Palladium-catalyzed 1,4-addition of secondary alkylphenylphosphines to α,β-unsaturated carbonyl compounds for the synthesis of phosphorus- and carbon-stereogenic compounds†
Chun
Li
b,
Qing-Long
Bian
b,
Sheng
Xu
b and
Wei-Liang
Duan
*a
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: wlduan@mail.sioc.ac.cn; Fax: (+86)-21-6416-6128; Tel: (+86)-21-5492-5203
bSchool of Chemistry & Molecular Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
First published on 21st April 2014
Abstract
Highly stereoselective 1,4-addition of alkylphenylphosphines to α,β-unsaturated carbonyl compounds catalyzed by PCP or NCN pincer–Pd complexes is described to synthesize chiral phosphorus compounds bearing both P- and C-stereogenic centers with good to excellent enantioselectivity (up to 99.6% ee).
Chiral phosphines are ligands widely used in catalytic reactions to control the reactivity and enantioselectivity.1 Among these phosphines, axially chiral, carbon-stereogenic, or planar-chiral phosphine ligands, such as binap, diop, and josiphos, are frequently used to construct optically active compounds from prochiral starting materials. By contrast, phosphorus (P)-chiral phosphines are relatively less employed in catalysis.2 The first reported chiral phosphine ligand contains only one P-stereogenic center,3 likely because the preparation of P-chiral compounds is synthetically more challenging compared with other compounds.4 Reported methods always involve the optical resolution of racemates or desymmetrization of prochiral compounds with stoichiometric amounts of chiral reagents. These methods also require multiple-step transformations. Direct construction of a P–C bond, along with P-chiral center formation via asymmetric catalysis, appears to be a promising approach to solving this synthetic problem.5,6 Several methods, including transition metal-catalyzed arylation and alkylation of secondary phosphines with good to excellent enantioselectivities, have been reported by Glueck et al. and Toste/Bergman et al., respectively.7 The asymmetric Michael addition of methylphenylphosphine to enones was recently reported to generate P- and C-chiral phosphorus compounds with up to 82% ee by Leung et al.8 However, certain limitations still in terms of the extent of stereoselectivity and generality of the substrates must be addressed. In the present study, we describe a palladium-catalyzed asymmetric addition of disubstituted phosphines to α,β-unsaturated carbonyl compounds for the synthesis of P- and C-stereogenic phosphorus compounds with good to excellent enantioselectivities.
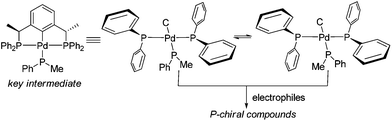
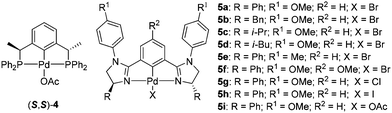
We previously reported the bisphosphine (PCP) pincer Pd-catalyzed asymmetric addition of diarylphosphines to electron-deficient alkenes for the synthesis of diverse chiral phosphine compounds.9 A Pd-diphenylphosphido intermediate was generated from the reaction of diphenylphosphine with the Pd catalyst and proposed as the active nucleophile toward electrophiles in the catalytic cycle.9a We hypothesized that the use of secondary phosphine nucleophiles bearing two different groups (e.g., methylphenylphosphine) could generate two Pd-phosphido diastereomers in a chiral environment induced by two methyl groups at the benzylic position of the catalyst. Subsequent functionalization of these intermediates could lead to the formation of P-stereogenic phosphorus compounds (Scheme 1).
