Bioinspired stiff and flexible composites of nanocellulose-reinforced amorphous CaCO3†
Tsuguyuki
Saito
a,
Yuya
Oaki
a,
Tatsuya
Nishimura
a,
Akira
Isogai
b and
Takashi
Kato
*a
aDepartment of Chemistry and Biotechnology, School of Engineering, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: kato@chiral.t.u-tokyo.ac.jp
bDepartment of Biomaterials Science, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan
First published on 21st January 2014
Abstract
Stiff yet flexible and strong transparent film materials mimicking the structure of crustacean exoskeletons were obtained from calcium carbonate and cellulose. These biobased materials have the composite structure of a three-dimensional nanoscale network of surface-carboxylated crystalline cellulose incorporated into an amorphous inorganic-based hybrid matrix of nanosegregated calcium carbonate and acidic polymers.
The production of innovative materials using renewable and abundant bioresources is an important challenge in materials science. Calcium carbonate and cellulose are accumulated in nature by fixing large amounts of CO2 from the atmosphere, and mass-consumed in our daily life, for instance in composite form as paper. However, it is not easy to develop such bioresources into innovative materials. In nature, various living organisms build up sophisticated and robust structures, using calcium carbonate or cellulose, to support and protect their own structures.1,2 If we control the micro- and nano-scale structures of synthetic bulk composite materials based on calcium carbonate and cellulose, structurally new green materials can be developed.
Biomimetic syntheses of calcium carbonate/organic hybrid materials have attracted much attention.3–10 For example, the synthesis of amorphous calcium carbonate (ACC)-based materials has recently been achieved.9,10 We found that transparent bulk ACC was obtained by drying colloidal precursors of ACC stabilized significantly with poly(acrylic acid) (PAA).9 This bulk material has hybrid structures of nanosegregated ACC and PAA. Transparent thin films supported by a solid substrate are formed by spreading and drying the ACC/PAA colloidal precursors onto the substrate. Recently Gebauer et al. reported a transparent ACC-based coating film that has nanoporous structures consisting of spherical ACC and short whisker-shaped cellulose with length ∼100 nm.10 In both cases,9,10 the preparation of free-standing tough films was not reported. Our intention is to use longer cellulose nanofibers with lengths of more than several micrometers as reinforcing 3D networks and PAA as a stabilizer for ACC colloids to obtain free-standing films that are mechanically tough and flexible. Cellulose nanofibrils have recently been used as fibrous reinforcements in nanocomposite materials and synthetic supports in functional materials.11–14
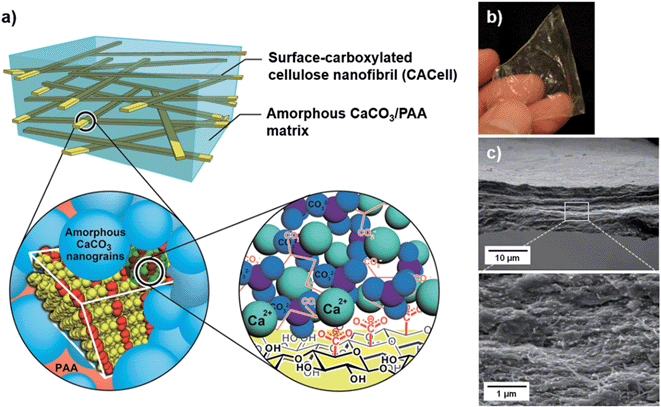
In the present study, we have developed mechanically strong, transparent and flexible ACC/PAA free-standing composite films reinforced with nanofibrillar cellulose. This composite structure mimics the organic/inorganic nanocomposite structures of crustacean exoskeletons (Fig. 1a). In the crustacean exoskeletons, the ACC component is bound to chitin nanofibrils at the interface through the acidic chitin-binding peptides.15,16 In our materials design, the reinforcing fibrils of carboxylated cellulose capable of interacting with Ca2+ are directly bound to ACC, which strengthens the interface of the fibrils and ACC. PAA has two functions:4 stabilization of the ACC and binding to both chitin and ACC, which further strengthen the interface.
A three-dimensional network of surface-carboxylated cellulose nanofibrils (CACell) was used for the preparation of composites (ESI, Fig. S1†).17 CACell/ACC/PAA composites (Fig. 1b) were obtained by percolating the colloidal precursors of ACC/PAA into the hydrogel sheet of CACell and then drying it. Fig. 1c presents scanning electron microscopy (SEM) images of the composite cross-sections of the network of cellulose nanofibrils densely filled with matrix-like ACC/PAA (see also ESI, Fig. S2 and S3†).
