Nano dispersion of 3D Cd(II) coordination polymer: synthetic blood plasma anticoagulant†
Darsi
Rambabu
,
Chullikkattil P.
Pradeep
and
Abhimanew
Dhir
*
School of Basic Sciences, Indian Institute of Technology, Mandi-175001, Himachal Pradesh, India. E-mail: abhimanew@iitmandi.ac.in; Fax: +91-01905-237924; Tel: +91-01905-237912
First published on 23rd January 2014
Abstract
A new 2,6-naphthalene dicarboxylate and quinoline based Cd(II) coordination polymer {[Cd(NDC)(QN)]}n (RAM 1) is designed and synthesized. The nano dispersion of RAM 1 behaves as an artificial blood plasma anticoagulant.
Exposure of thromboplastin to the endothelium upon injury initiates coagulation and leads to the formation of blood clots on the injured surface.1 However, the inhibition of blood coagulation to prevent mis-localized clotting of blood is another significant physiological process in human beings.2 This aim is accomplished by a number of circulating inhibitors within the serum which restrain the effect of thromboplastin. The Tissue Factor Pathway Inhibitor (TFPI, also known as lipoprotein associated coagulation inhibitor) plays a key role in the inhibition of thromboplastin.3 TFPI consists of full length truncated carboxyl groups4 and hydrophobic domains responsible for blood clotting inhibitory activity. However any malfunctioning of TFPI may cause disorders like deep vein thrombosis, pulmonary embolism, myocardial infarction and strokes.2
A group of pharmaceuticals called anticoagulants which prevent blood coagulation have been developed as medication for such disorders. Clinically used anticoagulant drugs have been approved which aim to reduce thromboplastin activity.5 Although these drugs have been successful in treating many thrombotic disorders, anticoagulation therapy continues to suffer from bleeding risks, a narrow therapeutic index, and other drug-specific adverse effects, which highlight the critical need to develop agents with better therapeutic profiles. Therefore researchers are making continuous efforts for the development of new synthetic anticoagulants based on organic molecules. Recently, Pinto et al. reported new organic motifs as synthetic anticoagulants.6 As the synthesis of sophisticated organic molecules requires arduous synthetic procedures and high costs, we aimed to develop an organic–inorganic hybrid system which is easy to synthesize, low cost and can act as a synthetic anticoagulant.
Among the various organic motifs utilized for biological purposes, we identified that naphthalene and quinoline have shown potential applications. Recently, naphthalene and quinoline derivatives alone and as conjugates have shown good antimycobacterial activity against mycobacterium tuberculosis H37Rv.7 Minarini et al. reported naphthalene diimides as anticancer agents.8 For several decades, quinoline and its derivatives have been investigated as antimalarial agents.9 Among the various metal ions used for bio-applications, cadmium is one of the least explored. It has been considered as environmentally hazardous and its effects upon long term exposure are substantially controversial. Cadmium poisoning due to continual consumption through food and water has been reported.10 However, Zhang et al. recently reported that cadmium, in its complexed state, exerts a favorable anti-tumor effect in breast cancer cells.11 Similarly, Andelkovic et al. reported the anti proliferative activity of new cadmium complexes with an IC50 value of less than 10 μM for all cell lines.12 Therefore it is likely that cadmium may exert a paradoxical effect and its effects perhaps depend upon the form it exists in. In the complexed state it may exert a favorable effect.11,12
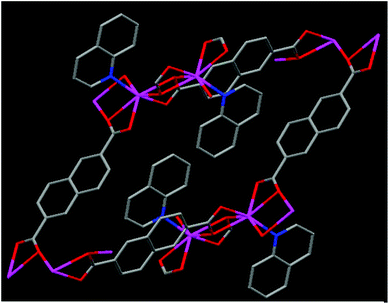
Our research aims to develop new organic, inorganic and hybrid systems for novel applications.13 In the present manuscript, keeping in view the chemical features of TFPI, the need for new anticoagulants, the potential uses of naphthalene and quinoline moieties and the possibility of the role of cadmium ions in their complexed state for biological applications, we designed and synthesized a new Cd(II) 3D coordination polymer {[Cd(NDC)(QN)]}n (specified as RAM 1) (Fig. 1) utilizing 2,6-naphthalene dicarboxylate (NDC) and quinoline (QN) as ligands (for experimental details see ESI S3†) and proved its potential as a synthetic anticoagulant (vide infra). To the best of our knowledge the use of a synthetic organic–inorganic hybrid system as an anticoagulant is unprecedented.