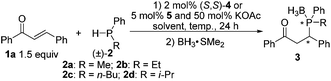
We initiated the reaction of alkylphenylphosphines with chalcone in the presence of pincer–Pd catalysts. The reaction of methylphenylphosphine 2a with enone 1a in the presence of catalyst (S,S)-410,11 only afforded products with low ee and dr (Table 1, entry 1).12 Solvent screening and temperature reduction to −30 °C did not lead to satisfactory results (entries 2–5). Changing the methyl group to ethyl and n-butyl groups in the P nucleophiles increased the ee and diastereomeric ratios of products (entries 6 and 7). Interestingly, introduction of an isopropyl group to substrate 2d significantly improved the ee of the product to 99% as well as its diastereoselectivity (entry 8; dr > 10/1). To increase the stereoselectivity in the reaction of methylphenyl phosphine with chalcone, bisimidazoline (NCN) pincer catalysts 5a–5i were synthesized and examined.13 Catalyst (S,S)-5a derived from (S)-phenylglycinol bearing a 4-methoxyphenyl group on the nitrogen atom afforded two diastereomers with good ee (entry 9: 74 and 83% ee; 1.3/1 dr) at −30 °C.14 By changing the substituted groups on the imidazoline ring to benzyl, isopropyl, and isobutyl groups, catalysts 5b–5d produced products with decreased enantioselectivity (entries 10–12). Investigations on temperature effects showed that reaction at room temperature yields products with 81% and 91% ee (entry 14). By changing the substituted group to 4-tolyl on the nitrogen atom or installation of a methoxy group at the phenyl ring of the catalyst, catalysts (S,S)-5e and 5f did not exert beneficial effects on the ee and dr (entries 15 and 16). The use of catalysts 5g and 5h featuring chloride or iodide anions caused a decrease in ee (entries 17 and 18). Changing KOAc to K2CO3 or removing KOAc in the reaction led to decreased ee (entries 19 and 20). Catalyst 5i bearing acetate anions also afforded products with lower ee than 5a (entry 21) via a slightly peculiar manner, the reason for which remains unclear at this point. Afterward, extensive examination of the solvents revealed that dichloromethane is optimal compared with other solvents (entries 22–25) at room temperature; toluene and tert-amyl alcohol afforded products with slightly low ee. Unexpectedly, using toluene as the solvent at −5 °C, products with the highest ee were isolated (entry 27: 87% and 96% ee). Further temperature reduction to −10 °C decreased the ee of both diastereomers (entry 28: 86% and 79% ee). For P nucleophiles 2b–2c, catalyst 5a only produced the corresponding products with moderate ee compared with catalyst 4 (entries 29–31).
| Entry | Phosphine | Catalyst | Solvent | Temp (°C) | Yielda (%) | drb | eec (%) |
|---|---|---|---|---|---|---|---|
a Isolated yields.
b Determined by 1H NMR analysis of the crude product.
c Determined by HPLC with hexane–2-propanol.
d 50 mol% K2CO3 used.
e Without addition of KOAc.
|
|||||||
| 1 | 2a | (S,S)-4 | Toluene | rt | 83 | 1.7/1 | 22/47 |
| 2 | 2a | (S,S)-4 | Toluene | −5 | 55 | 1.9/1 | 32/43 |
| 3 | 2a | (S,S)-4 | Toluene | −30 | 96 | 3.0/1 | 28/32 |
| 4 | 2a | (S,S)-4 | CH2Cl2 | −30 | 83 | 3.9/1 | 56/28 |
| 5 | 2a | (S,S)-4 | THF | −30 | 88 | 2.5/1 | 40/47 |
| 6 | 2b | (S,S)-4 | Toluene | −5 | 96 | 11/1 | 86/65 |
| 7 | 2c | (S,S)-4 | Toluene | −5 | 85 | 4.7/1 | 58/86 |
| 8 | 2d | (S,S)-4 | Toluene | −5 | 80 | >10/1 | 99.5 |
| 9 | 2a | (S,S)-5a | CH2Cl2 | −30 | 74 | 1.3/1 | 74/83 |
| 10 | 2a | (S,S)-5b | CH2Cl2 | −30 | 93 | 1.7/1 | 53/75 |