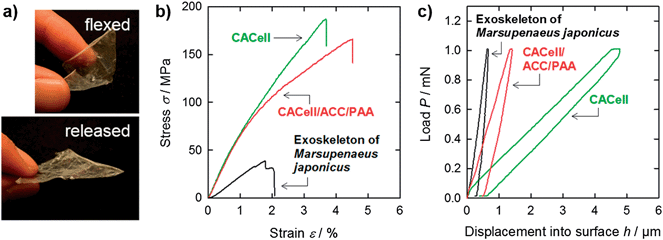
It is significant that stiff films of the synthetic CACell/ACC/PAA composites exhibit elastic flexibility similar to those of natural crustacean exoskeletons (Fig. 2a); a flexed film of the CACell/ACC/PAA composite quickly reverts to its original flat state after removing the flexural loading, even for a thin film thickness of about 10 μm. The unique composite structure of crystalline organic fibres (cellulose or chitin) and an amorphous inorganic-based matrix (ACC/PAA or ACC/protein) may induce the elastic flexibility of CACell/ACC/PAA composites and crustacean exoskeletons.
The tensile strength and Young's modulus of the CACell/ACC/PAA composites are 169 MPa and 7.3 GPa, respectively, as shown in Fig. 2b (red line); these values are several times higher than those of crustacean exoskeletons (Fig. 2b, black line and Table 1).18 As the crustacean exoskeletons, we used carapaces of Marsupenaeus japonicus, a type of decapod. The strength values of widely used polymeric films, such as polystyrene, polypropylene, polyethylene, poly(ethylene terephthalate), and poly(methyl methacrylate), are of the order of 10 MPa in most cases.19,20 The high mechanical strength of the CACell/ACC/PAA composites is based on that of cellulose nanofibrils. Neat CACell films have a tensile strength and Young's modulus of 195 MPa and 7.0 GPa, respectively (Fig. 2b, green line). These mechanical properties of neat CACell films are in the same range as those of the CACell/ACC/PAA composites. Furthermore, yield strength values, i.e., the stress at which a material begins to deform plastically, of the CACell/ACC/PAA composite and neat CACell films are almost the same: 77 MPa and 79 MPa, respectively (Fig. 2b). In the case of composite materials composed of polymer matrices and inorganic fillers,21,22 the yield strength of the polymer matrices often decreases on addition of rigid inorganic particles as a result of debonding of the particles from the matrices. The results for yield strength indicate that the ACC/PAA matrix binds the CACell (Fig. 1a) sufficiently to transfer the tensile loading.
| Density (g cm−3) | Moisture (%) | Tensile strength σ (MPa) | Young's modulus E (GPa) | Indentation hardness H (MPa) | Indentation modulus E (GPa) | |
|---|---|---|---|---|---|---|
| CACell | 1.1 | 9 | 195 ± 14 | 7.0 ± 1.2 | 2 ± 0.3 | 0.07 ± 0.02 |
| CACell/ACC/PAA | 1.8 | 17 | 169 ± 33 | 7.3 ± 0.5 | 65 ± 6 | 0.31 ± 0.03 |
| Exoskeleton of Marsupenaeus japonicas (chitin/ACC/protein) | 1.9 | 14 | 42 ± 9 | 2.9 ± 0.2 | 172 ± 23 | 1.87 ± 0.41 |
In indentation hardness tests, the CACell/ACC/PAA composites (Fig. 2c, red line) exhibit hardness over 30 times higher than that of neat CACell films (Fig. 2c, green line, and Table 1). This is a significant improvement in mechanical properties, achieved by forming composites with the ACC/PAA matrix. However, the indentation hardness of CACell/ACC/PAA composites is about one third that of crustacean exoskeletons (Fig. 2c, black line).23 Decapod crustaceans are equipped with a thin layer of calcium carbonate crystals at the outer surfaces of their carapaces, which results in the induction of hardness.24
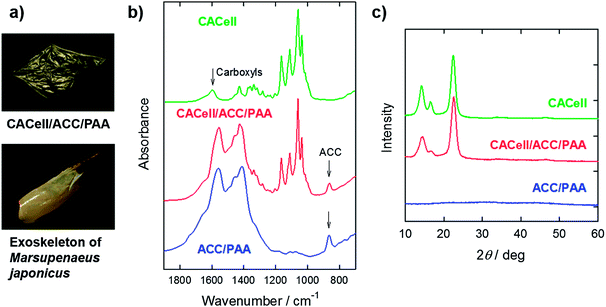
The carapaces of the decapod crustacean M. japonicus are optically transparent in a fresh and wet state (ESI, Fig. S4†). However, they inevitably become white and optically opaque during storage and drying processes (Fig. 3a) as a result of crystallization of unstable ACC or grain formation in the carapaces.25 In contrast, our synthetic composites prepared in the present study are transparent after drying. The Fourier-transform infrared (FTIR) spectrum of the CACell/ACC/PAA composites shows that the calcium carbonate component in the dried composites is amorphous (Fig. 3b, red line); the broad peak at 866 cm−1, characteristic of ACC, appears in the spectrum of the composite.9,26 The X-ray diffraction (XRD) pattern of the CACell/ACC/PAA composites presents no diffraction peaks of crystalline calcium carbonate (Fig. 3c). Moreover, the amorphous phase of the calcium carbonate component is preserved for at least one year under atmospheric conditions, and the composites remain transparent (ESI, Fig. S5†). These results show that the ACC components in our synthetic composites are stable.