The single crystal X-ray diffraction14 studies of RAM 1 revealed that it crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (for X-ray details see ESI S4 and S5†). The asymmetric unit contains one Cd(II) ion, 2 halves of the NDC ligand and one quinoline moiety (see ESI S6†). The Cd(II) centers in RAM 1 are 7 coordinated, bonding to six oxygen atoms from four NDC ligands and one N atom from a quinoline ligand (see ESI S7†). The carboxylate units of the NDC ligands are in a tridentate coordination mode bridging two Cd(II) ions. This tridentate bridging coordination mode through the carboxylate oxygen atoms O3 and O3#1 leads to the formation of a centro-symmetric four membered [Cd2(COO)4] ring in the crystal lattice (see ESI S7†). The Cd–Cd distance in this dimeric [Cd2(COO)4] unit is 4.0574(25) Å. The Cd–O bond distances vary in the range of 2.262–2.588 Å, which are comparable to those reported in similar systems.15 The Cd–N bond distance is 2.266(5) Å. The dimeric [Cd2(COO)4] units are inter-connected through the bridging carboxylic oxygens O2 and O2#3 and extend in a chain fashion in the crystal lattice (see ESI S6 and S7†). Four NDC ligands are disposed in four different directions around the dimeric [Cd2(COO)4] units (see ESI S8†). The coordinated quinoline moieties are arranged centro-symmetrically on adjacent Cd(II) centers in an alternate fashion (Fig. 1). The NDC ligands arranged around the chains formed from the [Cd2(COO)4] units further connect each chain with four of its neighboring chains three dimensionally, leading to the formation of a 3D framework in the crystal lattice. The coordinated quinoline moieties occupy the space created by the framework structure (see ESI S9†). Thermal gravimetric analysis performed on RAM 1 reveals that it is thermally stable (see ESI S10†) up to 314 °C. The first weight loss of 25.6% was observed in the range 314–386 °C, which corresponds to the loss of the quinoline moiety. Between 447 and 631 °C, a second weight loss of 53.8% corresponding to the 2,6-naphthalene dicarboxylate ligand (NDC) was observed. A CdO residue was left behind.
space group (for X-ray details see ESI S4 and S5†). The asymmetric unit contains one Cd(II) ion, 2 halves of the NDC ligand and one quinoline moiety (see ESI S6†). The Cd(II) centers in RAM 1 are 7 coordinated, bonding to six oxygen atoms from four NDC ligands and one N atom from a quinoline ligand (see ESI S7†). The carboxylate units of the NDC ligands are in a tridentate coordination mode bridging two Cd(II) ions. This tridentate bridging coordination mode through the carboxylate oxygen atoms O3 and O3#1 leads to the formation of a centro-symmetric four membered [Cd2(COO)4] ring in the crystal lattice (see ESI S7†). The Cd–Cd distance in this dimeric [Cd2(COO)4] unit is 4.0574(25) Å. The Cd–O bond distances vary in the range of 2.262–2.588 Å, which are comparable to those reported in similar systems.15 The Cd–N bond distance is 2.266(5) Å. The dimeric [Cd2(COO)4] units are inter-connected through the bridging carboxylic oxygens O2 and O2#3 and extend in a chain fashion in the crystal lattice (see ESI S6 and S7†). Four NDC ligands are disposed in four different directions around the dimeric [Cd2(COO)4] units (see ESI S8†). The coordinated quinoline moieties are arranged centro-symmetrically on adjacent Cd(II) centers in an alternate fashion (Fig. 1). The NDC ligands arranged around the chains formed from the [Cd2(COO)4] units further connect each chain with four of its neighboring chains three dimensionally, leading to the formation of a 3D framework in the crystal lattice. The coordinated quinoline moieties occupy the space created by the framework structure (see ESI S9†). Thermal gravimetric analysis performed on RAM 1 reveals that it is thermally stable (see ESI S10†) up to 314 °C. The first weight loss of 25.6% was observed in the range 314–386 °C, which corresponds to the loss of the quinoline moiety. Between 447 and 631 °C, a second weight loss of 53.8% corresponding to the 2,6-naphthalene dicarboxylate ligand (NDC) was observed. A CdO residue was left behind.
