Finding function and form
Dirk
Trauner
Department of Chemistry, University of Munich, Butenandtstr. 5-13 (F4.086), D-81377 Munich, Germany. E-mail: dirk.trauner@cup.uni-muenchen.de
Nevertheless, the field is currently under fire, at least in societies that have traditionally supported it. On one hand, the total synthesis of natural products is portrayed as a routine operation that always results in success, provided enough time and money is spent. On the other hand, its ability to support biological investigations or provide access to drugs is called into question. It seems that as synthetic chemists we are caught between a rock and a hard place. Due to criticism and misconceptions such as these, total synthesis has become more difficult to justify to funding agencies and colleagues from other walks of science. The day when this fact can be safely ignored, as not to appear defensive, has long passed.
I think that this criticism will ebb and total synthesis will continue to thrive if chemists are able to deliver molecules - and deliver functions - without denying the intellectual challenges that come along with both. In this issue of Natural Product Reports, Baran (DOI: 10.1039/C3NP70090A) and Mulzer (DOI: 10.1039/C3NP70105K) argue that natural products can indeed be provided on useful scales if certain conditions are met. One of these is the development of better reactions, i.e., reactions that not only allow for new strategic disconnections but are also more economic, safer, and environmentally friendly. Carreira (DOI: 10.1039/C3NP70084D), Inoue (DOI: 10.1039/C3NP70103D), Kirschning (DOI: 10.1039/C3NP70108E), Krische (DOI: 10.1039/C3NP70076C), Menche (DOI: 10.1039/C3NP70093C), Nay (DOI: 10.1039/C3NP70077A), as well as Luo and Yang (DOI: 10.1039/C3NP70075E), provide examples of such powerful reactions and their strategic use in their respective reviews.
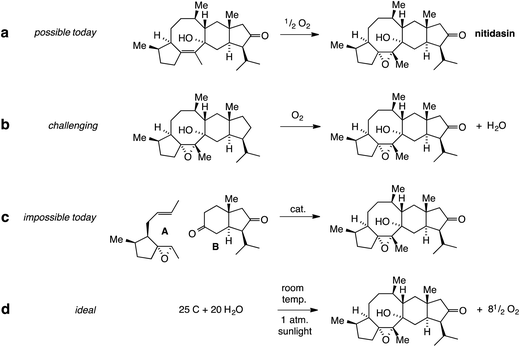
Indeed, the total synthesis of complex natural products has always provoked chemists to invent new reactions or at least think about them in conceptual terms. This is illustrated in Fig. 1, using the complex sesterterpenoid nitidasin as an example. The epoxidation of a double bond in the final stage of its synthesis (Fig. 1a) seems straightforward with the modern repertoire of selective reactions. On the other hand, the transformation shown in Fig. 1b is a much tougher challenge. Today, the discrimination between a methylene group and 37 other C,H-bonds, might be achieved using microbial oxidation but would be hard to do using small chemical reagents and catalysts. It is something that one can and should seriously think about.
Conceptually, one could take this a step further. The addition of epoxy alkene A to diketone B in Fig. 1c would enable a highly convergent total synthesis of nitidasin but is hardly realistic with today's methodology. The same is true for the “ideal synthesis” shown in Fig. 1d. While it can be written out as a balanced equation, it is unclear how such a transformation could be achieved and it remains a very distant dream. Paul Wender (DOI: 10.1039/C4NP00013G) would argue in his Highlight that this should not prevent us from striving toward this ideal and use it as a measure for the enormous challenges left in total synthesis.
In addition to inventing more powerful and economic reactions, syntheses must be continuously improved at a strategic level. Much of the criticism that has been levied at total synthesis stems from the misconception that it often amounts to a “mere stringing of known reaction steps”.2 In my opinion, a known reaction is one that has been published with specific substrates under specific conditions. This does not mean that it will also work on a more complex substrate that merely bears an analogous functional group. The literature is replete with cases where very well established reactions, such as Diels–Alder cycloadditions, ring-closing olefin metatheses, or even hydrogenations, have failed or given unexpected results.3 Of course, the status of reactions described for simple substrates is immediately elevated when they are shown to work with complex ones, where selectivity becomes a primary concern and steric hindrance rears its ugly head.