| 11 | 2a | (S,S)-5c | CH2Cl2 | −30 | 93 | 1.8/1 | 44/67 |
| 12 | 2a | (S,S)-5d | CH2Cl2 | −30 | 75 | 2.0/1 | 24/51 |
| 13 | 2a | (S,S)-5a | CH2Cl2 | 0 | 83 | 1.3/1 | 81/90 |
| 14 | 2a | (S,S)-5a | CH2Cl2 | rt | 87 | 1.4/1 | 81/91 |
| 15 | 2a | (S,S)-5e | CH2Cl2 | rt | 81 | 1.3/1 | 60/85 |
| 16 | 2a | (S,S)-5f | CH2Cl2 | rt | 83 | 1.4/1 | 78/83 |
| 17 | 2a | (S,S)-5g | CH2Cl2 | rt | 87 | 1.4/1 | 80/88 |
| 18 | 2a | (S,S)-5h | CH2Cl2 | rt | 94 | 1.5/1 | 61/75 |
| 19d | 2a | (S,S)-5a | CH2Cl2 | rt | 97 | 1.2/1 | 71/74 |
| 20e | 2a | (S,S)-5a | CH2Cl2 | rt | 94 | 1.4/1 | 61/80 |
| 21e | 2a | (S,S)-5i | CH2Cl2 | rt | 97 | 1.4/1 | 75/84 |
| 22 | 2a | (S,S)-5a | Toluene | rt | 70 | 1.3/1 | 82/89 |
| 23 | 2a | (S,S)-5a | t-AmOH | rt | 74 | 1.2/1 | 80/89 |
| 24 | 2a | (S,S)-5a | MeOH | rt | 33 | 2.5/1 | 15/25 |
| 25 | 2a | (S,S)-5a | DMF | rt | 77 | 1.5/1 | 58/71 |
| 26 | 2a | (S,S)-5a | Toluene | 0 | 80 | 1.3/1 | 83/95 |
| 27 | 2a | (S,S)-5a | Toluene | −5 | 98 | 1.1/1 | 87/96 |
| 28 | 2a | (S,S)-5a | Toluene | −10 | 95 | 0.9/1 | 86/79 |
| 29 | 2b | (S,S)-5a | Toluene | −5 | 88 | 2.7/1 | 36/34 |
| 30 | 2c | (S,S)-5a | Toluene | −5 | 98 | 2.2/1 | 66/7 |
| 31 | 2d | (S,S)-5a | Toluene | −5 | 81 | 1.6/1 | 12/73 |
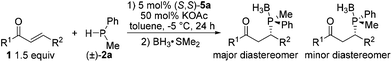
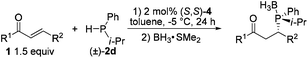
The scope of substrates was examined under optimum conditions, and the results are shown in Table 2. Methylphenylphosphine reacted with enones bearing diverse substituted groups (i.e., alkyl, methoxy, halide, and nitro) smoothly, thereby yielding a series of phosphorus compounds with good to excellent enantiomeric excess (Table 2: entries 1–9). For substrates bearing an isopropyl moiety at the β-position, the ee of products is lower than that in the case of aryl groups (entry 10). α,β-Unsaturated N-acyl pyrroles have been reported to be good acceptors in asymmetric addition reactions.15 The pyrrole group can be easily converted into various moieties.16 Experimental results indicate that various α,β-unsaturated N-acyl pyrroles show properties comparable to those of enones in terms of ee and dr (entries 11–17), which further extends the applicability of the current catalytic system. Table 3 summarizes the reactions of isopropylphenylphosphines with α,β-unsaturated enones and N-acyl pyrroles. All of the products were isolated with almost perfect enantioselectivities and moderate to good diastereomeric ratios (Table 3: entries 1–8; 98 to 99.6% ee). To illustrate the utility of the current method, a bisenone was reacted with a secondary phosphine 2d to yield a chiral bisphosphine bearing four stereogenic centers in 99% ee (Scheme 2); this biphosphine is a useful ligand for the preparation of P-chiral pincer metal catalysts.6q,17
| Entry | R1 | R2 | Yield (%)/(dr)a,b | ee of the major and minor productc,d (%) |
|---|---|---|---|---|
| a Isolated yields. b Determined by 1H NMR analysis of the crude product. c Determined by HPLC with hexane–2-propanol. d The absolute configurations of products were determined to be S,Sp for major diastereomers and S,Rp for minor diastereomers by X-ray crystal diffraction of 1,4-adducts in entries 9 and 17 (see ESI for details).18 | ||||
| 1 | Ph | Ph | 98 (1.1/1) | 87/96 |
| 2 | Ph | 4-MeC6H4 | 97 (1.1/1) | 88/95 |
| 3 | Ph | 4-MeOC6H4 | 92 (1.2/1) | 89/95 |
| 4 | Ph | 3-BrC6H4 | 94 (1.1/1) | 87/96 |
| 5 | Ph | 4-BrC6H4 | 98 (1.1/1) | 83/96 |
| 6 | Ph | 4-O2NC6H4 | 94 (1.2/1) | 87/97 |
