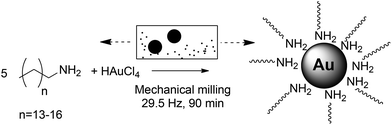
Mechanosynthesis of ultra-small monodisperse amine-stabilized gold nanoparticles with controllable size†
Monika J.
Rak
,
Nadim K.
Saadé
,
Tomislav
Friščić
and
Audrey
Moores
*
Centre for Green Chemistry and Catalysis, Department of Chemistry, McGill University, Montreal, Canada. E-mail: audrey.moores@mcgill.ca; Fax: +1-514-398-3797; Tel: +1-514-398-4654
First published on 29th October 2013
Abstract
We report a fast mechanochemical method for making gram amounts of monodisperse and ultra-small gold nanoparticles in the size range of ∼1–4 nm, without external reducing agents or bulk solvents.
Gold nanoparticles (AuNPs) have attracted much interest due to a broad range of applications including catalysis,1–3 microelectronics,4 biology,5 medicine,6,7 and sensors.8–10 Many properties of AuNPs heavily depend on three key parameters controlled during synthesis: namely the size, shape, and monodispersity. For instance, ultrasmall AuNPs (1–2 nm) were demonstrated to feature unusual magnetic properties.11 AuNPs are typically obtained using bottom-up solution-based syntheses which are based on a few seminal methods.12–16 Small, monodisperse AuNPs, and well defined Au clusters, are made using a variety of stabilizing ligands including phosphines,17 thiols,18–20 or amines.15,21
While these methods provide control over AuNP monodispersity,19,20 they rely on large amounts of solvents, ligands, external reducing agents, auxiliaries, phase transfer agents, and non-trivial purification steps.22 Additionally, the slow progress of nanoparticle-based technologies has been related to difficulties of scaling-up laboratory synthetic procedures.23
We now report a scalable, low-energy, and rapid (minutes to hours) synthesis of small amine-stabilized AuNPs (down to 1.3 nm) in gram quantities and without the need for additives and bulk solvents (Fig. 1). The methodology is based on a bottom-up assembly process under mild‡ mechanochemical milling24–30 in which amine ligands provide control over the size of AuNPs formed through in situ galvanic reduction of a gold(III) precursor by the steel milling assembly. It is important to note that a mechanochemical bottom-up approach to AuNPs was first investigated by Debnath et al., who obtained polydisperse AuNP samples with sizes 6 nm–30 nm (standard deviation averaging ∼28%) by reducing KAuCl4 with NaBH4 in the presence of poly(vinylpyrrolidone).31§
 | ||
| Fig. 1 Transmission electron micrographs of mechanochemically synthesized AuNPs stabilized by: (a) pentadecylamine, (b) hexadecylamine, (c) heptadecylamine and (d) octadecylamine. | ||
Our synthesis¶ (Scheme 1) is tunable by varying the ligand-to-precursor ratio, milling time, or the nature of the ligand. The AuNPs produced were fully characterized by Transmission Electron Microscopy (TEM), Matrix-Assisted Laser Desorption-Ionization Time-Of-Flight Mass Spectroscopy (MALDI-TOF MS), Ultraviolet-visible spectroscopy (UV-vis), Powder X-Ray Diffraction (PXRD) and X-Ray Photoelectron Spectroscopy (XPS). The AuNPs produced by this method do not require the traditional extensive purification as no additives are used in the synthesis.¶ We explored small molecules classically used as ligands in AuNP synthesis, i.e. thiols, amines, pyridines and imidazoles. Specifically, we compared four long chain amines (penta-, hexa-, hepta-, and octadecylamine), four N-heterocyclic molecules (4-dimethylaminopyridine (DMAP), 4,4′-bipyridine (4,4′-BIPY), imidazole, 1-methyl imidazole), two sulfur-based molecules (benzyl disulphide, ω-mercaptododecanoic acid) and a carboxylic acid (citrate).