To evaluate the anticoagulant behavior of RAM 1, we dispersed crystals of RAM 1 (2 mg in 100 mL) in H2O at the physiological pH (7.4) of blood in the human body using a HEPES buffer (this dispersion of RAM 1 was named DRAM 1). Recently, Mukherjee et al. utilized the dispersion of their metal organic framework for sensing nitro-aromatics.16 The nature of the crystals of RAM 1 before and after dispersion in H2O (DRAM 1) was checked by using powder XRD and IR techniques. The results showed that RAM 1 does not undergo decomposition in water (see ESI S11 and S12,† respectively).
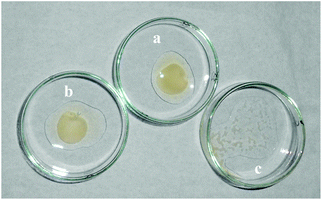
To evaluate the anticoagulation behavior of DRAM 1, a prothrombin time (PT) test was performed using blood plasma.17 The PT was observed by treating the plasma with a thromboplastin reagent (TP) (activated rabbit brain) in the absence and presence of 50 μL of DRAM 1. It was observed that the coagulation of plasma occurred in 18 seconds in the sample without DRAM 1 (Fig. 2a); on the contrary, no coagulation was observed in the sample containing DRAM 1 even after 24 h (Fig. 2c). We also carried out a blank experiment in which the thromboplastin reagent was added to the plasma in the presence of 50 μL of H2O (pH = 7.4, HEPES) where the coagulation occurred in 18 seconds (Fig. 2b). From these experiments, we concluded that DRAM 1 is interacting with the TP, inhibiting its activity and preventing coagulation. We also evaluated the anticoagulant effect of each component of RAM 1i.e. naphthalene dicarboxylate, quinoline and cadmium chloride. We observed that coagulation took place in the presence of each of these components independently which indicates that the anticoagulation property is the behavior of RAM 1 as a unit and not that of its independent components. We also carried out a fluorescence study of DRAM 1 with human serum albumin (HSA) with the same amount and under similar conditions as that with TP. We observed that there was no significant change in the fluorescence emission of DRAM 1 on addition of HSA (see ESI S14†).
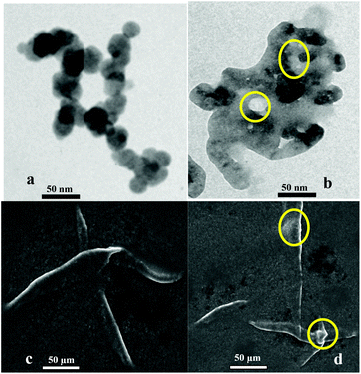
To obtain a deep insight into the binding behavior of DRAM 1 with TP, we carried out fluorescence and UV-vis studies on DRAM 1 with TP. DRAM 1 exhibited a significant fluorescence emission band at 370 nm when excited at 243 nm in H2O (pH = 7.4, HEPES) (Fig. 3) which may be ascribed to a chelation enhanced fluorescence (CHEF)18 effect as a result of the binding of cadmium with naphthalene and quinoline moieties. The λex at 243 was selected, as the λmax for DRAM 1 was observed at this wavelength in the absorption spectrum (see ESI S13†). On addition of 750 μL of TP, the fluorescence emission of DRAM 1 showed fluorescence quenching (Fig. 3). The UV-vis spectrum of DRAM 1 showed absorption bands at 243, 286, 297 and 313 nm in H2O (pH = 7.4, HEPES) (see ESI S13†). On addition of TP (215 μL), the band at 243 nm showed a significant increase in absorption intensity (see ESI S13†). The band at 286 nm started disappearing with the appearance of a new band at 274 nm, which gradually showed a blue shift to 269 nm on subsequent additions of TP (see ESI S13†). The bands at 297 nm and 313 nm also increased on consequent additions of TP. Therefore, changes in the fluorescence and UV-vis behavior of DRAM 1 confirm that there is an interaction between DRAM 1 and TP. Further, to investigate the mode of interaction, we carried out TEM and SEM studies on DRAM 1 in the presence and absence of TP. The TEM image of DRAM 1 showed nano-spheres attached to each other resulting in nano-fibrils, which are further attached to each other in an organized manner to form a network (Fig. 4a). In the presence of TP, the TEM of DRAM 1 showed irregular clusters in an unorganized pattern with significant bright areas (Fig. 4b).
