Pyridinium linkers and mixed anions in cationic metal–organic frameworks†
Jian-Bin
Lin
and
George K. H.
Shimizu
*
Department of Chemistry, University of Calgary, 2500 University Dr NW, Calgary AB T2N 1N4, Canada. E-mail: gshimizu@ucalgary.ca; Fax: +1 403 2899488; Tel: +1 403 2205347
First published on 7th March 2014
Abstract
Pyridinium linkers direct the formation of a cationic metal organic framework [Cu(ipq)]A (where H2ipq+ = 1-(3,5-dicarboxyphenyl)-4-(pyridin-4-yl)pyridinium, A− = NO3−, BF4−, BF4−/PF6−), CALF-32(A), where intra-pore anions can be varied and mixed to control porosity and gas uptake.
Metal organic frameworks (MOFs) are porous coordination polymers touted for their modular synthetic routes and options for systematic variation.1 MOFs as new solid sorbents could exhibit selective capture of CO2 and a low energy penalty for the release of the gas giving more efficient overall capture.2 Low partial pressure CO2 capture is enhanced by higher heats of adsorption. Metal ions with open sites3 or having active organic nitrogen sites4 elevate CO2–MOF interactions yielding higher heats of adsorption. Studies of charge-separated MOFs, where charged units other than the framework metals and ligating functional groups are incorporated into the linkers/pores, are much less common. Most of these efforts are focused on impregnation of secondary metal cations in the pores of anionic MOFs.5 While there are reports of anion exchange in MOFs,6 to our knowledge, there is no report on the doping of anions in a cationic framework for improving CO2 capture.
Pyridinium linkers are cationic with a highly electropositive quaternized nitrogen. When incorporated into a MOF, this charge could yield a framework with high affinity for the quadrupole of CO2 molecules.7 Moreover, the anions included in the pores could augment CO2 affinity by supplying additional polarizing sites or through stronger confinement effects. Here we report the design and synthesis of a MOF with cationic struts. The pyridinium-containing linker, 1-(3,5-dicarboxyphenyl)-4-(pyridin-4-yl)pyridinium hexafluoro-phosphate, (H2ipq)(PF6), was synthesized (Zincke reaction)8 and incorporated into the structurally characterized [Cu(ipq)]NO3·2DMF·2H2O, CALF-32(NO3) (CALF = Calgary Framework) and analogues with different included anions in the same parent framework. CALF-32(NO3) collapses upon activation but analogues with A− = BF4− and BF4−/PF6− combinations retain more open structures and demonstrate higher CO2 uptake and CO2 selectivity. Notably, CALF-32(BF4)0.6(PF6)0.4 has a surface area of almost 1000 m2 g−1.
The CALF-32 family ([Cu(ipq)]A·guest, A− = NO3−, BF4−, BF4−/PF6−) could be obtained solvothermally by reacting Cu(NO3)2 or Cu(BF4)2 with H2ipq+ in N,N-dimethylformamide (DMF) and ethanol at 80 °C for 12 h (see ESI†). CALF-32(NO3) crystallizes in the monoclinic space group P21/c, with one copper atom, one ipq− ligand, one nitrate anion, two DMF and two water molecules in the asymmetric unit. As shown in Fig. 1a, a pair of copper ions forms a paddlewheel unit with four carboxylate groups in the equatorial plane and two pyridyl nitrogen atoms from ipq− ligands in the axial sites. The overall structure exhibits a (3,6)-connected rtl topology considering the ligand and the paddlewheel cluster as three and six connecting nodes, respectively. The Cu complex of 5-(pyridin-4-yl)isophthalic acid (H2pip), the same linker as in the present study but with the pyridinium unit removed, has been reported and forms the same topology structure but with a neutral framework.9 In CALF-32(NO3), the NO3− anions are located in the large channel along the a-axis with O⋯H–C hydrogen bonding (O⋯C 3.3–3.6 Å) and electrostatic attractions between O and pyridinium N atoms (O⋯N 3.15(1) Å) (Fig. 1b). The NO3− anions partially separate the large channels into cages with a diameter of ca. 4.7 Å excluding vdW radii. These channels are further connected through 3.6 Å windows to give 3D pores (Fig. 1c and d). The solvent accessible volume of CALF-32(NO3) is 47% calculated by PLATON.10 Interestingly, although the synthesis had a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of NO3− and PF6−, the crystal structure shows exclusively nitrate anions in the pores, an observation further confirmed by 19F NMR of dissolved samples (Fig. S1a and b†). The guest solvent molecules were refined as DMF and water, and this was corroborated by the elemental analysis and thermogravimetric analysis (TGA) data. The TGA data show stability of CALF-32(NO3) to 210 °C after ca. 30% weight loss (Fig. S2†).