TEM images were taken for all samples (Fig. 1, S1 in ESI†) and were used to assess the diameters of AuNPs (measured as an average of 250 particles for each sample, ESI Table 1†). We noticed that the amines provided the best results, leading to ultra-small, monodisperse AuNPs with diameters between 1.3–4.2 nm. While larger amine-stabilized AuNPs are accessible through conventional solution routes, the size regime of herein reported ones has not yet been accessible.21
We varied a number of parameters in the synthesis: the amine chain length, reaction time, and ligand-to-gold ratio. As with previous observations for solution-made NPs,32,33 the size of the mechanochemically synthesized NPs decreased with increasing the length of the amine ligand chain. This may be rationalized by the longer chains hindering access to the surface of the NPs more effectively than the shorter ones hindering NP growth. Pentadecylamine was the only ligand of the series to feature a melting temperature low enough to be reached upon grinding.¶ Consequently, the resulting AuNPs were significantly larger (4.2 ± 1.2 nm) than those with longer chains. Upon excluding pentadecylamine, a linear correlation is obtained between the amine chain length and the AuNP size (Fig. S4 in ESI†) demonstrating the ability to rationally control nanoparticle size in the solid phase, in this solvent- and auxiliary-free approach.
We probed the effect of the metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio on AuNP size (see Fig. S2 in ESI†) using octadecylamine as a stabilizing ligand which demonstrated that higher ligand amounts resulted in smaller AuNPs. A gold
ligand ratio on AuNP size (see Fig. S2 in ESI†) using octadecylamine as a stabilizing ligand which demonstrated that higher ligand amounts resulted in smaller AuNPs. A gold![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio of 1
ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 yielded particles of 1.25 ± 0.19 nm, while the 1
5 yielded particles of 1.25 ± 0.19 nm, while the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio afforded larger particles of 1.92 ± 0.30 nm. The results are consistent with more ligand passivating more NP surface, thereby limiting NP growth and yielding smaller AuNPs. In order to establish whether the size of the herein synthesized AuNPs is at an equilibrium,34 we explored the effect of reaction time on their size and monodispersity. Increased reaction times led to an increase in the size of the octadecylamine-stabilized AuNPs (Fig. S5 in ESI†). Consequently, the size of the nanoparticles can also be controlled by changing the reaction time, while maintaining high monodispersity.
2 ratio afforded larger particles of 1.92 ± 0.30 nm. The results are consistent with more ligand passivating more NP surface, thereby limiting NP growth and yielding smaller AuNPs. In order to establish whether the size of the herein synthesized AuNPs is at an equilibrium,34 we explored the effect of reaction time on their size and monodispersity. Increased reaction times led to an increase in the size of the octadecylamine-stabilized AuNPs (Fig. S5 in ESI†). Consequently, the size of the nanoparticles can also be controlled by changing the reaction time, while maintaining high monodispersity.
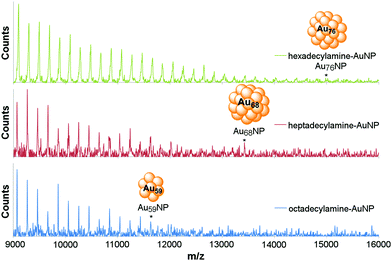
The TEM analysis was corroborated by UV-Vis spectra which only showed plasmon absorption bands for samples with amine-AuNP sizes >2 nm (Fig. S6 and S7 in ESI†) and mass spectroscopy.19,20,35–40 Optimized MALDI-TOF of hexa-, hepta- and octadecylamine afforded an excellent fragmentation pattern of the AuNPs, with peaks separated by 197.09 unit increments consistent with the mass of one gold atom (Fig. 2 and S16 in ESI† – calculation performed on the octadecylamine sample). The peaks of highest mass were measured to be M(C18) = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022.081 m/z, M(C17) = 13
022.081 m/z, M(C17) = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425.411 m/z, and M(C16) = 11
425.411 m/z, and M(C16) = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 627.572 m/z, corresponding to the bare clusters Au59, Au68 and Au76 respectively. C18–AuNPs (Au59 by MS, 1.25 nm by TEM) compared well with Au55 cluster reported to have a diameter of 1.2 nm.41 C17–AuNPs (Au68 by MS, 1.5 nm by TEM) were in good agreement with Au67 cluster reported to have a diameter of 1.4 nm.42 This result is the first example of MS characterization of amine-stabilized AuNPs. The extensive fragmentation in the MS spectra is not surprising, as amines are much weaker ligands to the gold surface and, thus, less efficient in stabilizing AuNPs compared to the extensively studied thiolates.
