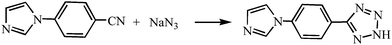Two luminescent Cu(I) coordination polymers based on the 1-(4-tetrazolephenyl)imidazole ligand for sensing of nitrobenzene†
Tian
Wen
,
De-Xiang
Zhang
,
Qing-Rong
Ding
,
Hua-Bin
Zhang
and
Jian
Zhang
*
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: zhj@fjirsm.ac.cn
First published on 7th May 2014
Abstract
Two Cu(I) coordination polymers based on the in situ generated 1-(4-tetrazolephenyl)imidazole ligand show intriguing ABW and (4,8)-connected topologies, and both of them are promising luminescent materials for sensing of nitrobenzene.
In the past few decades, metal–organic frameworks (MOFs) or coordination polymers (CPs) have attracted dramatic interest mainly owing to their aesthetic structures and potential applications as functional materials in many fields.1–3 As a special class, luminescent CPs are of particular interest due to their potential applications as chemical sensors,4 light-emitting devices,5 biomedical imaging agents,6etc.
From the viewpoint of crystal engineering, the fascinating architectures and functionalities of CPs are due to coordination modes of metal ions and ligand geometries. The rational design strategy of CPs based on how to choose metal ions and organic bridging ligands has received a great deal of attention.7 Up to now, many N-heterocyclic bridging ligands (e.g. imidazolate, triazolate, tetrazolate, etc.) have been documented to represent a unique class of building subunits.8 In our previous research, we found that CPs based on (n-pyridyl)tetrazolate ligands may have exceptional luminescence behavior.9 To enlarge the π-system of these (n-pyridyl)tetrazolate ligands, a new 1-(4-tetrazolephenyl)imidazole (= Htpi) ligand is considered to build new functional CPs. The rotation of two N-heterocyclic rings (tetrazole and imidazole) between the central benzene ring is expected to provide special structures and functions. To the best of our knowledge, such an unusual tpi ligand is for the first time designed and applied for the synthesis of CPs.
In this work, two three-dimensional Cu(I) CPs, [Cu(tpi)]n (1) and [Cu2I(tpi)]n (2), have been successfully synthesized and structurally characterized by single crystal X-ray diffraction.‡,§ They show intriguing ABW and (4,8)-connected topologies, respectively, and both of them are promising luminescent materials for sensing of nitrobenzene. The phase purity of 1 and 2 has been demonstrated by powder X-ray diffraction (PXRD) (Fig. S1–2†). The two compounds are stable in air and insoluble in common solvents (Fig. S3†).
The Htpi ligand was in situ synthesized via the “click” reaction between 1-(4-cyanophenyl)imidazole and NaN3 (Scheme 1). In situ ligand synthesis has been well established as a novel and effective way for the preparation of coordination compounds.10 Although a large number of tetrazolate-based coordination polymers have been reported via such an in situ click solvothermal synthesis,10,11 this Htpi ligand is for the first time in situ designed to construct new CPs. Moreover, compounds 1 and 2 could not be obtained by direct reactions of Cu(I) and Htpi.
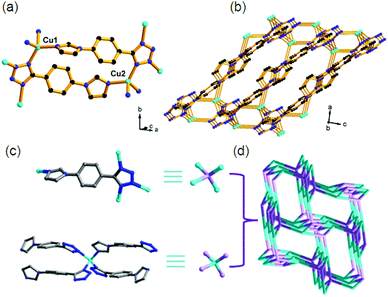
Compound 1 features a three-dimensional (3D) framework with a special 4-connected zeotype ABW topology. In the structure of 1, each Cu+ center is coordinated by one imidazole N donor and three tetrazole N donors, displaying distorted tetrahedral coordination geometry (Fig. 1a). Each tpi ligand links four Cu+ centers through three tetrazole N donors and one imidazole N donor (Fig. 1c). Thus, both the Cu+ center and tpi ligand can be reduced to 4-connected nodes. Finally, the tetrahedral Cu+ center is connected by the μ4-tpi ligands into a dense 3D framework (Fig. 1b). Topological analysis indicates that the structure of 1 possesses a 4-connected network with the zeotype ABW topology (Fig. 1d).12
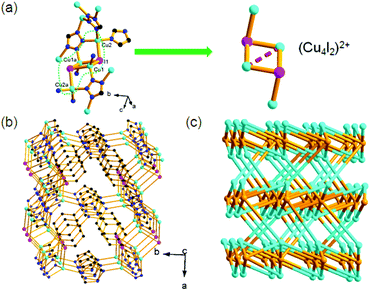
One prominent structural feature of compound 2 is the presence of the cationic (Cu4I2)2+ units. The shortest Cu⋯Cu distance in this unit is 3.375 Å, showing an obvious Cu⋯Cu interaction. This Cu4I2 unit is a part of the neutral cubane-like Cu4I4 unit and it is usual in the family of copper(I)–iodide clusters.11e There are two types of tetrahedral Cu+ centers linked by the μ3-I− ions to form the (Cu4I2)2+ unit (Fig. 2a). Each (Cu4I2)2+ unit is coordinated by eight μ5-tpi ligands. Unlike 1, all five N donors from the tpi ligand take part in the coordination with Cu+ centers in 2. The μ5-tpi ligands link the (Cu4I2)2+ units into a dense 3D framework (Fig. 2b). Here, the (Cu4I2)2+ units can be considered as the 8-connected nodes and the μ5-tpi ligands are regarded as the 4-connected nodes. Topological analysis shows that compound 2 has a (4,8)-connected topological network with a Schläfli symbol of (43·63)2(46·618·84) (Fig. 2c).
