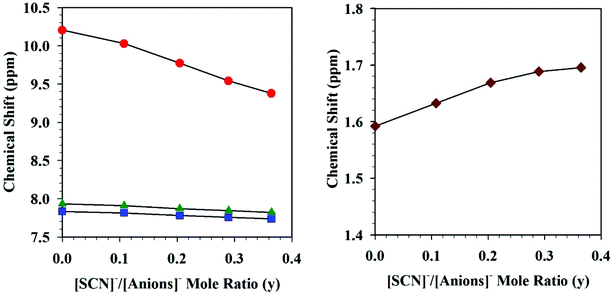 | ||
Fig. 3
1H NMR chemical shifts of the imidazolium ring protons (left: H-2 ( ), H-4 ( ), H-4 ( ), and H-5 ( ), and H-5 ( )) and acetate methyl proton (right: )) and acetate methyl proton (right:  ) for [NH4]x[C2C1im](1−x)[SCN]y[OAc](1−y). (Drawn from NMR data in ref. 21; Zero = [C2C1im][OAc]; water content <1200 ppm.) ) for [NH4]x[C2C1im](1−x)[SCN]y[OAc](1−y). (Drawn from NMR data in ref. 21; Zero = [C2C1im][OAc]; water content <1200 ppm.) | ||
In comparison, the NMR data reported by Kirchner et al. for a three-ion DSIL, [C2C1im][Cl]x[SCN](1−x) showed an almost linear increase in upfield shift for the H-2, H-4, and H-5 protons of the imidazolium ring with increasing Cl−/[C2C1im]+ mole ratio.56 Based on computer simulations, they showed that the Cl− and [SCN]− anions compete for the same interaction sites of the cations. In this case, based on the choice of anions, it is interesting to note that the physical properties such as density were shown to be ideal. If in fact the behavior of DSILs is controlled by the presence of specific interactions related to the competition of different ions for the same interactions sites, we would suggest future studies to choose appropriately different ions to accentuate these interactions making the changes more apparent in the experimental data.
MacFarlane et al. also discussed Walden plots for DSILs, in the presence of phosphonium, pyrrolidinium, or imidazolium cations with [NTf2]− or [N(CN)2]− anions (Fig. 4).26 Typically, deviations from the ideal line obtained in a Walden plot are interpreted as a reflection of the degree of ion association in the medium.76 In addition, it is known that the Walden plot can also be affected by the radius of the ions in solution.10,77 As shown in Fig. 4, the DSILs containing the most bulky ions ([P6,6,6,14]+ and [C6C1pyr]+) exhibited the most significant deviations from the ideal KCl line. These results further suggest that steric effects of the ions are important in these DSILs.
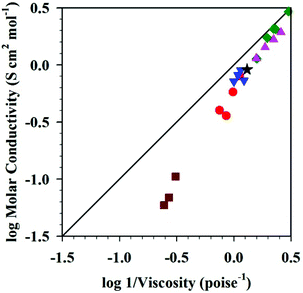 | ||
Fig. 4 Walden plots of [C3C1pyr][NTf2] (★), [C3C1pyr]x[P6,6,6,14](1−x)[NTf2] ( ), [C3C1pyr]x[C6C1pyr](1−x)[NTf2] ( ), [C3C1pyr]x[C6C1pyr](1−x)[NTf2] ( ), [C3C1pyr]x[C4C1pyr](1−x)[NTf2] ( ), [C3C1pyr]x[C4C1pyr](1−x)[NTf2] ( ), [C2C1im]x[C3C1pyr](1−x)[NTf2] ( ), [C2C1im]x[C3C1pyr](1−x)[NTf2] ( ), [C3C1pyr][N(CN)2]x[NTf2](1−x) ( ), [C3C1pyr][N(CN)2]x[NTf2](1−x) ( ), and the 0.1 M KCl(aq) line (___). For each series, values from left to right correspond to x = 0, 0.25, 0.5, 0.75, and 1, except for [C3C1pyr]x[P6,6,6,14](1−x)[NTf2] that shows only values for x = 0, 0.25, 0.5, and 1. (Drawn with permission from the data in Fig. 7, 9 and 10 in ref. 26 as provided by the authors; Copyright 2013 American Chemical Society.) ), and the 0.1 M KCl(aq) line (___). For each series, values from left to right correspond to x = 0, 0.25, 0.5, 0.75, and 1, except for [C3C1pyr]x[P6,6,6,14](1−x)[NTf2] that shows only values for x = 0, 0.25, 0.5, and 1. (Drawn with permission from the data in Fig. 7, 9 and 10 in ref. 26 as provided by the authors; Copyright 2013 American Chemical Society.) | ||
Here again, it appears that the steric effects of the ions and their specific associations in space (mainly driven by Coulombic interactions) lead to unique DSIL properties, different from the IL parents and different for each different mole ratio of the ions. Overall, the available Walden plot results support the idea that the physical properties essentially depend on these same factors, the compositions of the ions and the respective ionic interactions present in solution, and not on the original ionic interactions present in the individual ILs used to prepare the mixture.
3. Thermal behavior of DSILs
In 2006, Earle et al. reported the distillation of ILs and some ‘50/50 mixtures of ILs’.78Table 5 reports the composition of the undistilled residue and the distillate, analyzed by 1H NMR after an optimized distillation processing of equimolar ‘mixtures of ILs’. Entry 5 clearly shows that the composition of the residue and the distillate contained different mole ratios of each ion. Interestingly, the distillate was composed of 30% [C2C1im]+, 70% [N2,2,2,2]+, 57% [CF3SO3]−, and 43% [NTf2]−, corresponding to a ratio of ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for the cations, but ca. 1
2 for the cations, but ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the anions.
1 for the anions.
| Entry | IL A | IL B | Composition of residue (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B)a B)a |
Composition of distillate (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B)a B)a |
|---|---|---|---|---|
| a Mole ratios. b The undistilled residue contained 56% [C2C1im]+, 44% [N2,2,2,2]+, 47% [CF3SO3]–, and 53% [NTf2]–; the distillate contained 30% [C2C1im]+, 70% [N2,2,2,2]+, 57% [CF3SO3]–, and 43% [NTf2]– (mol%). | ||||
| 1 | [C2C1im][NTf2] | [C16C1im][NTf2] | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 76 |
76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 2 | [C2C1im][NTf2] | [C6C1im][NTf2] | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
| 3 | [C4C1pyr][NTf2] | [N2,2,2,2][NTf2] | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 53 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
| 4 | [C4C1im][NTf2] | [C4C1im][PF6] | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | [C2C1im][CF3SO3] | [N2,2,2,2][NTf2] | ||
Given the data discussed for DSILs above and the potential for unique ion–ion interactions which can be optimized differently in the bulk for each ion, this result should be further investigated to determine if unique DSIL properties are responsible for the observed volatility. It would be interesting to study whether the DSIL [C2C1im]0.30[N2,2,2,2]0.70[CF3SO3]0.57[NTf2]0.43 could be prepared and distilled completely without a change in composition.
Jones et al. reported the distillation of [C2C1im]0.5[C8C1im]0.5[NTf2] and [C2C1im]x[C8C1im](1−x)[EtSO4]y[NTf2](1−y).79 Distillation of the first DSIL led to partial separation of the two ILs, exhibiting an increase in mole fraction of [C2C1im][NTf2] in the distillate with increasing temperature. However, only a small amount of distillate was collected for the second four-ion DSIL. The authors concluded that the ethylsulfate anion was unstable and decomposed during distillation, probably through dealkylation of the sulfate oxygen. In addition, they stated that the vapor phase consisted of all possible combinations of neutral ion pairs (NIPs) from the liquid ‘mixture’.79
3.1. Thermal stability
Egashira et al. reported the thermogravimetric analysis (TGA) of [C2C1im][NTf2], [C2C1im][BF4], [C2C1im][CF3SO3], and two DSILs prepared by mixing these ILs, [C2C1im][CF3SO3]0.5[NTf2]0.5 and [C2C1im][BF4]0.5[NTf2]0.5.69 The DSILs exhibited unique properties not expected from the IL parents. For example, the thermal decomposition of [C2C1im][CF3SO3]0.5[NTf2]0.5 was more similar to [C2C1im][CF3SO3] than [C2C1im][NTf2], suggesting the thermal decomposition was controlled by the [CF3SO3]− anion. In contrast, the thermal decomposition of [C2C1im][BF4]0.5[NTf2]0.5 required higher temperatures than in [C2C1im][BF4].Spectroscopy carried out to understand these trends indicated that the C-2 (imidazolium cation) hydrogen (H-2) in the 1H NMR spectra of [C2C1im][BF4]0.5[NTf2]0.5 (δ = 8.63 ppm) shifted upfield compared to those of either single anion salt (δ = 8.87 ppm for [C2C1im][BF4] and δ = 8.86 ppm for [C2C1im][NTf2]). The coexistence of [BF4]− and [NTf2]− in this DSIL allows competition between the two anions for the cations, changing the local structure (i.e., the mode of interaction between the cations and anions) from both IL parents. These results suggest that the cations can optimize interactions with different anions in DSILs in such a way that each anion interacts with the cations in the most favorable way (e.g., strongly Coulombic vs. directionally hydrogen bonded) for that ion. However, this study only presented the comparison of the two IL parents and one DSIL (x = 0.5). Clearly other intermediate mole ratios are needed to fully understand the interactions between these ions based on spectroscopic data.69
Another study on [C2C1im][EtSO4]x[NTf2](1−x) reported that the thermal stability of the DSILs rich in [EtSO4]− (a better nucleophile than [NTf2]−) remained quite constant from x = 0.5 to x = 1 (Fig. 5),46 however, the larger the excess of [NTf2]− anions, the higher was the onset of decomposition temperatures. Since the more nucleophilic anion ([EtSO4]−) more easily interacts with the imidazolium cation, decomposition at lower temperatures as the proportion of this anion increases might be expected. However, this example clearly highlights the importance of the presence of the other anion, since the decomposition temperatures were not linear with the variation of the mole ratio and a threshold mole ratio of [EtSO4]− was required to affect the decomposition onset temperature. Here again, the results suggest unique properties for specific ion combinations and argue away from basing all analyses on the properties of the IL parents.