627.572 m/z, corresponding to the bare clusters Au59, Au68 and Au76 respectively. C18–AuNPs (Au59 by MS, 1.25 nm by TEM) compared well with Au55 cluster reported to have a diameter of 1.2 nm.41 C17–AuNPs (Au68 by MS, 1.5 nm by TEM) were in good agreement with Au67 cluster reported to have a diameter of 1.4 nm.42 This result is the first example of MS characterization of amine-stabilized AuNPs. The extensive fragmentation in the MS spectra is not surprising, as amines are much weaker ligands to the gold surface and, thus, less efficient in stabilizing AuNPs compared to the extensively studied thiolates.
We further characterized the AuNPs using XPS and PXRD. The XPS of the long chain amine ligand-stabilized AuNPs established the complete reduction of Au(3+) to Au(0) during milling. Two Au4f peaks are visible at binding energies consistent with reduced gold43,44 (Fig. S8–11 in ESI†). Although milling the ligand alone did not induce significant changes to its PXRD pattern, milling the ligands with the gold precursor resulted in the appearance of X-ray reflections characteristic of Au(0), in agreement with XPS results (Fig. S14 and S15 in ESI†).
To establish the reducing role of the steel milling assembly in AuNP synthesis, we attempted the synthesis in a poly(methyl)methacrylate (PMMA) jar with alumina balls. Milling resulted in a bright yellow mixture without AuNP formation. Replacing alumina balls with steel ones resulted in an immediate colour change and AuNP appearance. This led us to conclude the reduction of Au(III) to Au(0) took place through galvanic reduction by stainless steel of the milling equipment. The steel balls size was observed to diminish in size over several syntheses of AuNPs through this method. Because of the key role of the steel assembly in this synthesis, we verified by XPS that amine-stabilized AuNPs made by this method are free of iron contamination.
We also tested other ligands typically used in AuNPs synthesis (Table 1, Fig. 1). TEM images of DMAP- and 4,4′-BIPY-based samples evidenced small and spherical AuNPs of diameters of 2.5 ± 0.4 and 2.1 ± 0.5 nm respectively. However, characterization by XPS and PXRD (Fig. S12–S13 in ESI†) gave no evidence of Au(0). With these two ligands, the reduction is incomplete under milling conditions and the AuNPs observed by TEM presumably originated from reduction under the electron beam. Imidazole-based systems by TEM did not afford small or well defined particles (Table 1 and Fig. S1 in ESI†). Interestingly, sulfur-based ligands also gave poor TEM results (Table 1 and Fig. S1 in ESI†) and we hypothesize that the stability of thiolate binding to gold, responsible for their success in solution phase synthesis, has a detrimental effect under milling conditions because the growth of the AuNPs is impeded by the strongly bonded thiols. The less strongly bound amines, however, are able to detach and facilitate access of Au atoms to the NP core.
| Ligand | AuNP aspects and diameter (nm) |
|---|---|
a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand 1 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HAuCl4 milled at 29.5 Hz for 90 min.
b Spherical small NPs are observed by TEM, but XPS analysis demonstrated that mechanosynthesis only afforded partial reduction. HAuCl4 milled at 29.5 Hz for 90 min.
b Spherical small NPs are observed by TEM, but XPS analysis demonstrated that mechanosynthesis only afforded partial reduction.