The solid-state photoluminescence properties of 1 and 2 were investigated at room temperature (Fig. 3). Particularly, in order to gain insight into their photoluminescence, a free Htpi ligand was synthesized according to the reported literature.13 The emission bands of Htpi are centered at about 467 nm (λex = 360 nm), which may be attributed to the ligand-centered π–π* electronic transitions. Upon irradiation of ultraviolet light at 360 nm, the emission maximum wavelengths for 1 and 2 are 505 nm and 532 nm, respectively. The emissions of 1 and 2 can be tentatively assigned to the [Cu → π*(ipz)] metal-to-ligand charge transfer (MLCT), which are similar to those observed in some documented Cu–tetrazolate compounds.11,14 However, the red shift of the bands in the spectrum of 1 compared to those of 2 probably involved with Cu⋯Cu [3d → 4s] cluster-centered (CC) excited states. The short Cu⋯Cu interactions (3.375 Å) in the structure of 2 might also contribute to the photoemission, as proved for other Cu(I) coordination polymers.11e
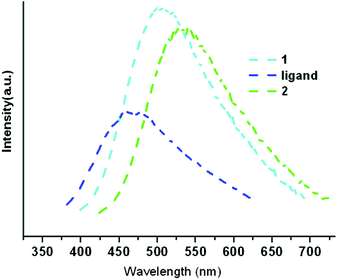 | ||
| Fig. 3 The solid-state emission spectra (λex = 360 nm) for 1, Htpi ligand and 2 at room temperature. | ||
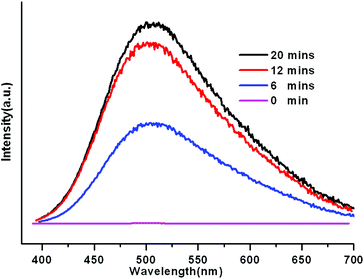
Encouraged by our previous work, the Cu(I) CPs may be considered promising candidates as nitrobenzene sensors. At room temperature, we performed the sensing experiments of nitrobenzene using solid-state samples of 1 and 2. Fortunately, both of them showed excellent fluorescence quenching behavior upon immersing them in nitrobenzene. As a typical example, the quenching experiments of 1 are described in detail. With the nitrobenzene vapor volatilizing, the fluorescence intensity begins to increase and finally reaches the highest value after the total removal of nitrobenzene in about 20 minutes (Fig. 4). However, when 1 was dispersed in some other solvents (methanol, ethanol, toluene, etc.), no obvious luminescence change was observed (Fig. S4†). The distinct fluorescence quenching response of 1 to nitrobenzene might be attributed to the electrostatic interactions and the electron deficiency.15 Compound 2 also displayed similar sensing behavior to nitrobenzene (Fig. S5–6†). The inspiring results suggest that they could be exceptional luminescent probes for sensing nitrobenzene.
In summary, a rigid 1-(4-tetrazolephenyl)imidazole ligand is first in situ synthesized and employed to construct two novel 3D Cu(I) coordination polymers with interesting structural topologies and photoluminescence properties. Moreover, compound 1 is a promising sensing material for detection of nitrobenzene.
We acknowledge the support for this work from the 973 Program (2011CB932504 and 2012CB821705) and NSFC (21221001, 91222105).
Notes and references
- (a) P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232 CrossRef PubMed; (b) Y. Kang, F. Wang, J. Zhang and X. Bu, J. Am. Chem. Soc., 2012, 134, 17881 CrossRef CAS PubMed.
- (a) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (b) D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257 RSC; (c) F. Wang, Z.-S. Liu, H. Yang, Y.-X. Tan and J. Zhang, Angew. Chem., Int. Ed., 2011, 50, 450 CrossRef CAS PubMed.