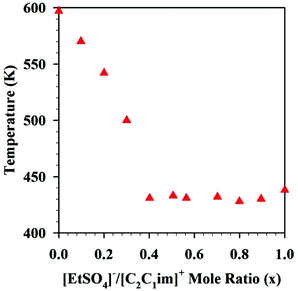 | ||
| Fig. 5 Decomposition temperatures of [C2C1im][EtSO4]x[NTf2](1−x) as a function of mole ratio. (Drawn with permission from the data in Fig. 6 in ref. 46 Copyright 2013 American Chemical Society.) | ||
3.2. Melting point
In a general way, the melting points or glass transition temperatures of two-ion ILs are widely used properties, directly linked to the structures of these ILs.5,80,81 For example, Holbrey and Seddon first showed that the melting points of two-ion ILs, based on the 1-alkyl-3-methylimidazolium cation with tetrafluoroborate or hexafluorophosphate anions, could be changed in a regular fashion by changing the size and symmetry of the cation (i.e., that ILs could be designed and tuned for such properties).82 Dunstan and Caja observed the same trends with the two-ion ILs 1-ethyl-3-methylimidazolium hexafluorophosphate ([C2C1im][PF6]) and 1-ethyl-2-methylpyrazolium hexafluorophosphate ([C2C1pyraz][PF6]),83 but obtained melting points below the melting point of either two-ion IL parent ([C2C1im][PF6]) by preparing [C2C1im]x[C2C1pyraz](1−x)[PF6] DSILs with different ionic compositions. The use of cations with significant differences in their structures, and the variation of the abundance of each ion (i.e., designing of the ionic composition) allowed access to unique combinations of ions with new melting points, lower than those of the IL parents.83MacFarlane et al. reported DSC data for [C3C1pyr][N(CN)2]x[NTf2](1−x), [C3C1pyr]x[C4C1pyr](1−x)[NTf2], [C2C1im]x[C3C1pyr](1−x)[NTf2], [C3C1pyr]x[C6C1pyr](1−x)[NTf2], [C3C1pyr]x[P6,6,6,14](1−x)[NTf2] which exhibited several crystallization exotherms, indicating that different crystallization processes were occurring at the same time while the two-ion IL parents showed single devitrification exotherms (Fig. 6).26 Interestingly, all the [P6,6,6,14]x[C3C1pyr](1−x)[NTf2] DSILs, containing a particularly large cation, were glass-forming. The authors concluded that using relatively similar structures of cations (occupied volume) may provide a means of lowering melting points and extending the low temperature liquid range of DSILs. However, this study also showed that the unique ionic combinations do not always lead to expected unique crystallization events. This aspect of DSILs remains unclear, but emphasizes the importance of collecting new data for these systems. It thus remains a research challenge to gain and interpret new data for DSILs to understand the specific combination of ion–ion interactions leading to the observed behavior.
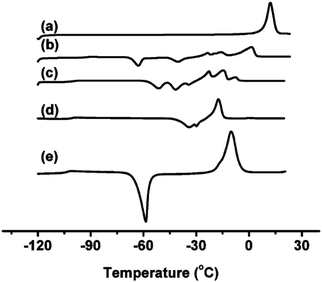 | ||
| Fig. 6 DSC data of [C3C1pyr][N(CN)2]x[NTf2](1−x)x = (a) 0; (b) 0.25; (c) 0.50; (d) 0.75; (e) 1.00. (Reprinted with permission from ref. 26; Copyright 2013 American Chemical Society.) | ||
In 2008 using differential scanning calorimetry data (DSC), our group proposed a method for ready determination of the eutectic point compositions for mixtures of two-ion ILs. In addition, we proposed a synthetic strategy to directly form eutectics of ILs (DSILs) to tune bulk properties, based on the properties provided by each cation, each anion, and the variation of ion proportions (Fig. 7).18 For example, [C1C1im][4-NO2-tri]0.64[4,5-diNO2-im]0.36 was prepared by reacting 1-butyl-3-methyl-1H-imidazol-3-ium-2-carboxylate with 0.64 eq. of 4-nitro-1,2,3-triazole and 0.36 eq. of 4,5-dinitroimidazole (Scheme 1a). As expected, this specific DSIL exhibited the same properties as the identical ion composition obtained by mixing 0.64 mole ratio [C1C1im][4-NO2-tri] with 0.36 mole ratio [C1C1im][4,5-diNO2-im]. This example clearly demonstrated that DSILs do not have to be prepared by ‘mixing’ as one would have to do in the study of molecular solvent mixtures, and that the properties so obtained are governed by the ionic composition, not the parent ILs (see section 6.2.2).
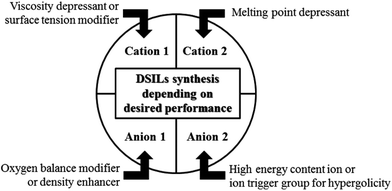 | ||
| Fig. 7 Synthetic strategy to prepare DSILs to tune bulk energetic properties.18 | ||
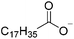
3.3. Crystallization behavior
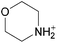
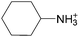
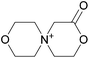
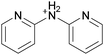
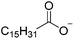
The concept of DSILs may have support from literature dating back to the 1960s. Mod et al. reported studies on the freezing point behavior of “ternary reciprocal salt pair systems” composed of morpholinium, cyclohexylammonium, and 2,2′-dipyridylammonium cations with stearate and palmitate anions: [Morph][C15H31COO]x[C17H35COO](1−x), [C6H11-NH3][C15H31COO]x[C17H35COO](1−x), [Morph]x[C6H11-NH3](1−x)[C15H31COO], [Morph]x[C6H11-NH3](1−x)[C17H35COO], [Morph]x[C6H11-NH3](1−x)[C15H31COO]y[C17H35COO](1−y), [(py)2-NH2][C15H31COO]x[C17H35COO](1−x), and [Morph]x[(py)2-NH2](1−x)[C15H31COO]y[C17H35COO](1−y).84,85 The authors described these as ‘stable salt pairs’ and studied their thermal properties as a function of the mole ratios of the constituent ions. Their phase diagrams allowed prediction of the crystallization properties depending on the ion compositions of the different salts, and the temperature. Interestingly, the phase diagrams revealed that in some regions two-ion salts crystallized (e.g., [Morph][C15H31COO], [Morph][C17H35COO], [(py)2-NH2][C15H31COO], and [(py)2-NH2][C17H35COO]) and in others, double salts crystallized (e.g., [(py)2-NH2][C15H31COO]x[C17H35COO](1−x), and [Morph]x[(py)2-NH2](1−x)[C15H31COO]y[C17H35COO](1−y)). These results would seem to confirm that different types of interactions are present according to the mole ratios of ions and ion type which leads to different nucleation events.On this same basis, the thermodynamic properties of [C2C1im]x[C6C1im](1−x)[NTf2] studied by adiabatic calorimetry were recently reported by Paulechka et al., where it was concluded that the crystalline state of the DSILs86 was less stable than that of the parent component crystals.87,88 Moreover, they determined that the specific heat capacity of the DSILs was significantly higher than the heat capacity calculated for the same composition of both IL parents.88 Conversely, the total enthalpy of the crystal to liquid transition was significantly lower than the additive value.86 These results suggest that DSIL configurations favor solid solution formation substituted in the cationic sublattice. However, it is important to note that the authors assumed a hypothetical starting point that the mixture of [C2C1im][NTf2] and [C6C1im][NTf2] was ideal in order to build the phase diagram, and this approximation has not yet been proven.
Kunze et al. prepared DSILs from N-butyl-N-methylpyrrolidinium ([C4C1pyr]+) and N-propyl-N-methylpyrrolidinium ([C3C1pyr]+) cations, and (fluorosulfonyl)imide derivatives ([N(SO2F)2]−, [NTf2]−, [N(SO2C2F5)2]−, and [N(S2O4C5F12)]−) as anions.27 DSC heating and cooling traces showed that the anionic species are responsible for the crystallization of the ILs and DSILs. The same results were observed in the presence of only the [NTf2]− anion (two-ion IL), only the [N(SO2F)2]− anion (two-ion IL), or both at 50 mol% (DSIL). Only the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio of each anion was tested in this study and other intermediate ratios need to be investigated to better understand the thermal behavior. However, the authors concluded that if the cation is able to fit into the anionic matrix, the system can crystallize. If the cations do not fit, due to size and configuration, only a glassy state will be achieved.
1 mole ratio of each anion was tested in this study and other intermediate ratios need to be investigated to better understand the thermal behavior. However, the authors concluded that if the cation is able to fit into the anionic matrix, the system can crystallize. If the cations do not fit, due to size and configuration, only a glassy state will be achieved.
Related to this conclusion, NMR studies reported in the literature for [C2C1im][BF4]0.5[NTf2]0.5 (see section 3.1),69 [C4C1pyr][NTf2]x[N(S2O4C5F12)](1−x), [C4C1pyr][NTf2]x[N(SO2C2F5)2](1−x), and [C4C1pyr][N(SO2C2F5)2]x[N(S2O4C5F12)](1−x) indicated that the local structure was changed compared to the IL parents, showing a certain degree of nanostructuring, most likely in the form of short-range fluorine–fluorine and cation–anion contacts.51 Indeed, 2D heteronuclear NOESY experiments (detection of heteronuclear through-space Nuclear Overhauser Effect (NOE) connectivities between non-bonded nuclei) specifically showed some significant changes to the local structure brought about by the simultaneous presence of anionic components differing in fluorine content, steric hindrance, and symmetry. The NOE data suggests formation of domains in DSILs, and particularly [C4C1pyr][N(SO2C2F5)2]x[N(S2O4C5F12)](1−x) showed important deviations in physical properties compared to the average mixing of the corresponding IL parents, attributed to the marked difference in size and fluorine content of its two anions.
Overall, the thermal properties reported for DSILs are in agreement with the double salt concept that we propose. The phase transition temperatures of two-ion ILs are governed by van der Waals forces and electrostatic interactions.89 As we have discussed previously, we believe that these forces can be optimized such that each ion in a DSIL can have different overall interactions. These optimized interactions can lead to new physical and thermal properties of these salts, thus the DSIL concept provides an approach to the development of new properties not obtained in two-ion ILs. However, some of the reported thermal behavior remains difficult to explain (e.g., Fig. 6), and the unique ionic combinations do not always seem to lead to unique crystallization events. Based on the low number of reported DSILs and their characterization, clearly more fundamental studies are needed to understand the role of both van der Waals and electrostatic interactions in the organization of these salts.
4. Excess molar enthalpy
Experimental and simulation data on the enthalpy of two-ion ILs has confirmed that they are structured media.90 By definition, liquid mixtures that obey Raoult's Law are considered as ideal solutions and in this case, the enthalpy of mixing is equal to zero. Based on this law, the measurement of excess molar enthalpy of a DSIL, compare to the enthalpy of mixing of the corresponding IL parents is an important factor to better understand DSILs properties. We discuss below the excess molar enthalpies of three-ion DSILs (Table 6), but the comparison of each system is difficult since the experimental conditions are often very different, and some caution is urged in trying to draw too many firm conclusions too soon.| Entry | DSIL | Excess molar enthalpiesa (J mol−1) | H2O content (ppm) | Temperature (K) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cation | Anion 1 | Anion 2 | x = 0.25 | x = 0.50 | x = 0.75 | ||||
| 1 | [C2C1im] | [MeSO4]x | [BF4](1−x) | −235 | −360 | −230 | N/A | 303.15 | 57 |
| 2 | [C4C1im] | [BF4]x | [PF6](1−x) | −90 | −210 | −70 | N/A | 303.15 | 57 |
| 3 | [C4C1im] | [NO3]x | [ClO4](1−x) | +3900 | +1650 | +900 | N/A | 373.00 | 62 |
| 4 | [123tri] | [NO3]x | [ClO4](1−x) | −1500 | −2000 | −1750 | N/A | 373.00 | 62 |
| 5 | [124tri] | [NO3]x | [ClO4](1−x) | N/A | −900 | N/A | N/A | 373.00 | 62 |
| Cation 1 | Cation 2 | Anion | x = 0.25 | x = 0.50 | x = 0.75 | ||||
|---|---|---|---|---|---|---|---|---|---|
| a Excess molar enthalpies (J mol−1) calculated from the Gibbs–Helmholtz model.91 | |||||||||
| 6 | [C2C1im]x | [C8C1im](1−x) | [NTf2] | N/A | +310 | N/A | <70 | 298.15 | 58 |
| 7 | [C2C1im]x | [C10C1im](1−x) | [NTf2] | N/A | +560 | N/A | <70 | 298.15 | 58 |
| 8 | [C4C1im]x | [C8C1im](1−x) | [NTf2] | N/A | +290 | N/A | <70 | 298.15 | 58 |
| 9 | [C8C1im]x | [C10C1im](1−x) | [NTf2] | N/A | +180 | N/A | <70 | 298.15 | 58 |
| 10 | [C4C1im]x | [C10C1im](1−x) | [NTf2] | N/A | +500 | N/A | <70 | 298.15 | 58 |
| 11 | [C6C1im]x | [C10C1im](1−x) | [NTf2] | N/A | +250 | N/A | <70 | 298.15 | 58 |
| 12 | [C2C1im]x | [C6C1im](1−x) | [BF4] | +190 | +300 | +220 | N/A | 303.15 | 57 |
| 13 | [C4C1im]x | [C6C1im](1−x) | [BF4] | +60 | +80 | +40 | N/A | 303.15 | 57 |
| 14 | [123tri]x | [124tri](1−x) | [NO3] | N/A | +330 | N/A | N/A | 373.00 | 62 |
| 15 | [123tri]x | [124tri](1−x) | [ClO4] | N/A | −12 | N/A | N/A | 373.00 | 62 |
| 16 | [123tri]x | [C4C1im](1−x) | [NO3] | N/A | +1705 | N/A | N/A | 373.00 | 62 |
| 17 | [123tri]x | [C4C1im](1−x) | [ClO4] | N/A | +480 | N/A | N/A | 373.00 | 62 |
| 18 | [124tri]x | [C4C1im](1−x) | [NO3] | +2500 | +2071 | +600 | N/A | 373.00 | 62 |
The excess enthalpy is related to the breaking and making of interactions in a mixing process. A positive excess enthalpy implies that attractive interactions between ions in DSILs are, on average, weaker than those between the ions in the IL parents, whereas a negative excess enthalpy indicates the opposite. For example, Navia et al. reported small positive excess enthalpies of mixing for DSILs of different cations with a common anion such as [C2C1im]x[C6C1im](1−x)[BF4] and [C4C1im]x[C6C1im](1−x)[BF4] for all compositions, with a maximum observed when x = 0.5 (Table 6, entries 12 and 13), indicating a destabilizing effect of mixing in these DSILs compared to the IL parents.57 However, a small negative excess enthalpy was reported for DSILs with different anions and a common cation such as [C2C1im][MeSO4]x[BF4](1−x) and [C4C1im][BF4]x[PF6](1−x) (Table 6, entries 1 and 2), indicating more energetically favorable interactions between the ions in the DSILs.