1 ratio of NO3− and PF6−, the crystal structure shows exclusively nitrate anions in the pores, an observation further confirmed by 19F NMR of dissolved samples (Fig. S1a and b†). The guest solvent molecules were refined as DMF and water, and this was corroborated by the elemental analysis and thermogravimetric analysis (TGA) data. The TGA data show stability of CALF-32(NO3) to 210 °C after ca. 30% weight loss (Fig. S2†).
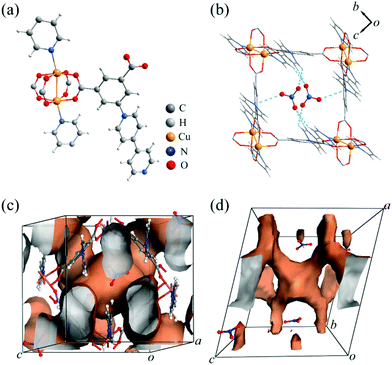 | ||
| Fig. 1 (a) Coordination environment, (b) embedding of NO3− anions in the channel view along the a-axis, 3D (c) porous channels and (d) accessible guest surface in CALF-32(NO3). | ||
Upon activation under a range of conditions (multiple solvent combinations and heating cycles but excluding supercritical CO2), CALF-32(NO3) was observed to lose crystallinity as verified by the powder X-ray diffraction (PXRD) (Fig. S3†) in all cases. N2 sorption at 77 K exhibited no uptake but CO2 sorption at 195 K showed type-I behaviour with a Langmuir surface area of only 118 m2 g−1 (Table S2 and Fig. S4†). This is attributed to the contraction of the framework after activation compared to the single-crystal structure.
To investigate the effect of the impregnated anions on the framework stability and gas sorption properties, the BF4− anion was introduced into the CALF-32 framework using Cu(BF4)2 and H2ipqBF4 as reactants. PXRD showed that the BF4− salt gave the isomorphous phase CALF-32(BF4) (Fig. S3†). TGA showed that CALF-32(BF4) was stable up to 240 °C, 30 °C higher than CALF-32(NO3) (Fig. S2†). While CALF-32(NO3) showed no N2 uptake, for CALF-32(BF4), the N2 sorption at 77 K showed a type-I isotherm with a Langmuir surface area of 248 m2 g−1 (Table S2 and Fig. S4†). PXRD showed the shift of the [100] peak to higher angle attributed to shrinkage of the a-axis (Fig. S3†). The loss of other peaks may be due to distortion of the framework and displacement of anions. The CO2 sorption isotherm measured at 195 K showed a two-step adsorption behavior with a 2nd step gate opening pressure at P/P0 = 0.028. This may be ascribed to the expansion of the framework at higher temperature compared with the N2 sorption.11 The Langmuir surface area calculated from the first step is 206 m2 g−1 and the total surface area is 408 m2 g−1 (Table S2 and Fig. S4†).
Based on the outcomes of the CALF-32(BF4) study, it was hypothesized that a larger anion could help prevent structural collapse and enhance porosity. Numerous attempts were made to prepare the isomorphous CALF-32(PF6) using Cu(PF6) and H2ipqPF6 but these invariably gave mixtures of different structures. However, by varying the reactant ratio of Cu(BF4)2 and H2ipqPF6 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, two CALF-32 analogues were obtained with mixed BF4−/PF6− anions. Solution 19F NMR on dissolved complexes reproducibly gave BF4−/PF6− ratios of 4
3, two CALF-32 analogues were obtained with mixed BF4−/PF6− anions. Solution 19F NMR on dissolved complexes reproducibly gave BF4−/PF6− ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by integrating the BF4− and PF6− peaks (Fig. S1d/e†) for the 1
2 by integrating the BF4− and PF6− peaks (Fig. S1d/e†) for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 metal/ligand ratios, respectively. Although the stabilities of CALF-32(BF4)0.8(PF6)0.2 and CALF-32(BF4)0.6(PF6)0.4 from TGA were similar to CALF-32(BF4) (Fig. S2†), they both showed improved gas sorption abilities. For CALF-32(BF4)0.8(PF6)0.2, the PXRD pattern was similar to the pure BF4− compound after activation (partial collapse) but N2 sorption at 77 K gave a type-I isotherm with a Langmuir surface area of 317 m2 g−1, 20% higher than the pure BF4− MOF (Table S2 and Fig. S4†). CO2 sorption at 195 K showed the same two-step pattern observed for the BF4− system with Langmuir surface areas 314 m2 g−1 and 600 m2 g−1 calculated from the first step and total sorption, respectively (Table S2 and Fig. S4†). The gate opening pressure of the 2nd step is P/P0 = 0.039.