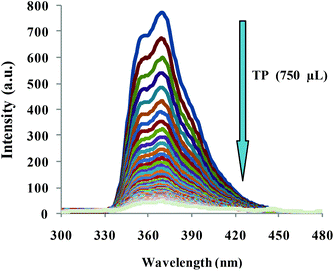 | ||
| Fig. 3 Fluorescence spectra of DRAM 1 on addition of 750 μL of TP in H2O (pH = 7.4, HEPES), λex = 243 nm. | ||
We propose that the inhibition of the activity of TP is due to its adsorption on the surface of DRAM 1. TP primarily contains proteins. Protein adsorption on solid surfaces is a well known phenomenon.19 Protein adsorption is a process driven by different protein-solid surface forces including van der Waals, hydrophobic and electrostatic forces regulated by parameters such as pH, temperature, ionic strength and the nature of the solvent.20 Thus, we ascribe the bright areas in the TEM images (Fig. 4b) as specific areas on the surface of DRAM 1 where TP adsorption has occurred. The interaction between TP and DRAM 1 was also observed with the help of SEM analysis. In the SEM analysis the surface of DRAM 1 showed fibrils (Fig. 4c) on which such bright areas where protein adsorption has taken place were clearly observed (Fig. 4d).
Thus, we conclude that the surface of DRAM 1 interacts with TP inhibiting its activity and hence acts as a synthetic blood plasma anticoagulant. Estimation of the anticoagulation behavior of similar analogues incorporating different metal ions is in progress. The discovery of such organic–inorganic hybrid nano-systems could be helpful in the development of new synthetic anti-coagulants at the commercial level and for their potential application in medical equipment such as blood transfusion bags and renal dialysis equipment.
AD acknowledges the Department of Science and Technology, India for the INSPIRE faculty award and AMRC IIT Mandi for laboratory facilities. DRB acknowledges the Council of Scientific and Industrial Research (CSIR) India for the research fellowship.
Notes and references
- B. Furie and B. C. Furie, J. Clin. Invest., 2005, 115, 3355 CrossRef CAS PubMed.
- R. W. Colman, V. J. Marder, A. W. Clowes, J. N. George and S. Z. Goldhaber, Hameostasis and Thrombosis: Basic principles and clinical practice, 2006, Lippincott Williams and Wilkins, Philadelphia, 5th edn, ISBN 0-7817-4996-4 Search PubMed.
- C. A. Sprecher, W. Kisiel, S. Mathewes and D. C. Foster, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 3353 CrossRef CAS.
- I. Warshawsky, G. Bu, A. Mast, J. E. Saffitz, G. J. Broze Jr. and A. L. Shwartz, J. Clin. Invest., 1995, 95, 1773 CrossRef CAS PubMed.
- (a) K. A. Bauer, New anticoagulants. Hematology (Am. Soc. Hematol., Educ. Program Book), 2006, p. 450 Search PubMed; (b) B. L. Henry and U. R. Desai, Anticoagulants, in Burger's Medicinal Chemistry, Drug Discovery and Development, ed. D. J. Abraham and D. P. Rotella, John Wiley, N. J. Hoboken, 7th edn, 2010, p. 365 Search PubMed.
- (a) M. Correia-da-Silva, E. Sousa, B. Duarte, F. Marques, F. Carvalho, F. Cunha-Ribeiro and M. M. M. Pinto, J. Med. Chem., 2011, 54, 5373 CrossRef CAS PubMed; (b) M. Correia-da-Silva, E. Sousa, B. Duarte, F. Marques, F. Carvalho, L. M. Cunha-Ribeiro and M. M. M. Pinto, J. Med. Chem., 2011, 54, 95 CrossRef CAS PubMed.
- R. S. Upadhayaya, J. K. Vandavasi, R. A. Kardile, S. V. Lahore, S. S. Dixit, H. S. Deokar, P. D. Shinde, M. P. Samah and J. Chattopadhyaya, Eur. J. Med. Chem., 2010, 45, 1854 CrossRef CAS PubMed.