Based on the reported excess molar enthalpies as a function of composition (Table 6) and general rules of thermodynamics for liquid mixtures,92,93 DSILs should not be considered as ideal mixtures of the IL parents. Additionally, differences in size (e.g., Table 6, entry 7) and polarity (e.g., Table 6, entries 3–5) between the ions appear to be two important factors to help explain the observed trends in excess molar enthalpies.92 However, with so little data reported to date, it is difficult to draw other relevant conclusions here.
5. Solvent properties of DSILs
We have discussed previously in this review how the physical and thermal properties could be explained by some of the possible ionic interactions in DSILs, mainly based on steric and electrostatic effects, and how these data might support the double salt ionic liquid concept. From the few results reported, hydrogen bonding does not seem to greatly affect the physical properties of DSILs; however, we wanted to know if hydrogen bonding interactions could be important for the solvent properties of DSILs, as they are for two-ions ILs.94,95ILs have been widely studied as solvents for more than a decade,96,97 showing sometimes dramatic efficiency in terms of solubility, compared to classical molecular solvents. Many examples have appeared demonstrating the solvent properties of ILs for organic chemistry and catalysis,5,98 biomass processing,99–101 nanoparticle dispersion,102 electrochemical applications,72 analytical chemistry,103 nuclear fuel reprocessing,104 and many other areas.11,105,106 In this part of the review, we will discuss the solvent properties of DSILs through reported examples of the solubility of chemicals, gases, solvent, and drugs, and through the study of available polarity parameters.
5.1. Solubility studies in DSILs
Finotello et al. reported the solubility of CO2, CH4, and N2 at 40 °C and ambient pressure in [C2C1im][BF4]x[NTf2](1−x).109 Generally, the Henry's constant increased with increasing [BF4]− content (Table 7) and the authors demonstrated that the selectivity for CO2 over N2 and CH4 is higher in [C2C1im][BF4]0.9[NTf2]0.1 and [C2C1im][BF4]0.95[NTf2]0.05 than the parent ILs or other compositions of [C2C1im][BF4]x[NTf2](1−x). These two DSILs provided the smallest molar volumes in the study, leading the authors to suggest that control over IL molar volume could be used to enhance CO2 selectivity. Although improvements of CO2 solubility using DSILs have not been demonstrated yet, given that higher selectivity has been demonstrated we believe that DSILs may be beneficial in CO2 capture processing, since the cost, the toxicity, or other interesting parameters of DSILs can be tuned as a function of ionic composition.
| Henry's constant H (atm) | ||||
|---|---|---|---|---|
| Entry | IL or DSIL | CO2 | N2 | CH4 |
| 1 | [C2C1im][BF4] | 100 | 3800 | 2000 |
| 2 | [C2C1im][BF4]0.95[NTf2]0.05 | 94 | 5000 | 1900 |
| 3 | [C2C1im][BF4]0.90[NTf2]0.10 | 91 | 4500 | 1800 |
| 4 | [C2C1im][BF4]0.75[NTf2]0.25 | 85 | 4000 | 1600 |
| 5 | [C2C1im][BF4]0.50[NTf2]0.50 | 65 | 2400 | 980 |
| 6 | [C2C1im][BF4]0.25[NTf2]0.75 | 58 | 1700 | 740 |
| 7 | [C2C1im][BF4] | 50 | 1200 | 560 |
Baltazar et al. also investigated the use of [C4C1im][Cl]x[NTf2](1−x) as gas chromatography stationary phases for improving the separation selectivity of alcohols and aromatic compounds.112 The GC solvation parameter model was used in this study to examine the solvation interactions between the DSILs and the different probe molecules containing varied functional groups. This study showed that enrichment of the DSILs with Cl− anions resulted in a stationary phase with enhanced dipole-type and hydrogen bond basicity interactions (Cl−vs. [NTf2]− in terms of relative hydrogen bond basicity). The examples shown in Table 8 clearly show that the separation selectivity of mixtures of alcohols and aromatic analytes were improved by tuning the composition of the DSIL stationary phase.
| Entry | Analyte pair | IL or DSIL | |||
|---|---|---|---|---|---|
| [C4C1im][Cl]0.25[NTf2]0.75 | [C4C1im][Cl]0.5[NTf2]0.5 | [C4C1im][Cl]0.75[NTf2]0.25 | [C4C1im]Cl | ||
| a The selectivity α is defined by the ratio of retention factors (k1 and k2, respectively for the two compounds being separated), according the following equations: k = (tr − tm)/tm and α = k1/k2 where tr is the retention time, and tm is the void time. | |||||
| 1 | 1,2-Dichlorobenzene/octylaldehyde | 3.8 | 2.5 | 1.5 | 1.0 |
| 2 | Cyclohexanol/cyclohexanone | 14.6 | 9.4 | 4.7 | 0.74 |
| 3 | Cyclohexanol/ethyl phenyl ether | 13.9 | 9.8 | 5.4 | 0.89 |
| 4 | Benzonitrile/1-pentanol | 1.2 | 1.3 | 1.9 | 10.0 |
The solubility of organic solvents has also been investigated in DSILs for use in separations. Recently, García et al. reported the liquid–liquid extraction of toluene from heptane using [C2C1im]x[4-C1-C4py](1−x)[C2HF4SO3]y[NTf2](1−y) at 313.2 K and atmospheric pressure,113 based on the high capacity of 4-methyl-N-butylpyridinium bis(trifluoromethylsulfonyl)imide ([4-C4-C1py][NTf2]) for extraction of toluene114 and the high selectivity of 1-ethyl-3-methylimidazolium 1,1,2,2-tetrafluoroethanesulfonate ([C2C1im][C2HF4SO3]) in comparison to the widely-used polar aprotic solvent sulfolane.115 Although no chemical explanation was provided in this study, the specific DSIL studied, [C2C1im]0.7[4-C4-C1py]0.3[C2HF4SO3]0.7[NTf2]0.3, allowed efficient extraction of toluene from heptane with higher separation factors than that of other organic solvents (e.g., sulfalone) and one of the IL parents, namely [4-C4-C1py][NTf2] (Fig. 8).
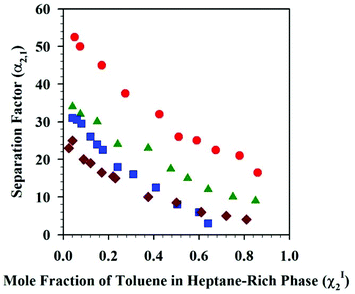 | ||
Fig. 8 Separation factors at 313.2 K and atmospheric pressure for the systems: heptane + toluene + [C2C1im]0.7[4-C4-C1py]0.3[C2HF4SO3]0.7[NTf2]0.3 ( ), or [4-C4-C1py][NTf2] ( ), or [4-C4-C1py][NTf2] ( ), or [C2C1im][C2HF4SO3] ( ), or [C2C1im][C2HF4SO3] ( ), or [C2C1im][C2HF4SO3], or sulfonane ( ), or [C2C1im][C2HF4SO3], or sulfonane ( ). The separation factors are α2,1 = (χ2II × χ1I)/(χ2I × χ1II) where χ is the mole fraction, superscripts I and II refer to the heptane-rich and IL-rich phases, respectively, and subscripts 1 and 2 refer to heptane and toluene, respectively. (Redrawn from Fig. 6 in ref. 113; Copyright 2013, with permission from Elsevier.) ). The separation factors are α2,1 = (χ2II × χ1I)/(χ2I × χ1II) where χ is the mole fraction, superscripts I and II refer to the heptane-rich and IL-rich phases, respectively, and subscripts 1 and 2 refer to heptane and toluene, respectively. (Redrawn from Fig. 6 in ref. 113; Copyright 2013, with permission from Elsevier.) | ||
This work represents an interesting example where the solvent properties of the individual two-ion ILs were used to select which ILs to combine. One was chosen for high capacity and one for selectivity. It was observed that the resulting properties could be tuned between those of the two individual ILs, allowing optimization of an appropriate selectivity higher than found for one of the original ILs and a higher capacity than observed for the other one. More studies of this type are needed to understand how the combination of ions worked so that future DSILs can be designed from scratch for specific applications.
 | ||
| Fig. 9 Ternary phase diagram of [C2C1im]x[C6C1im](1−x)[BF4] and C14E5 mixtures at 333 K where the phase boundaries were determined from cloud point curves (open circles) and from the composition analysis of two coexisting phases (filled circles). The dotted line connecting two filled circles corresponds to a tie line. The composition is expressed in mol%. (Redrawn from the diagram in ref. 116; Copyright 2013, with permission from Elsevier.) | ||
Analysis of the 1H NMR chemical shifts of the imidazolium protons suggested preferential interaction of the [C6C1im]+ cation with surfactant molecules in the DSILs through interaction between the hydrogen of the C-2 position of the imidazolium ring and the oxygen of POE, and between the hexyl group and the surfactant hydrocarbon chain. The cloud point observed at 333 K in [C2C1im]x[C6C1im](1−x)[BF4] may be attributable to the desolvation of the [C6C1im]+ cation from the surfactant chain caused by an increase in temperature. These results suggest that the ionic compositions of DSILs can be tuned to support the self-assembly of amphiphilic compounds.