| 7 | Ph | 4-CF3C6H4 | 86 (1.3/1) | 81/95 |
| 8 | 4-BrC6H4 | Ph | 88 (1.2/1) | 89/94 |
| 9 | Me | 4-BrC6H4 | 98 (1.7/1) | 89/94 |
| 10 | Ph | i-Pr | 91 (2.6/1) | 72/85 |
| 11 | 1-Pyrrolyl | Ph | 84 (1.2/1) | 86/93 |
| 12 | 1-Pyrrolyl | 4-MeC6H4 | 97 (1.2/1) | 84/93 |
| 13 | 1-Pyrrolyl | 2-Naphthyl | 91 (1.2/1) | 85/93 |
| 14 | 1-Pyrrolyl | 4-MeOC6H4 | 95 (1.1/1) | 83/92 |
| 15 | 1-Pyrrolyl | 4-NO2C6H4 | 97 (1.1/1) | 85/93 |
| 16 | 1-Pyrrolyl | 3-BrC6H4 | 98 (1.2/1) | 84/95 |
| 17 | 1-Pyrrolyl | 4-BrC6H4 | 96 (1.2/1) | 86/96 |
| Entry | R1 | R2 | Yield (%)/(dr)a,b | eec,d (%) |
|---|---|---|---|---|
| a Isolated yields. b Determined by 1H NMR analysis of the crude product. c Determined by HPLC with hexane–2-propanol. d The absolute configurations of products were determined to be S,Rp by X-ray crystal diffraction of the 1,4-adduct in entry 1 (see ESI for details).18 | ||||
| 1 | Ph | Ph | 80 (>10/1) | 99.5 |
| 2 | Ph | 4-MeOC6H4 | 84 (>10/1) | 98 |
| 3 | Ph | 4-NO2C6H4 | 85 (>10/1) | 99 |
| 4 | 3-BrC6H4 | Ph | 88 (>10/1) | 99.4 |
| 5 | 1-Pyrrolyl | Ph | 83 (7/1) | 98 |
| 6 | 1-Pyrrolyl | 4-BrC6H4 | 80 (5/1) | 99.6 |
| 7 | 1-Pyrrolyl | 3-BrC6H4 | 81 (6/1) | 99 |
| 8 | 1-Pyrrolyl | 2-Naphthyl | 78 (5/1) | >99.5 |
In summary, we have described the enantioselective addition of alkylphenylphosphines to α,β-unsaturated carbonyl compounds catalyzed by PCP and NCN pincer Pd catalysts to yield chiral phosphorus compounds bearing both P- and C-stereogenic centers with good to excellent enantioselectivity under mild conditions. Further studies will elucidate the stereochemistry-determining factors and applications of P-chiral compounds as ligands to transition metals in catalysis.
We thank the National Basic Research Program of China (973 Program 2010CB833300), the NSFC (20902099, 21172238), and SIOC for funding this work.
Notes and references
- (a) Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications, ed. A. Börner, Wiley-VCH, Weinheim, vol. 1–3, 2008 Search PubMed; (b) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed; (c) X. Cui and K. Burgess, Chem. Rev., 2005, 15, 3272 CrossRef PubMed; (d) J.-H. Xie, S.-F. Zhu and Q.-L. Zhou, Chem. Rev., 2011, 111, 1713 CrossRef CAS PubMed; (e) D.-S. Wang, Q.-A. Chen, S.-M. Lu and Y.-G. Zhou, Chem. Rev., 2012, 112, 2557 CrossRef CAS PubMed.
- For recent examples of P-chiral ligands in catalysis, see: (a) T. Imamoto, J. Watanabe, Y. Wada, H. Masuda, H. Yamada, H. Tsuruta, S. Matsukawa and K. Yamaguchi, J. Am. Chem. Soc., 1998, 120, 1635 CrossRef CAS; (b) I. D. Gridnev, N. Higashi, K. Asakura and T. Imamoto, J. Am. Chem. Soc., 2000, 122, 7183 CrossRef CAS; (c) W. Tang and X. Zhang, Angew. Chem., Int. Ed., 2002, 41, 1612 CrossRef CAS; (d) W. Tang, W. Wang and X. Zhang, Angew. Chem., Int. Ed., 2003, 42, 943 CrossRef CAS PubMed; (e) W. Tang, W. Wang, Y. Chi and X. Zhang, Angew. Chem., Int. Ed., 2003, 42, 3509 CrossRef CAS PubMed; (f) T. Imamoto, K. Sugita and K. Yoshida, J. Am. Chem. Soc., 2005, 127, 11934 CrossRef CAS PubMed; (g) T. Imamoto, Y. Saitoh, A. Koide, T. Ogura and K. Yoshida, Angew. Chem., Int. Ed., 2007, 46, 8636 CrossRef CAS PubMed; (h) A. M. Taylor, R. A. Altman and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 9900 CrossRef CAS PubMed; (i) T. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa and I. D. Gridnev, J. Am. Chem. Soc., 2012, 134, 1754 CrossRef CAS PubMed; (j) G. Liu, X. Liu, Z. Cai, G. Jiao, G. Xu and W. Tang, Angew. Chem., Int. Ed., 2013, 52, 4235 CrossRef CAS PubMed.