But, even if reactions were general and their success could be predicted with a high level of confidence, their successful orchestration would still be a huge intellectual achievement. A total synthesis is not a compilation of known steps, just as The Night Watch is not a pile of known pigments, Madame Bovary is not a sequence of known words, and Beethoven's Opus 111 is not a string of known notes (Fig. 2). How the colors are layered and how they interact with each other, how the words and phrases are aligned, and how individual sounds are arranged does matter! The same is true for total synthesis, where reactions simply cannot be applied in random order. Baran (DOI: 10.1039/C3NP70090A), Cossy (DOI: 10.1039/C3NP70062C), Dixon (DOI: 10.1039/C3NP70115H), and Mulzer (DOI: 10.1039/C3NP70105K) illustrate this point in their respective reviews and provide examples for the beautiful orchestration of known reaction steps.
Unfortunately, while certain guidelines exist, synthetic orchestration does not follow simple rules that can be easily transferred from one target molecule to the next, like a known reaction can be transferred from one substrate to the other. One of the reasons for this is that targets are simply too diverse. Synthetic strategies that are important in the total synthesis of, say, macrolides, are rarely relevant to the synthesis of polycyclic alkaloids or the total chemical synthesis of glycoproteins. Like protein folding, retrosynthetic analysis is too complex to follow manageable algorithms based on first principles. Indeed, attempts to program machines to orchestrate synthetic pathways have been less successful than originally hoped for. There is not a single example where a synthetic sequence suggested by a machine has been better than the result of human creativity. In a time where computers routinely beat chess grandmasters and robots have become competitive in table tennis, this is both disappointing and reassuring.
If we stopped training our students in the art and science of total synthesis, our ability to deliver molecules would quickly fade away and science as a whole would suffer. Gratifyingly, highly talented students are still attracted to this field - and for a good reason. In our curricula, courses on synthetic design are often the point where the intellectual fabric underlying organic chemistry becomes fully apparent. It is where our students realize that synthetic organic chemistry does not amount to cramming vocabulary, but also contains beautiful grammar and syntax. It gets even better when a different language, viz. biosynthesis, is introduced and compared with how we assemble natural products in the laboratory. Very quickly, students realize that these are really only two dialects of the universal language of chemistry, that innumerable equivalences exist, and that both can be used to (literally) express the molecules that we want.
Finally, synthetic chemists are in a unique position to define those very molecules that we want. In future years, the emphasis of synthetic chemistry, also natural product synthesis, will shift from delivering structures to delivering functions. Both go hand in hand and should ideally be done by the same person or team, since, to paraphrase Richard Feynman, “a molecule that I cannot make I do not understand”. As we make complex natural products, we better understand their conformational characteristics, biosynthetic connections, and reactivities and we learn where and how to modify them. These modifications could be structural simplifications that dramatically cut the synthetic effort needed or structural extensions, for example to increase binding affinity, attach a fluorophore or an affinity label. Chemists that are steeped in the philosophy of total synthesis will play a central role in these efforts. As this special issue of Natural Product Reports shows, there is no lack of good chemists to provide the molecules needed for progress in science and society!
References:
- P. Wang, S. Dong, J.-H. Shieh, S. Peguero, R. Hendrickson, M. A. S. Moore and S. J. Danishefsky, Science, 2013, 342, 1357 CrossRef CAS PubMed.
- R. Huisgen, The Adventure Playground of Mechanisms and Novel Reactions, in Profiles, Pathways and Dreams, ed. J. I. Seaman, American Chemical Society, 1994 Search PubMed.
- For an instructive example, see: M.-P. Benson, G. Collin, J. O'Neil, B. Fasching, M. B. D. Fenster, C. Godbout, K. Radkowski, R. Goddard and A. Fürstner, Angew. Chem., Int. Ed., 2009, 48, 9946 CrossRef PubMed and 9940.
| This journal is © The Royal Society of Chemistry 2014 |