3 metal/ligand ratios, respectively. Although the stabilities of CALF-32(BF4)0.8(PF6)0.2 and CALF-32(BF4)0.6(PF6)0.4 from TGA were similar to CALF-32(BF4) (Fig. S2†), they both showed improved gas sorption abilities. For CALF-32(BF4)0.8(PF6)0.2, the PXRD pattern was similar to the pure BF4− compound after activation (partial collapse) but N2 sorption at 77 K gave a type-I isotherm with a Langmuir surface area of 317 m2 g−1, 20% higher than the pure BF4− MOF (Table S2 and Fig. S4†). CO2 sorption at 195 K showed the same two-step pattern observed for the BF4− system with Langmuir surface areas 314 m2 g−1 and 600 m2 g−1 calculated from the first step and total sorption, respectively (Table S2 and Fig. S4†). The gate opening pressure of the 2nd step is P/P0 = 0.039.
For CALF-32(BF4)0.6(PF6)0.4, after activation, the PXRD pattern showed partial structural collapse but did retain some degree of crystallinity (Fig. S3†). Notably, the Langmuir surface area is much higher than those of the other three CALF-32 derivatives and reaches 955 m2 g−1 from N2 sorption at 77 K and 1186 m2 g−1 from CO2 sorption at 195 K. The pore volume calculated from N2 sorption at P/P0 = 0.95 is 0.35 cm3 g−1. This is the same value for the pore volume obtained from an in silico constructed model based on the single-crystal structure of CALF-32(NO3) and altering the NO3− anions to (BF4−)0.6(PF6−)0.4.
Comparing CO2 sorption at 273 K and 1 bar, the capacity of CALF-32(NO3) is only 3.4 wt% (0.8 mmol g−1). CALF-32(BF4), with the slightly higher Langmuir surface area, adsorbs almost double the CO2 of CALF-32(NO3) and reaches 6.6 wt% (1.5 mmol g−1) at 273 K and 1 bar. More interestingly, the doping of the PF6− anion significantly increases the CO2 uptake, which ranges from 8.8 wt% (2.0 mmol g−1) for CALF-32(BF4)0.8(PF6)0.2 to 21 wt% (4.8 mmol g−1) for CALF-32(BF4)0.6(PF6)0.4 at 273 K and 1 bar (Table S2 and Fig. S5†).
At 298 K and 1 bar, CALF-32(BF4)0.6(PF6)0.4 can store up to 15 wt% (3.4 mmol g−1) of CO2. This is lower than MOFs with open metal sites, such as MOF-74-M/CPO-27-M (24–36 wt% or 5.5–8.2 mmol g−1),3a but it is comparable to other high performing MOFs even functionalized with organic amines, such as CuBTTri-mmen (15.4 wt% or 3.5 mmol g−1),12 Bio-MOF-11 (15.2 wt% or 3.5 mmol g−1)4a and Zn2(ox)(atz)2 (14.3 wt% or 3.3 mmol g−1) at 298 K and 1 bar.4c,13 CALF-32(BF4)0.6(PF6)0.4 performs better than the neutral isostructural framework [Cu(pip)], which adsorbs 13.5 wt% (3.1 mmol g−1) of CO2 at 298 K and 1 bar with no anions in the pores.14
Regarding the partial collapse of the structures, the excellent CO2 adsorption in CALF-32(BF4)0.6(PF6)0.4 could be repeated more than once on the same sample indicating that, while partial collapse is occurring, the extent of the collapse is attenuated. Although none of the CALF-32 family could retain the exact framework without some degree of collapse, the PXRD and gas sorption experiments confirm that the doping of PF6− anions limits the contraction of the framework and significantly enhances porosity. Recently, outstanding CO2 capture ability has been demonstrated by Zaworotko, Eddaoudi et al., in a system in which SiF62− dianions play a key role in defining the pore character.15
The effect of incorporating pyridinium and anions to augment guest affinity is further confirmed by the CO2 adsorption enthalpies. The zero-coverage adsorption enthalpies were estimated from the CO2 sorption isotherms at 273 K and 298 K by using the virial equation. Obtained values ranged from 31 to 35 kJ mol−1 for the CALF-32 derivatives with the highest value for CALF-32(BF4)0.6(PF6)0.4 (Table S2 and Fig. S6/7†). For comparison, the CO2 adsorption enthalpy of neutral [Cu(pip)] is 28 kJ mol−1.14 The enhanced CO2 heat of adsorption and total uptake would benefit from the pyridinium in the framework and BF4−/PF6− anions in the pores.15 While many factors contribute to the isosteric heat of adsorption, and with the partial structural collapse precise assignment of these factors is not possible, the higher value is as expected based on the uptakes.