|
|
| Pentadecylamine (C15) | Spherical – 4.2 ± 1.2 |
| Hexadecylamine (C16) | Spherical – 1.8 ± 0.3 |
| Heptadecylamine (C17) | Spherical – 1.5 ± 0.2 |
| Octadecylamine (C18) | Spherical – 1.3 ± 0.2 |
| 4-Dimethylaminopyridine (DMAP) | Incomplete reductionb |
| 4,4′-Bipyridine (4,4′ BIPY) | Incomplete reductionb |
| Imidazole | Large irregular particles |
| 1-Methylimidazole | Large irregular particles |
| Benzyl disulfide | Irregular aggregates |
| ω-Mercaptododecanoic acid | Films formed on TEM grid |
| Citrate | Polydisperse large particles ∼500 nm |
To test the scale up capabilities of the synthesis, a gram-scale reaction was performed with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 Au(III)
5 Au(III)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octadecylamine ratio. Specifically, the jar was loaded with 1 g of material and 0.863 g of AuNPs was efficiently collected. TEM analysis revealed no noticeable difference from the smaller scale batch (in terms of size and shape) (Fig. S3 in ESI†).
octadecylamine ratio. Specifically, the jar was loaded with 1 g of material and 0.863 g of AuNPs was efficiently collected. TEM analysis revealed no noticeable difference from the smaller scale batch (in terms of size and shape) (Fig. S3 in ESI†).
In summary, we established an efficient solvent-free methodology for rapid synthesis of ultra-small gold nanoparticles, scalable to at least one gram. The synthesis allows the control over the size of final gold nanoclusters by variation of amine ligands, ligand-to-gold ratio, or milling time. TEM images of the product show a high level of NP monodispersity. Compared to existing syntheses, this approach drastically suppresses the need for solvent and strong reducing agents, with minimal energy consumption (at the laboratory scale the total power input is ca. 50 W per sample, i.e. comparable to the power level of a light bulb). Conventional methods typically utilise >15 liters of solvent per gram of product.22 The herein presented methodology is solvent-free and, even after toluene treatment required for analysis, consumption of solvent remains at 5 mL per gram,¶i.e. three orders of magnitude lower than for conventional approaches. In this study, amines appear to be superior ligands for AuNPs than either thiols or disulphides, contrary to solution-based synthesis. The ultra-small amine-stabilized AuNPs have not been accessible through solution-based techniques, and we believe this makes the presented method particularly appealing for applications involving making of gold seeds for growth of rods, wires or shaped particles.45,46 At last, keeping in mind MS characterization of metal NPs is a recent technique, we highlight the first successful MALDI-TOF analysis of amine-stabilized AuNPs.
Acknowledgements
We acknowledge the Fonds de Recherche sur la Nature et les Technologies (FRQNT) Centre for Green Chemistry and Catalysis, FRQNT Centre for Self-Assembled Chemical Structures, FRQNT Nouveaux Chercheurs fund, Natural Science and Engineering Research Council (NSERC) Discovery Grant, Canada Foundation for Innovation (CFI), the Canada Research Chairs (CRC), NSERC CREATE in Green Chemistry and McGill University for financial support. We are grateful to Dr A. S. Wahba for help with MS measurements, Dr D. Liu for TEM measurements and Prof. D. Ronis for fruitful discussions.Notes and references
- M. Haruta, Nature, 2005, 437, 1098–1099 CrossRef CAS PubMed.
- G. J. Hutchings and M. Haruta, Appl. Catal., A, 2005, 291, 2–5 CrossRef CAS PubMed.
- M. Haruta, Chem. Rec., 2003, 3, 75–87 CrossRef CAS PubMed.
- G. Schmid and U. Simon, Chem. Commun., 2005, 697–710 RSC.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkilany, E. C. Goldsmith and S. C. Baxter, Acc. Chem. Res., 2008, 41, 1721–1730 CAS.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS PubMed.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- H. Y. Lee, H. Son, J. M. Lim, J. Oh, D. Kang, W. S. Han and J. H. Jung, Analyst, 2010, 135, 2022–2027 RSC.