- A. Milelli, V. Tumiatti, M. Micco, M. Rosini, G. Zuccari, L. Raffaghello, G. Bianchi, V. Pistoia, J. F. Díaz, B. Pera, C. Trigili, I. Barasoain, C. Musetti, M. Toniolo, C. Sissi, S. Alcaro, F. Moraca, M. Zini, C. Stefanelli and A. Minarini, Eur. J. Med. Chem., 2012, 57, 417 CrossRef CAS PubMed.
- (a) K. C. Rice, J. Med. Chem., 1976, 19, 887 CrossRef CAS PubMed; (b) T. J. Egan, R. Hunter, C. H. Kaschula, H. M. Marques, A. Misplon and J. Walden, J. Med. Chem., 2000, 43, 283 CrossRef CAS PubMed; (c) A. P. Gorka, A. C. de Dios and P. D. Roepe, J. Med. Chem., 2013, 56, 5231 CrossRef CAS PubMed.
- K. Nogawa, et al. , BioMetals, 2004, 17, 581 CrossRef CAS PubMed.
- Z. Zhang, C. Bi, D. Buac, Y. Fan, X. Zhang, J. Zuo, P. Zhang, N. Zhang, L. Dong and Q. P. Dou, J. Inorg. Biochem., 2013, 123, 1 CrossRef CAS PubMed.
- S. Bjelogrlic, T. Todorovic, A. Baachi, M. Zec, D. Sladic, T. Srdic-Rajic, D. Radanovic, S. Radulovic, G. Pelizzi and K. Andelkovic, J. Inorg. Biochem., 2010, 104, 673 CrossRef CAS PubMed.
- (a) M. Devi, A. Dhir and C. P. Pradeep, Dalton Trans., 2013, 42, 7514 RSC; (b) A. K. Gupta, A. Dhir and C. P. Pradeep, Dalton Trans., 2013, 42, 12819 RSC.
- Crystal data for RAM 1: C12H10N2O6, M = 278.22, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.0724(4) Å, b = 9.7915(4) Å, c = 14.0849(7) Å, α = 89.657(3), β = 77.662(4), γ = 82.933(3)°; U = 2040.2(4) Å3, Dc = 1.524 Mg m−3, Z = 2, F(000) = 576, λ = 0.71073 Å, μ = 0.125 mm−1, total/unique reflections = 10
, a = 9.0724(4) Å, b = 9.7915(4) Å, c = 14.0849(7) Å, α = 89.657(3), β = 77.662(4), γ = 82.933(3)°; U = 2040.2(4) Å3, Dc = 1.524 Mg m−3, Z = 2, F(000) = 576, λ = 0.71073 Å, μ = 0.125 mm−1, total/unique reflections = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904/4233 [R(int) = 0.0271], T = 150(2) K, θ range = 2.92 to 25.00ο, final R indices [I > 2σ(I)] R1 = 0.0374, wR2 = 0.0818, R indices (all data) R1 = 0.0594, wR2 = 0.0922. CCDC 923235 contains the supplementary crystallographic data for this paper.
904/4233 [R(int) = 0.0271], T = 150(2) K, θ range = 2.92 to 25.00ο, final R indices [I > 2σ(I)] R1 = 0.0374, wR2 = 0.0818, R indices (all data) R1 = 0.0594, wR2 = 0.0922. CCDC 923235 contains the supplementary crystallographic data for this paper. - H. A. Habib, A. Hoffmann, H. A. Hoppe and C. Janiak, Dalton Trans., 2009, 1742 RSC; D. P. Martin, R. M. Supkowski and R. L. LaDuca, Dalton Trans., 2009, 514 RSC.
- B. Gole, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 12137 RSC.
- World Health Organization, WHO technical report series no 889, 1999, annex 3, p. 64.
- J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780 CrossRef CAS PubMed.
- J. J. Ramsden, Chem. Soc. Rev., 1995, 24, 73 RSC.
- (a) J. D. Andrade, Surface and interfacial Aspects of Biomedical Polymers, Plenum Press, New York and London, 1985, pp. 10–21. ISBN 0-306-41742-1 Search PubMed; (b) A. Cooper, Conformational Fluctuation and Change in Biological Macromolecules, Science Progress, Oxford, 1980, vol. 66, 473 Search PubMed; (c) C. Tanford, The Hydrophobic Effect, Wiley, New York, 1981 Search PubMedJ. Porath, Pure Appl. Chem., 1979, 51, 1549 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 923235. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3qi00013c |
| This journal is © the Partner Organisations 2014 |