- For early reported P-chiral ligands, see: (a) L. Horner, H. Siegel and H. Büthe, Angew. Chem., Int. Ed. Engl., 1968, 7, 942 CrossRef CAS; (b) W. S. Knowles and M. J. Sabacky, Chem. Commun., 1968, 1445 RSC; (c) W. S. Knowles, M. J. Sabacky and B. D. Vineyard, J. Chem. Soc., Chem. Commun., 1972, 10 RSC; (d) W. S. Knowles, M. J. Sabacky, B. D. Vineyard and D. J. Weinkauff, J. Am. Chem. Soc., 1975, 97, 2567 CrossRef CAS.
- For reviews on preparation of P-stereogenic phosphines, see: (a) K. M. Pietrusiewicz and M. Zablocka, Chem. Rev., 1994, 94, 1375 CrossRef CAS; (b) K. V. L. Crépy and T. Imamoto, Top. Curr. Chem., 2003, 229, 1 CrossRef; (c) K. V. L. Crépy and T. Imamoto, Adv. Synth. Catal., 2003, 345, 79 CrossRef; (d) P.-H. Leung, Acc. Chem. Res., 2004, 37, 169 CrossRef CAS PubMed; (e) A. Grabulosa, J. Granell and G. Muller, Coord. Chem. Rev., 2007, 251, 25 CrossRef CAS PubMed; (f) O. I. Kplodiazhnyi, Tetrahedron: Asymmetry, 2012, 23, 1 CrossRef PubMed. For representative examples, see: (g) W. B. Farnham, R. K. Murray Jr. and K. Mislow, J. Am. Chem. Soc., 1970, 92, 5809 CrossRef CAS; (h) J. Donohue, N. Mandel, W. B. Farnham, R. K. Murray Jr., K. Mislow and H. P. Benschop, J. Am. Chem. Soc., 1971, 93, 3792 CrossRef; (i) T. Imamoto, T. Oshiki, T. Onozawa, T. Kusumoto and K. Sato, J. Am. Chem. Soc., 1990, 112, 5244 CrossRef CAS; (j) S. Jugé, M. Stephan, J. Laffitte and J. Genet, Tetrahedron Lett., 1990, 31, 6357 CrossRef; (k) T. Oshiki and T. Imamoto, J. Am. Chem. Soc., 1992, 114, 3975 CrossRef CAS; (l) A. R. Muci, K. R. Campos and D. A. Evans, J. Am. Chem. Soc., 1995, 117, 9075 CrossRef CAS; (m) B. Wolfe and T. Livinghouse, J. Am. Chem. Soc., 1998, 120, 5116 CrossRef CAS; (n) K. Nagata, S. Matsukawa and T. Imamoto, J. Org. Chem., 2000, 65, 4185 CrossRef CAS; (o) T. Imamoto, S.-I. Kikuchi, T. Miura and Y. Wada, Org. Lett., 2001, 3, 87 CrossRef CAS; (p) Q. Xu, C.-Q. Zhao and L.-B. Han, J. Am. Chem. Soc., 2008, 130, 12648 CrossRef CAS PubMed; (q) G. Wang, R. Shen, Q. Xu, M. Goto, Y. Zhao and L.-B. Han, J. Org. Chem., 2010, 75, 3890 CrossRef CAS PubMed; (r) Y. Zhou, G. Wang, Y. Saga, R. Shen, M. Goto, Y. Zhao and L.-B. Han, J. Org. Chem., 2010, 75, 7924 CrossRef CAS PubMed; (s) Z. S. Han, N. Goyal, M. A. Herbage, J. D. Sieber, B. Qu, Y. Xu, Z. Li, J. T. Reeves, J.-N. Desrosiers, S. Ma, N. Grinberg, H. Lee, H. P. R. Mangunuru, Y. Zhang, D. Krishnamurthy, B. Z. Lu, J. J. Song, G. Wang and C. H. Senanayake, J. Am. Chem. Soc., 2013, 135, 2474 CrossRef CAS PubMed; (t) O. Berger and J.-L. Montchamp, Angew. Chem., Int. Ed., 2013, 52, 11377 CrossRef CAS PubMed.