- S. O. Obare, R. E. Hollowell and C. J. Murphy, Langmuir, 2002, 18, 10407–10410 CrossRef CAS.
- J. Liu and Y. Lu, J. Am. Chem. Soc., 2003, 125, 6642–6643 CrossRef CAS PubMed.
- S. Trudel, Gold Bull., 2011, 44, 3–13 CrossRef CAS PubMed.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC.
- W. W. Weare, S. M. Reed, M. G. Warner and J. E. Hutchison, J. Am. Chem. Soc., 2000, 122, 12890–12891 CrossRef CAS.
- D. V. Leff, L. Brandt and J. R. Heath, Langmuir, 1996, 12, 4723–4730 CrossRef CAS.
- S. D. Perrault and W. C. W. Chan, J. Am. Chem. Soc., 2009, 131, 17042–17043 CrossRef CAS PubMed.
- G. H. Woehrle, M. G. Warner and J. E. Hutchison, J. Phys. Chem. B, 2002, 106, 9979–9981 CrossRef CAS.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS PubMed.
- A. C. Dharmaratne, T. Krick and A. Dass, J. Am. Chem. Soc., 2009, 131, 13604–13605 CrossRef CAS PubMed.
- P. R. Nimmala and A. Dass, J. Am. Chem. Soc., 2011, 133, 9175–9177 CrossRef CAS PubMed.
- X. Lu, H.-Y. Tuan, B. A. Korgel and Y. Xia, Chem.–Eur. J., 2008, 14, 1584–1591 CrossRef CAS PubMed.
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS PubMed.
- T. Tsuzuki, Int. J. Nanotechnol., 2009, 6, 567–578 CrossRef CAS.
- R. B. N. Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC.
- T. Friščić, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. Kimber, V. Honkimaki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 CrossRef PubMed.
- V. Sepelak, S. Begin-Colin and G. Le Caer, Dalton Trans., 2012, 41, 11927–11948 RSC.
- P. Baláž, M. Achimovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- G. Štefanić, S. Krehula and I. Štefanić, Chem. Commun., 2013, 49, 9245–9247 RSC.
- D. Debnath, S. H. Kim and K. E. Geckeler, J. Mater. Chem., 2009, 19, 8810–8816 RSC.
- M. J. Schadt, W. Cheung, J. Luo and C.-J. Zhong, Chem. Mater., 2006, 18, 5147–5149 CrossRef CAS.
- B. L. V. Prasad, S. I. Stoeva, C. M. Sorensen and K. J. Klabunde, Langmuir, 2002, 18, 7515–7520 CrossRef CAS.
- P. Baláž, in Mechanochemistry in nanoscience and minerals engineering, Springer, Berlin, London, 2008, pp. 177–256 Search PubMed.
- E. S. Andreiadis, M. R. Vitale, N. Mezailles, X. Le Goff, P. Le Floch, P. Y. Toullec and V. Michelet, Dalton Trans., 2010, 39, 10608–10616 RSC.
- A. Dass, A. Stevenson, G. R. Dubay, J. B. Tracy and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 5940–5946 CrossRef CAS PubMed.
- H. Qian and R. Jin, Chem. Commun., 2011, 47, 11462–11464 RSC.
- H. Qian, Y. Zhu and R. Jin, ACS Nano, 2009, 3, 3795–3803 CrossRef CAS PubMed.
- T. G. Schaaff and R. L. Whetten, J. Phys. Chem. B, 1999, 103, 9394–9396 CrossRef CAS.
- A. Shivhare, S. J. Ambrose, H. Zhang, R. W. Purves and R. W. J. Scott, Chem. Commun., 2013, 49, 276–278 RSC.
- J. C. Garcia-Martinez and R. M. Crooks, J. Am. Chem. Soc., 2004, 126, 16170–16178 CrossRef CAS PubMed.
- M. Pumera, M. T. Castañeda, M. I. Pividori, R. Eritja, A. Merkoçi and S. Alegret, Langmuir, 2005, 21, 9625–9629 CrossRef CAS PubMed.