5.2. Polarity parameters of DSILs
In order to more fully understand how DSILs could help solubilize molecules, solvents, or gases, we need to look at the reported polarity parameters. Most of these types of studies are based on the use of solvatochromic dyes in two-ion ILs and the results are expressed in terms of Reichardt ET(30)123 and Kamlet–Taft parameters.66,124 Indeed, chemists usually attempt to understand solvent effects on chemical processes in terms of the solvent polarity, where weaker interactions such as hydrogen bonding become more important.D'Anna et al. analyzed the effects of DSILs on the properties of a solute carrying out a UV/Vis study using Nile Red as the probe to examine the dynamics and environment of the systems.63 The Nile Red absorbance values gradually increased with increasing x in [BzC4im]x[Bz(F5)C4im](1−x)[NTf2], but showed a maximum at x = 0.3 and a minimum at x = 0.8 for [BzC4im][BF4]x[NTf2](1−x). In such analyses, changes in slope are generally ascribed to changes in the microenvironment in which the solvatochromic probe is dissolved. Here more significant variations in solvent properties were observed for DSILs having two different anions. (Our analysis of three-dimensional organization of these DSILs was discussed in section 2.2.)
Men et al. investigated by X-ray photoelectron spectroscopy (XPS), the electronic interaction between the cation (pyrrolidinium or imidazolium) and anion ([NTf2]−, [PF6]− and I−) in DSILs composed of one cation and two anions.125 They studied the binding energy of the electronic interaction of the anion with the charge-bearing head group of the ILs and showed a correlation with the anion basicity. In addition, the authors stated that [C8C1Pyrr][NTf2]0.5[I]0.5 contains an “intimate mixture of cations and randomly distributed anions, not discrete pockets of the cation and one type of anion or the other.” This experiment does not support, or disprove, the formation of polar and non-polar domains within pyrrolidinium-based ILs, but it does eliminate the formation of discrete pockets of different ILs within the bulk DSILs, as would be the case in an aggregate or emulsion-based system. The authors also showed that the anion greatly affects the electronic environment of the cation and proposed that the underlying factors can be explained by treating Coulombic and van der Waals contributions independently.125
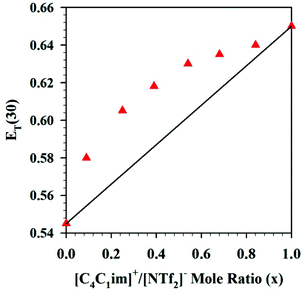 | ||
| Fig. 10 Variation of ET(30) with the mole ratio in [C4C1im]x[C4C1C1im](1−x)[NTf2] at 273 K. The bold line represents the average polarity curve based on the polarity of the two IL parents. (Drawn with permission from the data in ref. 130; Copyright 2013 American Chemical Society.) | ||
Very recently, the same authors investigated the same DSILs as solvents for the nucleophilic substitution of N-(p-fluorophenyldiphenylmethyl)-4-picolinium chloride using water and a series of alcoholic nucleophiles.131 They first showed the reduction of the hydrogen bond acidity of the two-ion ILs through the use of [C4C1C1im][NTf2] rather than [C4C1im][NTf2] as a solvent, yielded an increase in the rate constant of the reaction by testing different nucleophiles (water and some alcohols). Based on the Kamlet–Taft parameters of the IL parents, they showed that the solvation of aromatics was not strongly influenced by the aromaticity of the cation itself, confirming that the strong interactions between aromatic molecules and two-ion ILs result from ion-quadrupole rather than π–π interactions as shown before.132 Furthermore, the results of the studied reactions using DSILs were demonstrated to be unique, with water or selected alcohols exhibiting significant preferential solvation behavior resulting from electronic and steric effects from the alkyl group. This suggests some influence of the cations in the transition state of the reaction.
In summary, the use of ionic systems consisting of one IL with desirable solvation properties, such as strong hydrogen bond donating or accepting groups, and another IL with desired physical properties, such as low viscosity or melting point, is feasible and may enable new classes of chemical transformations to proceed more selectively and/or under milder conditions. While it is clear that the choice and proportion of each ion leads to DSILs with the desired properties, we do question whether it is really the properties of the IL parents that one needs to consider rather than simply the resultant ionic compositions.
Ohno et al. reported the Kamlet–Taft parameters of some amino acid-based DSILs as a function of ionic composition.136 Interestingly, the β parameter of the three studied DSIL types, [C2C1im][Ala]x[Val](1−x), [C2C1im][Asp]x[Lys](1−x), and [C2C1im][Glu]x[Lys](1−x), varied almost linearly with mole ratio (Fig. 11a). This suggests that the hydrogen bond acceptor strength is proportional to the abundance and the hydrogen bond acceptor strength of each anion. However, the α values of [C2C1im][Asp]x[Lys](1−x) change non-linearly with the ionic composition (Fig. 11b). Since the cation here should account for the observed α values, these values can decrease when stronger hydrogen bonding between anion and cation exists, reducing potential hydrogen bond donation from the cation to the dye probe.137 These results highlight the different interactions between the two anions and the common [C2C1im]+ cation in the studied DSILs.
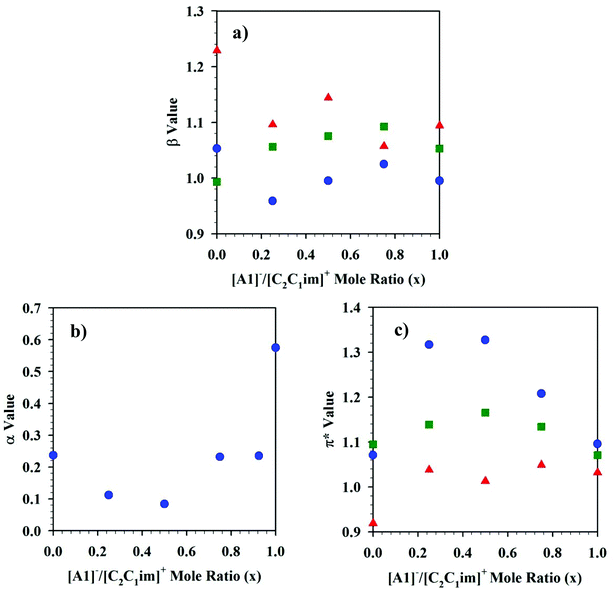 | ||
Fig. 11 Variation of β (a), α (b), and π* (c) parameters with the anion mole ratio of [C2C1im][Ala]x[Val](1−x) ( ; [A1]− = [Ala]), [C2C1im][Asp]x[Lys](1−x) ( ; [A1]− = [Ala]), [C2C1im][Asp]x[Lys](1−x) ( ; [A1]− = [Asp]), and [C2C1im][Lys]x[Glu](1−x) ( ; [A1]− = [Asp]), and [C2C1im][Lys]x[Glu](1−x) ( ; [A1]− = [Lys]). (Drawn from data in Fig. 1 in ref. 136 provided by the authors; Copyright 2013 the Chemical Society of Japan.) ; [A1]− = [Lys]). (Drawn from data in Fig. 1 in ref. 136 provided by the authors; Copyright 2013 the Chemical Society of Japan.) | ||
The π* parameters of [C2C1im][Ala]x[Val](1−x) are linear with mole ratio, contrary to [C2C1im][Asp]x[Lys](1−x) and [C2C1im][Glu]x[Lys](1−x) that have a maximum π* value when x = 0.25 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 anion ratio) and when x = 0.5 (1
3 anion ratio) and when x = 0.5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 anion ratio), respectively (Fig. 11c). These results indicate that the two latter DSIL types are highly polar, with π* values superior to those of the IL parents, but also greater than other known two-ion polar ILs.137,138
1 anion ratio), respectively (Fig. 11c). These results indicate that the two latter DSIL types are highly polar, with π* values superior to those of the IL parents, but also greater than other known two-ion polar ILs.137,138
These DSILs are also very viscous,136 and the authors thus studied the less viscous [P4,4,4,4][Asp]x[Lys](1−x), based on their previous work with [P4,4,4,4]+-based ILs.139 Based on the crystal structure of [P4,4,4,4][Glu], they discussed the possible interactions in amino acid ILs, and the importance of hydrogen bonding to explain the DSIL behavior. They concluded that even in the liquid state aggregations of ions via hydrogen bonding are possible, that the strength of hydrogen bonding between ions depended on the ion's capacities for hydrogen bonding, and that hydrogen bonding can contribute to lower ion mobility.
We would also strongly recommend that spectroscopic data be collected for the detailed study of such DSILs to help further understand the nature of the interactions between each ion in the DSILs before such conclusions are finalized. At a minimum, changes in the electronic environment of the ions can be readily determined.
Fletcher et al. conducted a complete study of solute–solvent and ion–ion interactions in [C4C1im][PF6]x[NTf2](1−x), [C2C1im]x[C4C1im](1−x)[NTf2], and [C2C1im]x[C4C1im](1−x)[PF6]y[NTf2](1−y) by investigating the polarity, dielectric, hydrogen bonding interactions, and microfluidity.28 Pyrene can be used as a fluorescent probe of polarity within organized media based on the ratio of monomer fluorescence intensities at bands I (0-0 band, 373 nm) and III (384 nm).140 In the above study, ideality was defined as the expected spectral response of the solvatochromic probe within a binary solvent mixture, obtained by the mole fraction weighted average of the probe's spectral responses in the two parent compounds.141 For the three DSILs studied, the corresponding ‘pyrene II/IIII’ values, a function of solvent dielectric (ε) and refractive index (n), were above the expected values, suggesting an increased local dielectric relative to ideal mixing of the two-ion ILs.
The deviations from ideality increased from [C2C1im]x[C4C1im](1−x)[NTf2] (maximum at x = 0.25), to [C4C1im][PF6]x[NTf2](1−x) (at x = 0.25), and finally [C2C1im]x[C4C1im](1−x)[PF6]y[NTf2](1−y) at x = y = 0.25.28 Anion coordination effects could explain the observed deviations in the DSILs with two different ions, compared to DSILs with a common anion. However, since pyrene cannot participate in hydrogen bonding, some of the results were not consistent and were poorly explained by the existence of microheterogeneity (as suggested by the authors) in DSILs, and need further investigations.
In addition, through the study of Kamlet–Taft parameters, the authors showed that deviations in π* values were statistically insignificant, and that the hydrogen bond acceptor basicity remained virtually unchanged across all mole fractions.28 On the other hand, the hydrogen bond acidity (α) of the studied DSILs showed clear deviations compared to the mixing average of the IL parents.
Overall, the results obtained for polarity parameters of ILs or DSILs may be biased by the selected probes or the chosen ions, and thus care must be taken before wide ranging conclusions are made. As we suggested earlier, phenomena dominated by Coulombic interactions might better be modeled using polarity scales based upon charged probes, whereas those for which Coulombic interactions are not significant can better be modeled using polarity scales based upon neutral probes.66 Thus, the reported results should be considered with caution since they use interaction-dependent techniques to discuss the polarity of DSILs whereas the interactions that govern DSILs are not really known and the number of possible interactions dramatically increases as one goes from two ions to three ions or more. Nonetheless, the reported studies have indicated that the use of DSILs may be a viable approach in the design and modification of these solvents toward specific ends. The caution here is that DSILs often exhibit properties not readily apparent from those of the individual IL parents.