- For reviews on the synthesis of chiral phosphorus compounds, see: (a) D. S. Glueck, Synlett, 2007, 2627 CrossRef CAS PubMed; (b) D. S. Glueck, Chem. – Eur. J., 2008, 14, 7108 CrossRef CAS PubMed; (c) J. S. Harvey and V. Gouverneur, Chem. Commun., 2010, 7477 RSC; (d) D. Zhao and R. Wang, Chem. Soc. Rev., 2012, 41, 2095 RSC. For leading examples, see: (e) I. Kovacik, D. K. Wicht, N. S. Grewal, D. S. Glueck, C. D. Incarvito, I. A. Guzei and A. L. Rheingold, Organometallics, 2000, 19, 950 CrossRef CAS; (f) A. D. Sadow, I. Haller, L. Fadini and A. Togni, J. Am. Chem. Soc., 2004, 126, 14704 CrossRef CAS PubMed; (g) C. Scriban, I. Kovacik and D. S. Glueck, Organometallics, 2005, 24, 4871 CrossRef CAS; (h) C. Scriban, D. S. Glueck, L. N. Zakharov, W. S. Kassel, A. G. DiPasquale, J. A. Golen and A. L. Rheingold, Organometallics, 2006, 25, 5757 CrossRef CAS; (i) A. D. Sadow and A. Togni, J. Am. Chem. Soc., 2005, 127, 17012 CrossRef CAS PubMed; (j) C. Genet, S. J. Canipa, P. O'Briena and S. Taylor, J. Am. Chem. Soc., 2006, 128, 9336 CrossRef CAS PubMed; (k) B. Join, D. Mimeau, O. Delacroix and A.-C. Gaumont, Chem. Commun., 2006, 3249 RSC; (l) G. Nishida, K. Noguchi, M. Hirano and K. Tanaka, Angew. Chem., Int. Ed., 2008, 47, 3410 CrossRef CAS PubMed; (m) P. Butti, R. Rochat, A. D. Sadow and A. Togni, Angew. Chem., Int. Ed., 2008, 47, 4878 CrossRef CAS PubMed; (n) J. S. Harvey, S. J. Malcolmson, K. S. Dunne, S. J. Meek, A. L. Thompson, R. R. Schrock, A. H. Hoveyda and V. Gouverneur, Angew. Chem., Int. Ed., 2009, 48, 762 CrossRef CAS PubMed; (o) L. Hong, W. Sun, C. Liu, D. Zhao and R. Wang, Chem. Commun., 2010, 2856 RSC; (p) W. Sun, L. Hong, C. Liu and R. Wang, Org. Lett., 2010, 12, 3914 CrossRef CAS PubMed; (q) M. Nielsen, C. B. Jacobsen and K. A. Jørgensen, Angew. Chem., Int. Ed., 2011, 50, 3211 CrossRef CAS PubMed; (r) S. Liu, Z. Zhang, F. Xie, N. Butt, L. Sun and W. Zhang, Tetrahedron: Asymmetry, 2012, 23, 329 CrossRef CAS PubMed.