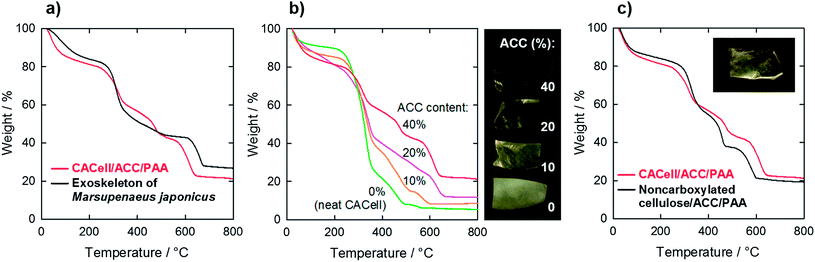
A thermogravimetric (TG) curve of the CACell/ACC/PAA composites shows a four-step weight-loss process (Fig. 4a, red line). Dehydration occurs first, and then cellulose, PAA, and calcium carbonate decompose at around 300 °C, 500 °C, and 600 °C, respectively.9 This TG curve is very similar in shape to that of the decapod carapaces (Fig. 4a, black line). In the case of the decapods, chitin, proteins, and calcium carbonate decompose in sequence.27 They consist of almost equal amounts by weight of organic and inorganic components,28 and are hard yet flexible and elastic. The claw and finger parts of the decapod exoskeletons contain more inorganic components, and are distinctly rigid and heavy. Our synthetic composites, CACell/ACC/PAA reported here, have a ratio of organic (cellulose and PAA) to inorganic (ACC) components which is the same as that of the decapod carapaces, i.e., a one-to-one ratio by weight. However, the CACell/ACC/PAA composites with smaller ratios of inorganic ACC become optically opaque (Fig. 4b). Composites of non-carboxylated cellulose and ACC/PAA are also opaque even if the ratios of organic to inorganic components are roughly one to one (Fig. 4c, and ESI, Fig. S6†). These properties are probably caused by voids forming inside the composites, in the former case because of lack of the ACC/PAA matrix to fill the network of cellulose nanofibrils and in the latter case as a result of insufficient adhesion between the non-carboxylated cellulose and the ACC/PAA matrix.
Conclusions
In summary, we have developed environmentally friendly nanocomposite films based on calcium carbonate and cellulose, both of which are CO2-fixing resources in nature. These biobased organic/inorganic composites are transparent and relatively hard, but flexible and strong. These unique characteristics cannot be obtained by simple mixing of cellulose and calcium carbonate. These transparent films mimicking the nanocomposite structures of crustacean exoskeletons show significant mechanical properties. They are promising for a range of applications such as protective packaging and in flexible display panels with impact resistance and shape persistency.Acknowledgements
This study was partially supported by a Grant-in-aid for Scientific Research (no. 22107003) on Innovative Areas of “Fusion Materials: Creative Development of Materials and Exploration of their Function through Molecular Control” (Area no. 2206) (T.K.), Scientific Research S (no. 21228007) (A.I.), and Encouragement of Young Scientists A (no. 23688020) (T.S.) from the Ministry of Education, Culture, Sports, Science and Technology.Notes and references
- J. W. C. Dunlop and P. Fratzl, Annu. Rev. Mater. Res., 2010, 40, 1 CrossRef CAS.
- M. A. Meyers, P.-Y. Chen, A. Y.-M. Lin and Y. Seki, Prog. Mater. Sci., 2008, 53, 1 CrossRef CAS PubMed.
- F. C. Meldrum and H. Cölfen, Chem. Rev., 2008, 108, 4332 CrossRef CAS PubMed.
- T. Kato, T. Sakamoto and T. Nishimura, MRS Bull., 2010, 35, 127 CrossRef CAS.