- A. N. Mansour, Surf. Sci. Spectra, 1994, 3, 197–201 CrossRef CAS.
- N. H. Turner and A. M. Single, Surf. Interface Anal., 1990, 15, 215–222 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Mater., 2001, 13, 2313–2322 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- M. Baláž, P. Baláž, G. Tjuliev, A. Zubrík, M. Sayagués, A. Zorkovská and N. Kostova, J. Mater. Sci., 2013, 48, 2424–2432 CrossRef.
- A. Pineda, A. M. Balu, J. M. Campelo, R. Luque, A. A. Romero and J. C. Serrano-Ruiz, Catal. Today, 2012, 187, 65–69 CrossRef CAS PubMed.
- A. Pineda, A. M. Balu, J. M. Campelo, A. A. Romero, D. Carmona, F. Balas, J. Santamaria and R. Luque, ChemSusChem, 2011, 4, 1561–1565 CrossRef CAS.
- E. Godočíková, L. Takacs, P. Baláž, J. Kováč, A. Šatka and J. Briančin, Rev. Adv. Mater. Sci., 2008, 18, 212–215 Search PubMed.
- M. Wang, X. Cao, Y. Huang, C. Guo, L. Huang, S. Yin and T. Sato, CrystEngComm, 2012, 14, 2950–2953 RSC.
- M. Senna, Int. J. Miner. Process., 1996, 44–45, 187–195 CrossRef CAS.
- A. Murugadoss, N. Kai and H. Sakurai, Nanoscale, 2012, 4, 1280–1282 RSC.
- H. Okatsu, N. Kinoshita, T. Akita, T. Ishida and M. Haruta, Appl. Catal., A, 2009, 369, 8–14 CrossRef CAS PubMed.
- S. A. Kondrat, G. Shaw, S. J. Freakley, Q. He, J. Hampton, J. K. Edwards, P. J. Miedziak, T. E. Davies, A. F. Carley, S. H. Taylor, C. J. Kiely and G. J. Hutchings, Chem. Sci., 2012, 3, 2965–2971 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3gc41827h |
| ‡ In contrast to high-speed milling of bulk materials, which is a well-known energy-intensive top-down approach for generating polydisperse NPs with sizes between 10 nm–100 nm, our approach is more similar to the mild bottom-up approaches which utilise reactive precursors47–49 to give well-defined NPs of binary chalcogenides.47–52 |
| § A two-step manual grinding procedure for making Ag–Au nanoclusters was recently reported which could not be extrapolated to pure AuNPs.53 Supported AuNPs have been made by grinding followed by calcination at 300 °C or higher.54,55 Au–Pd NPs were also reported.55 |
¶ In a typical experiment, the stabilizing amine ligand and HAuCl4 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand 1 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gold ratio, total weight 200 mg) are weighed into a 10 mL stainless steel milling jar. After adding two 7 mm stainless steel milling balls, the reaction mixture was milled for 90 min in a Retsch MM400 mill at 30 Hz. The resulting AuNPs are readily resuspended in a small amount of toluene (5 mL g−1) which allows their transfer, removal of any ligand excess, as well as magnetic separation of any iron shavings produced by the wear of the milling assembly. Based on previous studies, temperature of the jar after 90 min milling is evaluated to be between 33 to 35 °C. gold ratio, total weight 200 mg) are weighed into a 10 mL stainless steel milling jar. After adding two 7 mm stainless steel milling balls, the reaction mixture was milled for 90 min in a Retsch MM400 mill at 30 Hz. The resulting AuNPs are readily resuspended in a small amount of toluene (5 mL g−1) which allows their transfer, removal of any ligand excess, as well as magnetic separation of any iron shavings produced by the wear of the milling assembly. Based on previous studies, temperature of the jar after 90 min milling is evaluated to be between 33 to 35 °C. |
| This journal is © The Royal Society of Chemistry 2014 |