6. Uses of DSILs
6.1. Applications of DSILs
Applications which incorporate the use of DSILs have not been widely studied yet; the majority of the publications in this area have rather been directed to the study of their physicochemical properties. However, a few applications have been reported in the general areas of chemistry, industry, and sustainable development. These are grouped in Table 9, indicating the main advantages brought by using the DSILs.| Entry | Application | DSIL | Main advantages | Ref. |
|---|---|---|---|---|
| 1 | Electrochemistry | [C2C1im]x[C3C1pyr](1−x)[CF3SO3]y[NTf2](1−y), [C2C1im]x[BzC1im](1−x)[CF3SO3]y[NTf2](1−y), [C2C1im]x[BzC1im](1−x)[NTf2] | Reduction of viscosities and increasing conductivities in lithium ion battery applications | 142 |
| 2 | [C3C1im]x[C4C1im](1−x)[BF4]y[I](1−y) | Tuning the diffusion coefficient of triiodide with DSIL mole ratio | 143 | |
| 3 | [C3C1pyr]x[O-COO-azaspiro](1−x)[NTf2] | Elimination of dendrite formation; good cycling with high capacities | 144 | |
| 4 | [C14isoqui]x[N7,7,7,7](1−x)[N(SO2C2F5)2] | Change in the surface charge and in the structure of the electrical double layers | 145 | |
| 5 | [C3C1pip]x[C4C1im](1−x)[BF4]y[NTf2](1−y) | Increasing conductivity; decreasing the initial deposition–dissolution overpotentials | 146 | |
| 6 | CO2 absorption | [C2C1im][EtSO4]x[NTf2](1−x), [C2C1im]x[C4C2im](1−x)[EtSO4]y[NTf2](1−y) | Possibility of tuning the solubility of CO2 | 46 |
| 7 | [C2C1im]x[C4C1im]y[NH2C2C1im](1−x−y)[BF4] | Improvement in gas-liquid mass-transfer; five absorption/regeneration cycles at constant absorption capacity | 147 | |
| 8 | [C2C1im]x[C8C1im](1−x)[BF4]y[NTf2](1−y), [C4C1im]x[C8C1im](1−x)[BF4]y[NTf2](1−y) | A slightly better absorption capacity of the DSILs compared to IL parents | 108 | |
| 9 | Solvents in organic chemistry | [C4C1im][Cl]x[NTf2](1−x) | Higher catalytic activity than IL parents | 148 |
| 10 | [C4C1im][CF3SO3]x[NTf2](1−x) | Optimal activity and stability of lipase obtained in a DSIL | 149 | |
| 11 | [C4C1im][BF4]x[PF6](1−x) | Improvement of enzymatic performance | 150 | |
| 12 | [C2C1im]x[4-C1-C4py](1−x)[MeSO3]y[PF6](1−y) | Doubling conversion compared to IL parents | 151 | |
| 13 | Extraction/separation | [PF6]− salts of metal-ligating task specific cations (Fig. 12) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 combination with [C4C1im][PF6] 1 combination with [C4C1im][PF6] |
Reduced cost and viscosity of the TSIL used neat | 160 |
| 14 | [N8,8,8,H][NO3]x[NTf2](1−x) | Tuning hydrophobicity and viscosity of DSILs; enhancement of extractability and selectivity for metals | 152 | |
| 15 | [C4C1im]x[C8C1m](1−x)[BF4]y[PF6](1−y) | Tuning hydrophobicity of DSILs; increasing extraction efficiencies of solutes from aqueous phases | 153 | |
| 16 | Biomass processing | [C4C1im]x[C4H8SO3HC1im](1−x)[Cl]y[HSO4](1−y), [C4C1im]x[C4H8SO3HC1im](1−x)[OAc]y[HSO4](1−y), [C2C1im]x[C4H8SO3HC1im](1−x)[OAc]y[HSO4](1−y) | Cellulose dissolution efficiency and catalytic conversion improvement | 154 |
| 17 | [C4C1im][MeSO4]x[PF6](1−x) | Stabilization of nanoparticles; solubilization of lignin | 155 | |
| 18 | [C1C1im]0.25[C2C1im]0.5[C2C2im]0.25[OAc] [C1C1im]0.25[C4C1im]0.5[C4C4im]0.25[OAc] | Cellulose dissolution efficiency but with lower cost and avoidance of chloride anion in the synthetic scheme | 19 | |
| 19 | Energetic materials | [C1C1im][4,5-diNO2-im]x[4-NO2-tri](1−x) | Tuning of energetic properties | 18 |
| 20 | Inhibitors in oil and gas industry | [C2C1im][Cl]x[Br](1−x) | Better inhibition effects compared to IL parents | 156 |
| 21 | Gas chromatography | [C4C1im][Cl]x[NTf2](1−x) | Improvement of separation selectivity of alcohols and aromatic compounds | 112 |
| 22 | Polymeric DSILs | Improvement of separation selectivity of analytes such as ketones, aldehydes, and aromatic compounds | 110 | |
The DSIL approach was also used to reduce viscosities allowing improved transport properties of iodide-based two-ion ILs.157–159 Diffusion coefficient measurements of triiodide in [C3C1im]x[C4C1im](1−x)[BF4]y[I](1−y) at 298 K were reported based on four comparative electrochemical methods and these could find interesting applications for Grätzel-type dye-sensitized solar cells (Table 9, entry 2).143
Another investigation of specific DSILs as electrolytes in lithium battery cells was performed by Lane et al. where [C3C1pyr]x[O-COO-azaspiro](1−x)[NTf2] ([O-COO-azaspiro]+: 2-oxo-3,9-dioxa-6-azaspiro[5.5]undecan-6-ium cation) with added lithium salt (LiNTf2) was used to suppress dendrite formation (Table 9, entry 3).144 Indeed, the [O-COO-azaspiro]+ cation was already known to successfully suppress dendrite formation in [O-COO-azaspiro][NTf2], but its high melting point, poor capacity, and passivation of the electrode, prohibited its use alone. On the other hand, the pyrrolidinium cation was known to cause dendrite formation at the lithium electrode, resulting in shorting of the cell in [C3C1pyr][NTf2]. It was the combination of these two cations ([O-COO-azaspiro]+ and [C3C1pyr]+) in a DSIL with the common anion [NTf2]− that led to the elimination of dendrite formation and good cycling of the cell with high capacities.
The DSILs [C14isoqui]x[N7,7,7,7](1−x)[N(SO2C2F5)2] were investigated at the DSIL–water interface (Table 9, entry 4).145 The surface properties of the water interface were affected by the applied potential, resulting in changes in the surface charge and the structure of the electrical double layers, but were also changed by changing the mole ratio of the ions in the DSILs. Indeed, the interfacial tension measurements at both DSIL–water and DSIL–air interfaces suggested a phase transition for the DSIL phase at x = 0.2. Interestingly, [C14isoqui]+ is much more surface active than [N7,7,7,7]+ in the DSIL, and the gap in critical micelle concentration values and the phase transition points occurred at the specific mole ratio x = 0.2. The authors attributed this observation to the presence of a “certain common denominator that gives rise to the difference in surface and bulk properties in the phase transition of the electrolyte solutions,” although the exact chemical explanation was not provided.
Electrodeposition has also been reported using DSILs, where magnesium was electrodeposited from solutions of Mg(CF3SO3)2 in [C3C1pip]x[C4C1im](1−x)[BF4]y[NTf2](1−y) (Table 9, entry 5).146 Increasing the relative mole ratios of [C3C1pip]+ and [NTf2]− increased the conductivity of the electrolyte system and the initial deposition–dissolution overpotentials were found to be lower in the DSILs than in any of the IL parents. In particular, [C3C1pip]0.6[C4C1im]0.4[BF4]0.4[NTf2]0.6 exhibited good conductivity and the lowest initial overpotential. Interestingly, reversible electrodeposition was maintained over 200 cycles using this DSIL.
Other examples in organic chemistry have reported the efficiency of DSILs in enzymatic reactions. For example, the optimal activity and stability of lipase was obtained in [C4C1im][CF3SO3]0.5[NTf2]0.5 in the lipase-catalyzed synthesis of glucose fatty acid ester (Table 9, entry 10).149 The mole ratio also played an essential role in optimizing the enzymatic hydrolysis of penicillin using [C4C1im][BF4]x[PF6](1−x) (Table 9, entry 11).150 In the same way, the use of [C2C1im]0.5[4-C1-C4py]0.5[MeSO3]0.5[PF6]0.5 for the enzymatic synthesis of mono-6- and 6′-O-linoleyl-α-D-maltose led to double the conversions in comparison to two-ion ILs (Table 9, entry 12).151 No explanations were provided for the enhancement of enzymatic activity in the presence of DSILs, but the reported improvements in these reactions are worth further study.
 | ||
Fig. 12 Metal ligating task specific cations combined with [PF6]− and used neat or in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 combination with [C4C1im][PF6] to form task-specific ILs for the extraction of Hg2+ and Cd2+ from aqueous solution.160 1 combination with [C4C1im][PF6] to form task-specific ILs for the extraction of Hg2+ and Cd2+ from aqueous solution.160 | ||
DSILs have also recently been investigated for other extractions and separations. [N8,8,8,H][NO3]x[NTf2](1−x) systems were used as extractants for platinum-group elements, where recycling is of interest for applications with jewelry, electrical contacts, dentistry, and antipollution devices in automobiles (Table 9, entry 14).152 Interestingly, these DSILs exhibited new physical properties such as more hydrophobicity and lower viscosities than the IL parents, as well as higher extractabilities and selectivities for Pd(II) and Pt(IV) in acidic chloride media than general hydrophobic ILs. In addition, these results were controllable through the molar compositions of the DSILs.
The tunable balance between hydrophobicity and hydrophilicity based on DSIL composition was also studied in dispersive liquid–liquid micro-extraction (Table 9, entry 15).153,161 Here, [C4C1im]x[C8C1im](1−x)[BF4]y[PF6](1−y) allowed rapid extraction of two of the typical pyrethroid pesticides, namely permethrin and biphenthrin, from the aqueous phase into DSILs, compared to the use of two-ion ILs.
These interesting results will not be discussed further here, since they involve DSILs in aqueous solution, which is not only outside the scope of this review, but also it remains to be seen whether such solutions could not simply be a combination of solvated ions prepared separately. In order to clearly understand the role played by any DSIL in this kind of study, quantification of the different phases and phase ratios after separation should be rigorously investigated.
We also demonstrated a lower cost, halide free approach to the preparation of cellulose-dissolving ILs based on dialkylimidazolium acetate (Table 9, entry 18). A statistical mixture of symmetric and asymmetric dialkylimidazolium cations was prepared in a one pot synthesis by following the reaction depicted in Scheme 1b (section 1) yielding [C1C1im]0.25[C2C1im]0.5[C2C2im]0.25[OAc].19 This DSIL and [C1C1im]0.25[C4C1im]0.5[C4C4im]0.25[OAc] were shown to dissolve cellulose and other biopolymers with the same efficiency and capacities as the single cation ILs, but at significantly lower cost while avoiding any chloride anion in the synthetic scheme.
6.2 Why use DSILs?
In this last part of the review, we would like to discuss other new opportunities brought by the DSIL concept. Certainly, the numerous examples and applications described above, have already shown advantages of DSILs in tuning physical, thermal, and chemical properties and for improving selectivity, yield, and reactivity; but it is now important to determine other future opportunities for DSILs.Aparicio and Atilhan reported an ATR-FTIR study combined with molecular dynamics simulations of [3-C1-C4py]x[3-C1-C8py](1−x)[BF4] and [3-C1-C4py][BF4]x[N(CN)2](1−x). The data for the first DSIL was explained by considering lower apolar domains for the [3-C1-C4py]+-rich systems, leading to more efficient packing, lower steric hindrance, more dense and less viscous fluids, but without important structural changes.162 The second DSIL showed two regions in which the properties resembled those of the IL parents, separated by a critical concentration of [BF4]− anions (at x = 0.6). However, the changes in physical properties obtained from molecular dynamics simulations were almost linear with increasing mole ratio, and the comparison of vibrational spectra deconvoluted from two-ion ILs with that of the DSILs suggested that regions dominated by interactions between cation and [N(CN)2]− anions, when the DSIL is depleted in [BF4]−, or reverse for regions dominated by [BF4]−.