- (a) Z. Yin, Q. Wang and M.-H. Zeng, J. Am. Chem. Soc., 2012, 134, 4857 CrossRef CAS PubMed; (b) R. Vaidhyanathan, S. S. Iremonger, K. W. Dawson and G. K. H. Shimizu, Chem. Commun., 2009, 5230 RSC.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC; (b) Z. Xie, L. Ma, K. E. deKrafft, A. Jin and W. Lin, J. Am. Chem. Soc., 2010, 132, 922 CrossRef CAS PubMed; (c) T. Wen, D.-X. Zhang, J. Liu, R. Lin and J. Zhang, Chem. Commun., 2013, 49, 5660 RSC.
- (a) J. Ni, X. Zhang, Y.-H. Wu, L.-Y. Zhang and Z.-N. Chen, Chem. – Eur. J., 2011, 17, 1171 CrossRef CAS PubMed; (b) J. Ni, X. Zhang, N. Qiu, Y.-H. Wu, L.-Y. Zhang, J. Zhang and Z.-N. Chen, Inorg. Chem., 2011, 50, 9090 CrossRef CAS PubMed; (c) X. Zhang, J.-Y. Wang, J. Ni, L.-Y. Zhang and Z.-N. Chen, Inorg. Chem., 2012, 51, 5569 CrossRef CAS PubMed.
- (a) K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261 CrossRef CAS PubMed; (b) Z. Ning and H. Tian, Chem. Commun., 2009, 5483 RSC.
- (a) G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, Science, 2005, 309, 2040 CrossRef CAS PubMed; (b) Y.-B. Zhang, H.-L. Zhou, R.-B. Lin, C. Zhang, J.-B. Lin, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2012, 3, 642 CrossRef PubMed; (c) Y.-S. Wei, K.-J. Chen, P.-Q. Liao, B.-Y. Zhu, R.-B. Lin, H.-L. Zhou, B.-Y. Wang, W. Xue, J.-P. Zhang and X.-M. Chen, Chem. Sci., 2013, 4, 1539 RSC; (d) M. Kondo, M. Shimamura, S.-i. Noro, Y. Kimura, K. Uemura and S. Kitagawa, J. Solid State Chem., 2000, 152, 113 CrossRef CAS.
- (a) J. Ni, Y.-H. Wu, L.-Y. Zhang and Z.-N. Chen, Inorg. Chem., 2009, 48, 10202 CrossRef CAS PubMed; (b) J. Ni, X. Zhang, Y.-H. Wu, L.-Y. Zhang and Z.-N. Chen, Chem. – Eur. J., 2011, 17, 1171 CrossRef CAS PubMed; (c) J. Ni, X. Zhang, N. Qiu, Y.-H. Wu, L.-Y. Zhang, J. Zhang and Z.-N. Chen, Inorg. Chem., 2011, 50, 9090 CrossRef CAS PubMed; (d) X. Zhang, J.-Y. Wang, J. Ni, L.-Y. Zhang and Z.-N. Chen, Inorg. Chem., 2012, 51, 5569 CrossRef CAS PubMed.
- T. Wen, D.-X. Zhang and J. Zhang, Inorg. Chem., 2013, 52, 12 CrossRef CAS PubMed.
- (a) H. Zhao, Z.-R. Qu, H.-Y. Ye and R.-G. Xiong, Chem. Soc. Rev., 2008, 37, 84 RSC; (b) X.-M. Chen and M.-L. Tong, Acc. Chem. Res., 2007, 40, 162 CrossRef CAS PubMed.
- (a) T. Wen, X.-P. Zhou, D.-X. Zhang and D. Li, Chem. – Eur. J., 2014, 20, 644 CrossRef CAS PubMed; (b) T. Wen, X.-P. Zhou and D. Li, Dalton Trans., 2011, 40, 5684 RSC; (c) T. Wu, B.-H. Yi and D. Li, Inorg. Chem., 2005, 44, 4130 CrossRef CAS PubMed; (d) Z. Li, M. Li, S.-Z. Zhan, X.-C. Huang, S. W. Ng and D. Li, CrystEngComm, 2008, 10, 978 RSC; (e) M. Li, Z. Li and D. Li, Chem. Commun., 2008, 3390 RSC; (f) Z. Li, M. Li, X.-P. Zhou, T. Wu, D. Li and S. W. Ng, Cryst. Growth Des., 2007, 7, 1992 CrossRef CAS.