The amounts of N2 and CH4 adsorbed by the CALF-32 series are much lower than those of CO2. At 273 K and 1 bar, the N2 adsorption ranged from 0.1 wt% for CALF-32(NO3) to 0.8 wt% for CALF-32(BF4)0.6(PF6)0.4. For CH4 adsorption, CALF-32(BF4)0.6(PF6)0.4 adsorbs 2.1 wt% at 273 K and 1 bar, while the other three adsorb less than 0.5 wt% (Table S2 and Fig. S5†). The selectivities of CO2 over N2 and CH4 were estimated from ideal adsorbed solution theory (IAST)16 by fitting the isotherms with the Langmuir–Freundlich method (Fig. S8†). The N2 and CH4 mole fractions were chosen to be 0.87 and 0.5, respectively. As shown in Fig. 2 and Table S2,† the separation ratios of CO2/N2 and CO2/CH4 for CALF-32(NO3) are 63 and 19 at 273 K and 1 bar, significantly higher than those for [Cu(pip)] (26 and 5 for CO2/N2 and CO2/CH4, respectively).14 The selectivity of CO2 for CALF-32(BF4) is similar to the NO3 complex but dramatically increases to 157 for CO2/N2 and 47 for CO2/CH4 in CALF-32(BF4)0.8(PF6)0.2. These CO2 selectivities exhibited by CALF-32(BF4)0.8(PF6)0.2 are among the highest yet reported for a MOF either functionalized with open metal sites or amino groups.2a,15,17 Interestingly, the selectivities of CO2/N2 and CO2/CH4 for CALF-32(BF4)0.6(PF6)0.4 decrease to 98 and 14 at 273 K and 1 bar although this MOF has higher capacity for CO2.
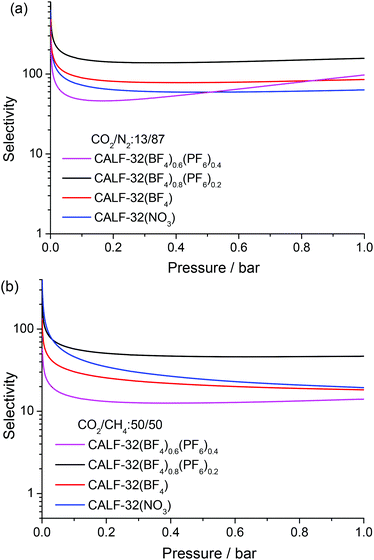 | ||
| Fig. 2 Selectivity for (a) CO2/N2: 13/87 and (b) CO2/CH4: 50/50 gas mixture in CALF-32(A) predicted by IAST at 273 K. | ||
We have reported a new MOF structure, CALF-32(A), incorporating cationic pyridinium units in the struts and different anions in the pores including mixtures of anions. Incorporation of larger anions into the pores is shown to augment the structural stability and the porosity. Most significantly, it was shown that mixed anion (BF4−/PF6−) species had by far the best performance for CO2 capture both in regard to capacity and selectivity. The isosteric heats of CO2 sorption in CALF-32(A) are increased to 31–35 kJ mol−1. Selectivities for gas capture are reported and CALF-32(BF4)0.8(PF6)0.2 seems to be the best for increasing the selectivity of CO2, while CALF-32(BF4)0.6(PF6)0.4 would be the best for uptake capacity at room temperature.