Computer simulations using an all-atom force field showed that [C2C1im]0.5[C6C1im]0.5[NTf2] contains microphase separation between polar and non-polar domains, but the global behavior of this DSIL was shown to be ideal.163 Thus, some simulation studies reported good agreement with predictions for several properties compared to ideality, but often, similar and common cations/anions were used, and the deviations would logically be small in such cases.60,163,164 In the same way, ideal behavior compared to the IL parents of DSILs composed of common [NTf2]− anions paired with imidazolium, pyridinium, and piperidinium cations (having the same range in sizes) was recently reported by Coutinho et al. based on surface tension measurements of ILs and the corresponding DSILs.165
Based on the numerous unexpected physicochemical properties reported throughout the examples of this review, we suggest that it is the non-ideal behavior which should be sought in order to take advantage of new properties proposed by DSILs. Clearly, the DSILs are not simple mixtures of ILs, and should not only be compared to mixtures of organic solvents because the individual ions which comprise each IL can interact independently with the other ions, and thus can lead to new and unexpected properties.
The DSILs [C1C1im]0.25[C2C1im]0.5[C2C2im]0.25[OAc] and [C1C1im]0.25[C4C1im]0.5[C4C4im]0.25[OAc] were likewise prepared in a one pot reaction, here by reacting 1 eq. each of two different alkylamines, aqueous formaldehyde, acetic acid, and aqueous glyoxal solution (see Scheme 1b in section 1).19 These DSILs could, of course also be prepared by first preparing three individual two ion ILs and mixing them in the appropriate ratios, but the cost (monetary and environmental) of doing so would be much greater.
We believe that other efficient ways will be established in order to prepare DSILs, since they are defined by the nature and the abundance of the ions composing them and do not retain the chemical identity of the original parent IL. Once the field stops focusing on the mixing of two or more ILs and instead focuses on the resulting DSILs and what these unique systems can accomplish, we are confident that the direct synthesis of DSILs will become commonplace.
Another interesting example is a study related to DSIL toxicity to Vibrio qinghaiensis (Q67).169 Aqueous solutions were prepared from the mixing of nine 1-alkyl-3-methylimidazolium based-ILs, with different alkyl chains lengths (ethyl, butyl, hexyl, and octyl) on the cations, and chloride, bromide, or alkylsulfates anions (Table 10, entries 1–9). Based on microplate toxicity analysis,170 Zhang et al. looked for antagonistic or synergistic toxicity effects compared to the toxicities of the IL parents by comparing EC50 values for aqueous solutions of [C4C1im][Cl]x[Br]y[MeSO4]z[C8H17SO4](1−x−y−z), [C8C1im][C5H11O2]x[C8H17SO4]1−x, [C4C1im]x[C8C1im]1−x[C8H17SO4], and [C2C1im]x[C4C1im]y[C6C1im]z[C8C1im](1−x−y−z)[Cl] (Table 10, entries 10–13). In the last solution (Table 10, entry 13), the authors found a good correlation with the effects obtained based on the individual IL parent solutions and their concentrations. However, the authors also highlighted that the presence of one specific ion cannot be responsible for all of the toxicity of a given solution, but rather that the new combinations of ions could change the toxicity.
| Entry | IL/solution | pEC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
|---|---|---|
| a The pEC50 is defined as the negative logarithm of the EC50 (which has units of molarity, mol L−1). The experimental procedure, called “microplate toxicity analysis” was reported previously.170 b Different molar concentrations of each ion were tested for each aqueous mixture. | ||
| Solutions of two-ion IL | ||
| 1 | [C2C1im]Cl | 1.01 |
| 2 | [C4C1im]Cl | 1.82 |
| 3 | [C4C1im]Br | 2.20 |
| 4 | [C4C1im][MeSO4] | 2.31 |
| 5 | [C4C1im][C8H17SO4] | 2.53 |
| 6 | [C6C1im]Cl | 3.09 |
| 7 | [C6C1im]Cl | 4.66 |
| 8 | [C8C1im][C8H17SO4] | 4.14 |
| 9 | [C8C1im][C5H11O2SO4] | 4.09 |
| Ion ratios in aqueous mixturesb | ||
| 10 | [C4C1im][Cl]x[Br]y[MeSO4]z[C8H17SO4](1−x−y−z) | 1.92–2.27 |
| 11 | [C2C1im]x[C4C1im]y[C6C1im]z[C8C1im](1−x−y−z)[Cl] | 1.14–2.19 |
| 12 | [C4C1im]x[C8C1im]1−x[C8H17SO4] | 2.61–3.04 |
| 13 | [C8C1im][C5H11O2SO4]x[C8H17SO4]1−x | 4.05–4.10 |
While in general this study does not make DSILs as we have defined them, since here two or four two-ion ILs were added separately to water, the resulting solutions would be the same as adding one DSIL to water and eventually such studies must be undertaken. Although further studies are needed to understand the results in terms of the nature and proportion of the ions on the mechanism of toxicity, this one example suggests that toxicity of a DSIL in solution should not be assumed to be a simple combination of any two-ion ILs. It would be interesting to study the toxicity of neat DSILs to investigate whether the design of DSILs less toxic than the IL parents is possible.
The use and study of DSILs are still in their infancy, perhaps due to the even larger number of ionic combinations than the already overwhelming number of two-ion ILs, not to mention that DSILs can be prepared by mixing not only ILs, but also an IL with higher melting salts or even eutectic mixtures of two or more higher melting salts. Based on the current literature and our personal experiences, we believe many new opportunities will arise leading to even more applications for ILs.
7. Conclusions and recommendations
We believe that the current literature shows ‘IL–IL mixtures’ should be conceptually considered as unique double salts, each defined by the nature and the abundance of each ion composing these systems. Despite the current paucity of data available for each property and the similarity of the reported systems, we have described the main trends found for the physical, thermal, and chemical properties of these ‘double salt ionic liquids’ (DSILs). The fact that both ideal and non-ideal behavior depends on the property studied, the nature of the ions, and the abundance of each ion, suggests that each DSIL is unique, and not a simple mixture of two-ion ILs. Thus, DSILs should not be defined by their method of preparation (mixing), but rather by their final ionic composition.The physical properties of DSILs such as viscosity, density, excess molar volume, etc., are mainly controlled by their composition and their intrinsic interactions. In fact, as several ions are present, the Coulombic forces seem to be even more important in these systems compared to two-ion ILs, and the steric effects of the ions lead to non-ideal properties. The importance of three-dimensional organization of ions in DSILs may be an important factor. The thermal properties may also be governed by van der Waals forces and electrostatic interaction forces, however, more fundamental studies are needed to clearly understand the role of each interaction in the organization of these salts.
Three-ion DSILs are currently the most studied, and some trends can start to be distinguished. In the two cation-based systems three-dimensional organization of the ions seems important, while for the two anion-based DSILs, the coordination abilities (basicity) of the anions seem to be important drivers. We strongly recommend more systematic use of spectroscopic analysis methods to support future studies of DSILs, in order to better understand the complex interactions between the various ions; with particular emphasis on the study of ionic combinations of ions with wildly different properties, sizes, etc. Some examples of potential design of melting point or phase transition behavior have been published, however, we believe that the most promising properties of DSILs are chemical, and it should be possible to design and modify DSILs toward specific ends. For example, DSILs often exhibit improved solvent properties not readily apparent from those of the two-ion IL parents.
Seddon stated that there are nearly one million simple ILs that can be easily prepared in the laboratory, leading to 1018 or more ILs if binary, ternary, etc. systems are considered.11 With the concept of double salts and their unique compositions as a function of ion type and abundance, there are in reality an infinite number of possible DSIL combinations. We are thus convinced that the DSIL concept will offer new and exciting properties to develop new approaches and new applications well beyond the current limitations of two-ion ILs.
In the general field of ILs to date, the emphasis has always been on finding an application where an IL can truly do something valuable, that no other system or approach can reasonably accomplish. The same might now be true of DSILs, where the most exciting chemistry and applications should be in areas that ILs have had limited success.
Abbreviations of ions
Acknowledgements
The authors thank the Novartis-Massachusetts Institute of Technology (MIT) Center for Continuous Manufacturing (CCM) for financial support of related projects.References
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- C. A. Angell, Y. Ansari and Z. Zhao, Faraday Discuss., 2012, 154, 9–27 RSC.
- N. V. Plechkova, R. D. Rogers and K. R. Seddon, Ionic Liquids: From Knowledge to Application, ACS Symposium Series, 2010 Search PubMed.
- S. Aparicio, M. Atilhan and F. Karadas, Ind. Eng. Chem. Res., 2010, 49, 9580–9595 CrossRef CAS.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2nd edn, 2008 Search PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- D. Rooney, J. Jacquemin and R. Gardas, Top. Curr. Chem., 2009, 290, 185–212 CrossRef CAS.
- D. R. MacFarlane, M. Forsyth, E. I. Izgorodina, A. P. Abbott, G. Annat and K. Fraser, Phys. Chem. Chem. Phys., 2009, 11, 4962–4967 RSC.
- J. D. Holbrey and K. R. Seddon, in Clean Products and Processes, ed. T. Matsunaga, Springer-Verlag, New York, 1999, vol. 1, p. 223 Search PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- Y. Marcus, Solvent Mixtures: Properties and Selective Solvation, Marcel Dekker, Inc., New-York, 2002 Search PubMed.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 4th edn, 2010 Search PubMed.
- K. N. Marsh, J. A. Boxall and R. Lichtenthaler, Fluid Phase Equilib., 2004, 219, 93–98 CrossRef CAS PubMed.
- H. Niedermeyer, J. P. Hallett, I. J. Villar-Garcia, P. A. Hunt and T. Welton, Chem. Soc. Rev., 2012, 41, 7780–7802 RSC.
- A. Stoppa, R. Buchner and G. Hefter, J. Mol. Liq., 2010, 153, 46–51 CrossRef CAS PubMed.
- M. Smiglak, N. J. Bridges, M. Dilip and R. D. Rogers, Chem.–Eur. J., 2008, 14, 11314–11319 CrossRef CAS PubMed.
- G. Gurau, H. Wang, Y. Qiao, X. Lu, S. Zhang and R. D. Rogers, Pure Appl. Chem., 2012, 84, 745–754 CrossRef CAS.
- R. D. Rogers, D. T. Daly and G. Guray, Methods for dissolving polymers using mixtures of different ionic liquids and compositions comprising the mixtures, Patent US2012/0216705 A1, 2012 Search PubMed.
- A. B. Pereiro, J. M. M. Araújo, F. S. Oliveira, C. E. S. Bernardes, J. M. S. S. Esperança, J. N. Canongia Lopes, I. M. Marrucho and L. P. N. Rebelo, Chem. Commun., 2012, 48, 3656–3658 RSC.
- R. L. Peck, N. G. Brink, F. A. Kuehl Jr., E. H. Flynn, A. Walti and K. Folkers, J. Am. Chem. Soc., 1945, 67, 1866–1867 CrossRef CAS.
- I. Freund, in Double Salts, Science Progress in the Twentieth Century, ed. N. H. Alcock and W. G. Freeman, 1907, vol. 2, pp. 135–159 Search PubMed.
- C. Christov and C. Balarew, J. Solution Chem., 1995, 24, 1171–1182 CrossRef CAS.