- (a) M. Maldonado, M. D. Oleksiak, S. Chinta and J. D. Rimer, J. Am. Chem. Soc., 2013, 135, 2641 CrossRef CAS PubMed; (b) Z. Su, Z. S. Bai, J. Xu, T.-a. Okamura, G. X. Liu, Q. Chu, X. F. Wang, W. Y. Sun and N. Ueyama, CrystEngComm, 2009, 11, 873 RSC; (c) Z. X. Chen, H. Y. Yang, M. L. Deng, Y. Ling, L. H. Weng and Y. M. Zhou, Dalton Trans., 2012, 41, 4079 RSC; (d) W. M. Meier, D. H. Olson and Ch. Baerlocher, Atlas of Zeolite Structure Types, International Zeolite Association, 4th edn, 1996 Search PubMed.
- J. Tao, Z.-J. Ma, R.-B. Huang and L.-S. Zheng, Inorg. Chem., 2004, 43, 6133 CrossRef CAS PubMed.
- (a) V. W. W. Yam and K. K.-W. Lo, Chem. Soc. Rev., 1999, 28, 323 RSC; (b) X.-C. Huang, J.-P. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2004, 126, 13218 CrossRef CAS PubMed.
- Z.-J. Zhang, S.-C. Xiang, X.-T. Rao, Q. Zheng, F. R. Fronczek, G.-D. Qian and B.-L. Chen, Chem. Commun., 2010, 46, 7205 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: TGA diagram, PXRD patterns, PL spectra of 1, and CIF files. CCDC 983653–983654. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00016a |
| ‡ Synthesis of 1: A mixture of Cu2O (0.014 g, 0.098 mmol), 1-(4-cyanophenyl)imidazole (0.020 g, 0.12 mmol), NaN3 (0.065 g, 1 mmol) and a few drops of NH3·H2O in the mixed water–isopropyl alcohol solvent (5/5 mL) was stirred for 15 min. It was then transferred and sealed in a 23 mL Teflon-lined stainless steel reactor, which was heated in an oven to 150 °C for 72 hours and then cooled to room temperature. Block crystals of 1 were obtained as the main product (53% yield). 2 was prepared using a similar procedure except replacing Cu2O with CuI (0.009 g, 0.047 mmol) and KI (0.009 g, 0.05 mmol). Block crystals of 2 were collected and dried in air (44% yield). |
§ Crystal data for 1: C20H14Cu2N12, M = 549.53, monoclinic, a = 19.1704(3) Å, b = 10.4373(2) Å, c = 9.3657(1) Å, α = 90°, β = 93.2850(1)°, γ = 90°, V = 1870.88(5) Å3, T = 293 K, space group P21/c, Z = 4, 7350 reflections measured, 3345 independent reflections (Rint = 0.0226). The final R1 value was 0.0286 (I > 2σ(I)). The final wR(F2) value was 0.0772 (I > 2σ(I)). The goodness of fit on F2 was 1.045. Anal. Calcd for 1: C, 43.67; H, 2.54; N, 30.57. Found: C, 43.51; H, 2.30; N, 30.34. IR (KBr pellet): 3110 (w), 1617 (m), 1535 (s), 1483 (s), 1441 (s), 1306 (s), 1058 (s), 1229 (m), 1170 (w), 1094 (m), 954 (m), 1188 (w), 845 (s), 716 (w), 618 (w). Crystal data for 2: C10H7Cu2IN6, M = 465.22, orthorhombic, a = 13.5477(11) Å, b = 20.560(2) Å, c = 8.7151(7) Å, V = 2427.5(4) Å3, T = 293 K, space group Pbca, Z = 8, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 reflections measured, 2875 independent reflections (Rint = 0.0341). The final R1 value was 0.0214 (I > 2σ(I)). The final wR (F2) value was 0.0544 (I > 2σ(I)). The goodness of fit on F2 was 1.002. Anal. Calcd for 2: C, 25.79; H, 1.50; N, 18.06. Found: C, 25.51; H, 1.17; N, 18.17. IR (KBr pellet): 2934 (w), 1607 (s), 1545 (s), 1488 (s), 1441 (m), 1344 (m), 1297 (s), 1053 (m), 1229 (w), 1115 (w), 954 (w), 850 (w), 845 (m), 785 (m), 654 (w). 520 reflections measured, 2875 independent reflections (Rint = 0.0341). The final R1 value was 0.0214 (I > 2σ(I)). The final wR (F2) value was 0.0544 (I > 2σ(I)). The goodness of fit on F2 was 1.002. Anal. Calcd for 2: C, 25.79; H, 1.50; N, 18.06. Found: C, 25.51; H, 1.17; N, 18.17. IR (KBr pellet): 2934 (w), 1607 (s), 1545 (s), 1488 (s), 1441 (m), 1344 (m), 1297 (s), 1053 (m), 1229 (w), 1115 (w), 954 (w), 850 (w), 845 (m), 785 (m), 654 (w). |
| This journal is © the Partner Organisations 2014 |