We acknowledge financial support of Carbon Management Canada.
Notes and references
- M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675 CrossRef CAS PubMed.
- (a) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed; (b) A. Samanta, A. Zhao, G. K. H. Shimizu, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 2012, 51, 1438 CrossRef CAS.
- (a) S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870 CrossRef CAS PubMed; (b) P. D. C. Dietzel, R. E. Johnsen, H. Fjellvag, S. Bordiga, E. Groppo, S. Chavan and R. Blom, Chem. Commun., 2008, 5125 RSC; (c) J. A. Mason, K. Sumida, Z. R. Herm, R. Krishna and J. R. Long, Energy Environ. Sci., 2011, 4, 3030 RSC.
- (a) J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 38 CrossRef CAS PubMed; (b) J.-B. Lin, J.-P. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2010, 132, 6654 CrossRef CAS PubMed; (c) R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Science, 2010, 330, 650 CrossRef CAS PubMed; (d) B. Zheng, J. Bai, J. Duan, L. Wojtas and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 133, 748 CrossRef CAS PubMed.
- H. J. Park and M. P. Suh, Chem. Sci., 2013, 4, 685 RSC.
- (a) X. Wang, Z. Wang and S. Gao, Sci. China: Chem., 2012, 55, 1055 CrossRef CAS; (b) J. Fu, H. Li, Y. Mu, H. Hou and Y. Fan, Chem. Commun., 2011, 47, 5271 RSC; (c) S. Wang, L. Li, J. Zhang, X. Yuan and C.-Y. Su, J. Mater. Chem., 2011, 21, 7098 RSC; (d) Q.-Y. Yang, K. Li, J. Luo, M. Pan and C.-Y. Su, Chem. Commun., 2011, 47, 4234 RSC; (e) L. Carlucci, G. Ciani, S. Maggini, D. M. Proserpio and M. Visconti, Chem.–Eur. J., 2010, 16, 12328 CrossRef CAS PubMed; (f) D. Liu, Q. Yang and C. Zhong, Mol. Simul., 2009, 35, 213 CrossRef CAS; (g) S. R. Halper, L. Do, J. R. Stork and S. M. Cohen, J. Am. Chem. Soc., 2006, 128, 15255 CrossRef CAS PubMed.
- M. Higuchi, D. Tanaka, S. Horike, H. Sakamoto, K. Nakamura, Y. Takashima, Y. Hijikata, N. Yanai, J. Kim, K. Kato, Y. Kubota, M. Takata and S. Kitagawa, J. Am. Chem. Soc., 2009, 131, 10336 CrossRef CAS PubMed.
- T. Zincke, G. Heuser and W. Möller, Ann. Chem., 1904, 333, 296 CrossRef.
- S. Xiang, J. Huang, L. Li, J. Zhang, L. Jiang, X. Kuang and C. Y. Su, Inorg. Chem., 2011, 50, 1743 CrossRef CAS PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
- H. J. Choi, M. Dinca and J. R. Long, J. Am. Chem. Soc., 2008, 130, 7848 CrossRef CAS PubMed.
- T. M. McDonald, D. M. D'Alessandro, R. Krishna and J. R. Long, Chem. Sci., 2011, 2, 2022 RSC.
- R. Vaidhyanathan, S. S. Iremonger, K. W. Dawson and G. K. H. Shimizu, Chem. Commun., 2009, 5230 RSC.
- L. Du, Z. Lu, K. Zheng, J. Wang, X. Zheng, Y. Pan, X. You and J. Bai, J. Am. Chem. Soc., 2012, 135, 562 CrossRef PubMed.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80 CrossRef CAS PubMed.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121 CrossRef CAS.
- K.-J. Chen, R.-B. Lin, P.-Q. Liao, C.-T. He, J.-B. Lin, W. Xue, Y.-B. Zhang, J.-P. Zhang and X.-M. Chen, Cryst. Growth Des., 2013, 13, 2118 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The additional experiment, crystallographic data, gas sorption and selective data, 19F NMR, TGA curves, PXRD, and sorption isotherms. CCDC 953575. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3qi00065f |
| This journal is © the Partner Organisations 2014 |