- C. Balarew and S. Tepavitcharova, Monatsh. Chem., 2003, 134, 721–734 CrossRef CAS.
- G. Annat, M. Forsyth and D. R. MacFarlane, J. Phys. Chem. B, 2012, 116, 8251–8258 CrossRef CAS PubMed.
- M. Kunze, S. Jeong, E. Paillard, M. Winter and S. Passerini, J. Phys. Chem. C, 2010, 114, 12364–12369 CAS.
- K. A. Fletcher, S. N. Baker, G. A. Baker and S. Pandey, New J. Chem., 2003, 27, 1706–1712 RSC.
- C. Hardacre, J. D. Holbrey, M. Nieuwenhuyzen and T. G. A. Youngs, Acc. Chem. Res., 2007, 40, 1146–1155 CrossRef CAS PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- A. Noda, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2001, 105, 4603–4610 CrossRef CAS.
- K. R. Seddon, A. Stark and M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275–2287 CrossRef CAS.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192–5200 RSC.
- R. Quinn, J. B. Appleby and G. P. Pez, J. Am. Chem. Soc., 1995, 117, 329–335 CrossRef CAS.
- W. Freyland, Liquid metals, molten salts, and ionic liquids: some basic properties, Coulombic Fluids, Springer Series in Solid-State Sciences, 2011, pp. 5–44 Search PubMed.
- A. M. Fernandes, M. A. A. Rocha, M. G. Freire, I. M. Marrucho, J. A. P. Coutinho and L. M. N. B. F. Santos, J. Phys. Chem. B, 2011, 115, 4033–4041 CrossRef CAS PubMed.
- C. Roth, T. Peppel, K. Fumino, M. Köckerling and R. Ludwig, Angew. Chem., Int. Ed., 2010, 49, 10221–10224 CrossRef CAS PubMed.
- K. Fumino, A. Wulf and R. Ludwig, Angew. Chem., Int. Ed., 2008, 47, 8731–8734 CrossRef CAS PubMed.
- K. Fumino, A. Wulf and R. Ludwig, Phys. Chem. Chem. Phys., 2009, 11, 8790–8794 RSC.
- K. Fumino, T. Peppel, M. Geppert-Rybczyńska, D. H. Zaitsau, J. K. Lehmann, S. P. Verevkin, M. Köckerling and R. Ludwig, Phys. Chem. Chem. Phys., 2011, 13, 14064–14075 RSC.
- E. I. Izgorodina and D. R. MacFarlane, J. Phys. Chem. B, 2011, 115, 14659–14667 CrossRef CAS PubMed.
- P. Bonhôte, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef PubMed.
- P. K. Katti and M. M. Chaudhri, J. Chem. Eng. Data, 1964, 9, 442–443 CrossRef CAS.
- L. Grunberg and A. H. Nissan, Nature, 1949, 164, 799–800 CrossRef CAS.
- E. C. Bingham, Fluidity and Plasticity, McGraw-Hill Book Company, Inc., New York, 1922 Search PubMed.
- A. M. Pinto, H. Rodríguez, Y. J. Colón, A. Arce Jr., A. Arce and A. Soto, Ind. Eng. Chem. Res., 2013, 52, 5975–5984 CrossRef CAS.
- P. Navia, J. Troncoso and L. Romaní, J. Solution Chem., 2008, 37, 677–688 CrossRef CAS.
- E. T. Fox, J. E. F. Weaver and W. A. Henderson, J. Phys. Chem. C, 2012, 116, 5270–5274 CAS.
- N. D. Khupse, S. R. Kurolikar and A. Kumar, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2010, 49, 727–730 Search PubMed.
- M. Montanino, M. Moreno, F. Alessandrini, G. B. Appetecchi, S. Passerini, Q. Zhou and W. A. Henderson, Electrochim. Acta, 2012, 60, 163–169 CrossRef CAS PubMed.
- F. Castiglione, G. Raos, G. B. Appetecchi, M. Montanino, S. Passerini, M. Moreno, A. Famulari and A. Mele, Phys. Chem. Chem. Phys., 2010, 12, 1784–1792 RSC.
- M. Larriba, S. García, P. Navarro, J. García and F. Rodríguez, J. Chem. Eng. Data, 2012, 57, 1318–1325 CrossRef CAS.
- W. Madelung and E. Kern, Justus Liebigs Ann. Chem., 1922, 427, 1–26 CrossRef CAS.
- A. P. Fröba, H. Kremer and A. Leipertz, J. Phys. Chem. B, 2008, 112, 12420–12430 CrossRef PubMed.
- A. Z. Tasic, B. D. Djordjevic, D. K. Grozdanic and N. Radojkovic, J. Chem. Eng. Data, 1992, 37, 310–313 CrossRef CAS.
- M. Brüssel, M. Brehm, A. S. Pensado, F. Malberg, M. Ramzan, A. Stark and B. Kirchner, Phys. Chem. Chem. Phys., 2012, 14, 13204–13215 RSC.
- P. Navia, J. Troncoso and L. Romaní, J. Chem. Eng. Data, 2007, 52, 1369–1374 CrossRef CAS.
- J. N. Canongia Lopes, T. C. Cordeiro, J. M. S. S. Esperança, H. J. R. Guedes, S. Huq, L. P. N. Rebelo and K. R. Seddon, J. Phys. Chem. B, 2005, 109, 3519–3525 CrossRef PubMed.
- D. Xiao, J. Rajesh Rajian, L. G. Hines Jr., S. Li, R. A. Bartsch and E. L. Quitevis, J. Phys. Chem. B, 2008, 112, 13316–13325 CrossRef CAS PubMed.
- M. B. Oliveira, M. Domínguez-Pérez, M. G. Freire, F. Llovell, O. Cabeza, J. A. Lopes-da-Silva, L. F. Vega and J. A. P. Coutinho, J. Phys. Chem. B, 2012, 116, 12133–12141 CrossRef CAS PubMed.
- H. Weingärtner, Angew. Chem., Int. Ed., 2008, 47, 654–670 CrossRef PubMed.
- H. J. Castejón and R. J. Lashock, J. Mol. Liq., 2012, 167, 1–4 CrossRef PubMed.
- F. D'Anna, S. Marullo, P. Vitale and R. Noto, ChemPhysChem, 2012, 13, 1877–1884 CrossRef CAS PubMed.
- J. Anglister and I. Z. Steinberg, Chem. Phys. Lett., 1979, 65, 50–54 CrossRef CAS.
- J. Dupont, Acc. Chem. Res., 2011, 44, 1223–1231 CrossRef CAS PubMed.
- M. A. Ab Rani, A. Brant, L. Crowhurst, A. Dolan, M. Lui, N. H. Hassan, J. P. Hallett, P. A. Hunt, H. Niedermeyer, J. M. Perez-Arlandis, M. Schrems, T. Welton and R. Wilding, Phys. Chem. Chem. Phys., 2011, 13, 16831–16840 RSC.
- J. H. Williams, Acc. Chem. Res., 1993, 26, 593–598 CrossRef CAS.
- D. A. Dougherty, Science, 1996, 271, 163–168 CAS.
- M. Egashira, S. Okada and J.-I. Yamaki, Solid State Ionics, 2002, 148, 457–461 CrossRef CAS.
- H. Every, A. G. Bishop, M. Forsyth and D. R. MacFarlane, Electrochim. Acta, 2000, 45, 1279–1284 CrossRef CAS.
- A. Jarosik, S. R. Krajewski, A. Lewandowski and P. Radzimski, J. Mol. Liq., 2006, 123, 43–50 CrossRef CAS PubMed.
- H. Ohno, M. Yoshizawa-Fujita and T. Mizumo, Electrochemical Aspect of Ionic Liquids, John Wiley & Sons, Inc., Hoboken, NJ, 2nd edn, 2011 Search PubMed.
- D. R. MacFarlane and K. R. Seddon, Aust. J. Chem., 2007, 60, 3–5 CrossRef CAS.
- C. A. Angell, N. Byrne and J.-P. Belieres, Acc. Chem. Res., 2007, 40, 1228–1236 CrossRef CAS PubMed.
- K. Ueno, H. Tokuda and M. Watanabe, Phys. Chem. Chem. Phys., 2010, 12, 1649–1658 RSC.
- W. Xu, E. I. Cooper and C. A. Angell, J. Phys. Chem. B, 2003, 107, 6170–6178 CrossRef CAS.
- K. J. Fraser, E. I. Izgorodina, M. Forsyth, J. L. Scott and D. R. MacFarlane, Chem. Commun., 2007, 3817–3819 RSC.
- M. J. Earle, J. M. S. S. Esperança, M. A. Gilea, J. N. Canongia Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS PubMed.
- A. W. Taylor, K. R. J. Lovelock, A. Deyko, P. Licence and R. G. Jones, Phys. Chem. Chem. Phys., 2010, 12, 1772–1783 RSC.
- J. A. Lazzús, Ind. Eng. Chem. Res., 2009, 48, 8760–8766 CrossRef.
- J. A. Lazzús, Fluid Phase Equilib., 2012, 313, 1–6 CrossRef PubMed.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133–2139 RSC.
- T. D. J. Dunstan and J. Caja, ECS Trans., 2007, 35, 21–32 Search PubMed.
- R. R. Mod, F. C. Magne and E. L. Skau, J. Am. Oil Chem. Soc., 1958, 35, 688–691 CrossRef CAS.
- R. R. Mod, F. C. Magne and E. L. Skau, J. Am. Oil Chem. Soc., 1962, 39, 444–447 CrossRef CAS.
- Y. U. Paulechka and G. J. Kabo, Russ. J. Phys. Chem. A, 2008, 82, 1412–1414 CrossRef.
- Y. U. Paulechka, A. V. Blokhin, G. J. Kabo and A. A. Strechan, J. Chem. Thermodyn., 2007, 39, 866–877 CrossRef CAS PubMed.
- A. V. Blokhin, Y. U. Paulechka and G. J. Kabo, J. Chem. Eng. Data, 2006, 51, 1377–1388 CrossRef CAS.
- S. Zhang, N. Sun, X. He, X. Lu and X. Zhang, J. Phys. Chem. Ref. Data, 2006, 35, 1475–1517 CrossRef CAS PubMed.
- L. M. N. B. F. Santos, J. N. Canongia Lopes, J. A. P. Coutinho, J. M. S. S. Esperança, L. R. Gomes, I. M. Marrucho and L. P. N. Rebelo, J. Am. Chem. Soc., 2007, 129, 284–285 CrossRef CAS PubMed.
- J. Liu, Y. Wang and W. Yan, J. Chem. Thermodyn., 2013, 64, 167–171 CrossRef CAS PubMed.
- J. E. Desnoyers and G. Perron, J. Solution Chem., 1997, 26, 749–755 CrossRef CAS.
- O. Redlich and A. T. Kister, Ind. Eng. Chem., 1948, 40, 345–348 CrossRef.
- R. Hayes, S. Imberti, G. G. Warr and R. Atkin, Angew. Chem., Int. Ed., 2013, 52, 4623–4627 CrossRef CAS PubMed.
- K. Dong and S. Zhang, Chem.–Eur. J., 2012, 18, 2748–2761 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275–297 CrossRef CAS.
- T. Welton, Coord. Chem. Rev., 2004, 248, 2459–2477 CrossRef CAS PubMed.
- D. A. Fort, R. C. Remsing, R. P. Swatloski, P. Moyna, G. Moyna and R. D. Rogers, Green Chem., 2007, 9, 63–69 RSC.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- P. S. Barber, C. S. Griggs, J. R. Bonner and R. D. Rogers, Green Chem., 2013, 15, 601–607 RSC.