- J. Aizenberg, A. Tkachenko, S. Weiner, L. Addadi and G. Hendler, Nature, 2001, 412, 819 CrossRef CAS PubMed.
- E. M. Pouget, P. H. H. Bomans, J. A. C. M. Goos, P. M. Frederik, G. de With and N. A. J. M. Sommerdijk, Science, 2009, 323, 1455 CrossRef CAS PubMed.
- A. Sugawara, T. Nishimura, Y. Yamamoto, H. Inoue, H. Nagasawa and T. Kato, Angew. Chem., Int. Ed., 2006, 45, 2876 CrossRef CAS PubMed.
- T. Sakamoto, Y. Nishimura, T. Nishimura and T. Kato, Angew. Chem., Int. Ed., 2011, 50, 5856 CrossRef CAS PubMed.
- Y. Oaki, S. Kajiyama, T. Nishimura, H. Imai and T. Kato, Adv. Mater., 2008, 20, 3633 CrossRef CAS.
- D. Gebauer, V. Oliynyk, M. Salajkova, J. Sort, Q. Zhou, L. Bergström and G. Salazar-Alvarez, Nanoscale, 2011, 3, 3563 RSC.
- J. R. Capadona, K. Shanmuganathan, D. J. Tyler, S. J. Rowan and C. Weder, Science, 2008, 319, 1370 CrossRef CAS PubMed.
- P. Laaksonen, A. Walther, J.-M. Malho, M. Kainlauri, O. Ikkala and M. B. Linder, Angew. Chem., Int. Ed., 2011, 50, 8688 CrossRef CAS PubMed.
- R. T. Olsson, M. A. S. Azizi Samir, G. Salazar-Alvarez, L. Belova, V. Ström, L. A. Berglund, O. Ikkala, J. Nogués and U. W. Gedde, Nat. Nanotechnol., 2010, 7, 584 CrossRef PubMed.
- C. Schütz, J. Sort, Z. Bacsik, V. Oliynyk, E. Pellicer, A. Fall, L. Wågberg, L. Berglund, L. Bergström and G. Salazar-Alvarez, PLoS One, 2012, 7, e45828 Search PubMed.
- H. Inoue, N. Ozaki and H. Nagasawa, Biosci., Biotechnol., Biochem., 2001, 65, 1840 CrossRef CAS.
- A. Sato, S. Nagasaka, K. Furihata, S. Nagata, I. Arai, K. Saruwatari, T. Kogure, S. Sakuda and H. Nagasawa, Nat. Chem. Biol., 2011, 7, 197 CrossRef CAS PubMed.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71 RSC.
- C. Sachs, H. Fabritius and D. Raabe, J. Struct. Biol., 2006, 155, 409 CrossRef CAS PubMed.
- N. A. Fleck, V. S. Deshpande and M. F. Ashby, Proc. R. Soc. A, 2010, 466, 2495 CrossRef CAS.
- M. F. Ashby, Acta Metall. Mater., 1993, 41, 1313 CrossRef CAS.
- W. C. J. Zuiderduin, C. Westzaan, L. Huétink and R. J. Gaymans, Polymer, 2003, 44, 261 CrossRef CAS.
- P. Podsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. Sup Shim, J. Xu, H. Nandivada, B. G. Pumplin, J. Lahann, A. Ramamoorthy and N. A. Kotov, Science, 2007, 318, 80 CrossRef CAS PubMed.
- D. Raabe, C. Sachs and P. Romano, Acta Mater., 2005, 53, 4281 CrossRef CAS PubMed.
- A. Al-Sawalmih, C. Li, S. Siegel, H. Fabritius, S. Yi, D. Raabe, P. Fratzl and O. Paris, Adv. Funct. Mater., 2008, 18, 3307 CrossRef CAS.
- A. Mikkelsen, S. B. Engelsen, H. C. B. Hansen, O. Larsen and L. H. Skibsted, J. Cryst. Growth, 1997, 177, 125 CrossRef CAS.
- L. Addadi, S. Raz and S. Weiner, Adv. Mater., 2003, 15, 959 CrossRef CAS.
- L. G. Alonso and D. O. Vega, J. Therm. Anal., 1990, 36, 1347 CrossRef.
- F. Boßelmann, P. Romano, H. Fabritius, D. Raabe and M. Epple, Thermochim. Acta, 2007, 463, 65 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of sample preparation and characterization and supplemental figures for structures of the CACell/ACC/PAA composites. See DOI: 10.1039/c3mh00134b |
| This journal is © The Royal Society of Chemistry 2014 |