- A. Wittmar, D. Ruiz-Abad and M. Ulbricht, J. Nanopart. Res., 2012, 14, 651–661 CrossRef.
- P. Sun and W. Armstrong, Anal. Chim. Acta, 2010, 661, 1–16 CrossRef CAS PubMed.
- S. H. Ha, R. N. Menchavez and Y.-M. Koo, Korean J. Chem. Eng., 2010, 27, 1360–1365 CrossRef CAS.
- D. D. Patel and J.-M. Lee, Chem. Rec., 2012, 12, 329–355 CrossRef CAS PubMed.
- R. D. Rogers, K. R. Seddon and S. Volkov, Green Industrial Applications of Ionic Liquids, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2002 Search PubMed.
- S. Supasitmongkol and P. Styring, Energy Environ. Sci., 2010, 3, 1961–1972 CAS.
- Z. Lei, J. Han, B. Zhang, Q. Li, J. Zhu and B. Chen, J. Chem. Eng. Data, 2012, 57, 2153–2159 CrossRef CAS.
- A. Finotello, J. E. Bara, S. Narayan, D. Camper and R. D. Noble, J. Phys. Chem. B, 2008, 112, 2335–2339 CrossRef CAS PubMed.
- Q. Zhao and J. L. Anderson, J. Sep. Sci., 2010, 33, 79–87 CrossRef CAS PubMed.
- C. Ragonese, D. Sciarrone, P. Q. Tranchida, P. Dugo and L. Mondello, J. Chromatogr., A, 2012, 1255, 130–144 CrossRef CAS PubMed.
- Q. Q. Baltazar, S. K. Leininger and J. L. Anderson, J. Chromatogr., A, 2008, 1182, 119–127 CrossRef CAS PubMed.
- S. García, J. García, M. Larriba, A. Casas and F. Rodríguez, Fluid Phase Equilib., 2013, 337, 47–52 CrossRef PubMed.
- J. García, S. García, J. S. Torrecilla and F. Rodríguez, Fluid Phase Equilib., 2011, 301, 62–66 CrossRef PubMed.
- S. García, J. García, M. Larriba, J. S. Torrecilla and F. Rodríguez, J. Chem. Eng. Data, 2011, 56, 3188–3193 CrossRef.
- T. Inoue and Y. Iwasaki, J. Colloid Interface Sci., 2010, 348, 522–528 CrossRef CAS PubMed.
- E. K. Paleologos, D. L. Giokas and M. I. Karayannis, TrAC, Trends Anal. Chem., 2005, 24, 426–436 CrossRef CAS PubMed.
- P. D. McCrary, P. A. Beasley, G. Gurau, A. Narita, P. S. Barber, O. A. Cojocaru and R. D. Rogers, New J. Chem., 2013, 37, 2196–2202 RSC.
- K. B. Smith, R. H. Bridson and G. A. Leeke, J. Chem. Eng. Data, 2011, 56, 2039–2043 CrossRef CAS.
- R. Ferraz, L. C. Branco, I. M. Marrucho, J. M. M. Araújo, L. P. N. Rebelo, M. N. Ponte, C. Prudêncio, J. P. Noronha and Z. Petrovski, Med. Chem. Commun., 2012, 3, 494–497 RSC.
- J. L. Shamshina, P. S. Barber and R. D. Rogers, Expert Opin. Drug Delivery, 2013, 10, 1367–1381 CrossRef CAS PubMed.
- H. Mizuuchi, V. Jaitely, S. Murdan and A. T. Florence, Eur. J. Pharm. Sci., 2008, 33, 326–331 CrossRef CAS PubMed.
- C. Reichardt, Green Chem., 2005, 7, 339–351 RSC.
- S. N. Baker, G. A. Baker and F. V. Bright, Green Chem., 2002, 4, 165–169 RSC.
- S. Men, K. R. J. Lovelock and P. Licence, Phys. Chem. Chem. Phys., 2011, 13, 15244–15255 RSC.
- K. Dimroth, C. Reichardt, T. Siepmann and F. Bohlmann, Justus Liebigs Ann. Chem., 1963, 661, 1–37 CrossRef CAS.
- C. Reichardt, Chem. Soc. Rev., 1992, 21, 147–153 RSC.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- R. W. Taft and M. J. Kamlet, J. Am. Chem. Soc., 1976, 98, 2886–2894 CrossRef CAS.
- C. C. Weber, A. F. Masters and T. Maschmeyer, J. Phys. Chem. B, 2012, 116, 1858–1864 CrossRef CAS PubMed.
- C. C. Weber, A. F. Masters and T. Maschmeyer, Org. Biomol. Chem., 2013, 11, 2534–2542 CAS.
- K. Shimizu, M. F. Costa Gomes, A. A. H. Pádua, L. P. N. Rebelo and J. N. Canongia Lopes, J. Phys. Chem. B, 2009, 113, 9894–9900 CrossRef CAS PubMed.
- M. J. Kamlet, J.-L. Abboud and R. W. Taft, J. Chem. Am. Soc., 1977, 99, 6027–6038 CrossRef CAS.
- M. J. Kamlet and R. W. Taft, J. Am. Chem. Soc., 1976, 98, 377–383 CrossRef CAS.
- D. W. Armstrong, L. He and Y.-S. Liu, Anal. Chem., 1999, 71, 3873–3876 CrossRef CAS.
- J. Kagimoto, K. Noguchi, K. Murata, K. Fukumoto, N. Nakamura and H. Ohno, Chem. Lett., 2008, 37, 1026–1027 CrossRef CAS.
- L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2003, 5, 2790–2794 RSC.
- S. Park and R. J. Kazlaukas, J. Org. Chem., 2001, 66, 8395–8401 CrossRef CAS PubMed.
- J. Kagimoto, K. Fukumoto and H. Ohno, Chem. Commun., 2006, 2254–2256 RSC.
- W. E. Acree Jr., D. C. Wilkins, S. A. Tucker, J. M. Griffin and J. R. Powell, J. Phys. Chem., 1994, 98, 2537–2544 CrossRef.
- Y. Marcus, Chem. Soc. Rev., 1993, 22, 409–416 RSC.
- M. A. Taige, D. Hilbert and T. J. S. Schubert, Z. Phys. Chem., 2012, 226, 129–139 CrossRef CAS.
- M. Zistler, P. Wachter, P. Wasserscheid, D. Gerhard, A. Hinsch, R. Sastrawan and H. J. Gores, Electrochim. Acta, 2006, 52, 161–169 CrossRef CAS PubMed.
- G. H. Lane, A. S. Best, D. R. MacFarlane, A. F. Hollenkamp and M. Forsyth, J. Electrochem. Soc., 2010, 157, A876–A884 CrossRef CAS PubMed.
- R. Ishimatsu, Y. Kitazumi, N. Nishi and T. Kakiuchi, J. Phys. Chem. B, 2009, 113, 9321–9325 CrossRef CAS PubMed.
- P. Wang, Y. NuLi, J. Yang and Z. Feng, Surf. Coat. Technol., 2006, 201, 3783–3787 CrossRef CAS PubMed.
- M. Wang, L. Zhang, L. Gao, K. Pi, J. Zhang and C. Zheng, Energy Fuels, 2013, 27, 461–466 CrossRef CAS.
- K. I. Tominaga, Catal. Today, 2006, 115, 70–72 CrossRef CAS PubMed.
- S. H. Lee, S. H. Ha, N. M. Hiep, W.-J. Chang and Y.-M. Koo, J. Biotechnol., 2008, 133, 486–489 CrossRef CAS PubMed.
- Y. Jiang, H. Xia, C. Guo, I. Mahmood and H. Liu, Biotechnol. Prog., 2007, 23, 829–835 CrossRef CAS.
- F. Fischer, M. Happe, J. Emery, A. Fornage and R. Schütz, J. Mol. Catal. B: Enzym., 2013, 90, 98–106 CrossRef CAS PubMed.
- S. Katsuta, Y. Yoshimoto, M. Okai, Y. Takeda and K. Bessho, Ind. Eng. Chem. Res., 2011, 50, 12735–12740 CrossRef CAS.
- R.-S. Zhao, X. Wang, F.-W. Li, S.-S. Wang, L.-L. Zhang and C.-G. Cheng, J. Sep. Sci., 2011, 34, 830–836 CrossRef CAS PubMed.
- J. Long, B. Guo, X. Li, Y. Jiang, F. Wang, S. C. Tsang, L. Wang and K. M. K. Yu, Green Chem., 2011, 13, 2334–2338 RSC.
- Y. Zhu, L. Chuanzhao, M. Sudarmadji, N. Hui Min, A. O. Biying, J. A. Maguire and N. S. Hosmane, Chem. Open, 2012, 1, 67–70 CAS.
- A. R. Richard and H. Adidharma, Chem. Eng. Sci., 2013, 87, 270–276 CrossRef CAS PubMed.
- C. Xi, Y. Cao, Y. Cheng, M. Wang, X. Jing, S. M. Zakeeruddin, M. Grätzel and P. Wang, J. Phys. Chem. C, 2008, 112, 11063–11067 CAS.
- Y. Cao, J. Zhang, Y. Bai, R. Li, S. M. Zakeeruddin, M. Grätzel and P. Wang, J. Phys. Chem. C, 2008, 112, 13775–13781 CAS.
- K. Fredin, M. Gorlov, H. Pettersson, A. Hagfeldt, L. Kloo and G. Boschloo, J. Phys. Chem. C, 2007, 111, 13261–13266 CAS.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J. H. Davis Jr. and R. D. Rogers, Chem. Commun., 2001, 135–136 RSC.
- R.-S. Zhao, X. Wang, L.-L. Zhang, S.-S. Wang and J.-P. Yuan, Anal. Methods, 2011, 3, 831–836 RSC.
- S. Aparicio and M. Atilhan, J. Phys. Chem. B, 2012, 116, 2526–2537 CrossRef CAS PubMed.
- K. Shimizu, M. Tariq, L. P. N. Rebelo and J. N. Canongia Lopes, J. Mol. Liq., 2010, 153, 52–56 CrossRef CAS PubMed.
- S. M. Hosseini, M. M. Alavianmehr, D. Mohammad-Aghaie, F. Fadaei-Nobandegani and J. Moghadasi, J. Ind. Eng. Chem., 2013, 19, 769–775 CrossRef CAS PubMed.
- M. B. Oliveira, M. Domínguez-Pérez, O. Cabeza, J. A. Lopes-da-Silva, M. G. Freire and J. A. P. Coutinho, J. Chem. Thermodyn., 2013, 64, 22–27 CrossRef CAS PubMed.
- M. Smiglak, J. D. Holbrey, S. T. Griffin, W. M. Reichert, R. P. Swatloski, A. R. Katritzky, H. Yang, D. Zhang, K. Kirichenko and R. D. Rogers, Green Chem., 2007, 9, 90–98 RSC.
- K. R. Seddon, Ionic liquids: Designer solvents for green synthesis, The Chemical Engineer, 2002, vol. 730, pp. 33–35 Search PubMed.
- J. H. Davis Jr., Chem. Lett., 2004, 33, 1072–1077 CrossRef.
- J. Zhang, S.-S. Liu, R.-N. Dou, H.-L. Liu and J. Zhang, Chemosphere, 2011, 82, 1024–1029 CrossRef CAS PubMed.
- Y.-H. Zhang, S.-S. Liu, H.-L. Liu and Z.-Z. Lui, Pest Manage. Sci., 2010, 66, 879–887 CAS.
| This journal is © The Royal Society of Chemistry 2014 |