- For catalytic asymmetric 1,4-addition of substituted phosphines or phosphine oxides to electron-deficient alkenes, see: (a) A. Carlone, G. Bartoli, M. Bosco, L. Sambri and P. Melchiorre, Angew. Chem., Int. Ed., 2007, 46, 4504 CrossRef CAS PubMed; (b) I. Ibrahem, R. Rios, J. Vesely, P. Hammar, L. Eriksson, F. Himo and A. Córdova, Angew. Chem., Int. Ed., 2007, 46, 4507 CrossRef CAS PubMed; (c) G. Bartoli, M. Bosco, A. Carlone, M. Locatelli, A. Mazzanti, L. Sambri and P. Melchiorre, Chem. Commun., 2007, 722 RSC; (d) X. Fu, Z. Jiang and C.-H. Tan, Chem. Commun., 2007, 5058 RSC; (e) I. Ibrahem, P. Hammar, J. Vesely, R. Rios, L. Eriksson and A. Córdova, Adv. Synth. Catal., 2008, 350, 1875 CrossRef CAS; (f) D. Zhao, L. Mao, D. Yang and R. Wang, J. Org. Chem., 2010, 75, 6756 CrossRef CAS PubMed; (g) S. Wen, L. Li, H. Wu, F. Yu, X. Liang and J. Ye, Chem. Commun., 2010, 4806 RSC; (h) Y. Huang, S. A. Pullarkat, L. Li and P.-H. Leung, Chem. Commun., 2010, 6950 RSC; (i) A. Russo and A. Lattanzi, Eur. J. Org. Chem., 2010, 6736 CrossRef CAS; (j) X. Luo, Z. Zhou, X. Li, X. Liang and J. Ye, RSC Adv., 2011, 1, 698 RSC; (k) M.-J. Yang, Y.-J. Liu, J.-F. Gong and M.-P. Song, Organometallics, 2011, 30, 3793 CrossRef CAS; (l) Y. Huang, R. J. Chew, Y. Li, S. A. Pullarkat and P.-H. Leung, Org. Lett., 2011, 13, 5862 CrossRef CAS PubMed; (m) D. Zhao, L. Wang, D. Yang, Y. Zhang and R. Wang, Chem. – Asian. J., 2012, 7, 881 CrossRef CAS PubMed; (n) Y. Huang, S. A. Pullarkat, S. Teong, R. J. Chew, Y. Li and P.-H. Leung, Organometallics, 2012, 31, 4871 CrossRef CAS; (o) Y. Huang, S. A. Pullarkat, R. J. Chew, Y. Li and P.-H. Leung, J. Org. Chem., 2012, 77, 6894 Search PubMed; (p) M. Hatano, T. Horibe and K. Ishihara, Angew. Chem., Int. Ed., 2013, 52, 4549 CrossRef CAS PubMed; (q) B. Ding, Z. Zhang, Y. Xu, Y. Liu, M. Sugiya, T. Imamoto and W. Zhang, Org. Lett., 2013, 15, 5476 CrossRef CAS PubMed.
- For catalytic asymmetric synthesis of chiral phosphines through arylation or alkylation of substituted phosphines, see: (a) J. R. Moncarz, N. F. Laritcheva and D. S. Glueck, J. Am. Chem. Soc., 2002, 124, 13356 CrossRef CAS PubMed; (b) V. S. Chan, I. C. Stewart, R. G. Bergman and F. D. Toste, J. Am. Chem. Soc., 2006, 128, 2786 CrossRef CAS PubMed; (c) C. Scriban and D. S. Glueck, J. Am. Chem. Soc., 2006, 128, 2788 CrossRef CAS PubMed; (d) N. F. Blank, J. R. Moncarz, T. J. Brunker, C. Scriban, B. J. Anderson, O. Amir, D. S. Glueck, L. N. Zakharov, J. A. Golen, C. D. Incarvito and A. L. Rheingold, J. Am. Chem. Soc., 2007, 129, 6847 CrossRef CAS PubMed; (e) V. S. Chan, R. G. Bergman and F. D. Toste, J. Am. Chem. Soc., 2007, 129, 15122 CrossRef CAS PubMed; (f) T. J. Brunker, B. J. Anderson, N. F. Blank, D. S. Glueck and A. L. Rheingold, Org. Lett., 2007, 9, 1109 CrossRef CAS PubMed; (g) C. Scriban, D. S. Glueck, J. A. Golen and A. L. Rheingold, Organometallics, 2007, 26, 1788 CrossRef CAS; (h) V. S. Chan, M. Chiu, R. G. Bergman and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 6021 CrossRef CAS PubMed; (i) T. W. Chapp, D. S. Glueck, J. A. Golen, C. E. Moore and A. L. Rheingold, Organometallics, 2010, 29, 378 CrossRef CAS.
- Y. Huang, S. A. Pullarkat, Y. Li and P.-H. Leung, Inorg. Chem., 2012, 51, 2533 CrossRef CAS PubMed.
- (a) J.-J. Feng, X.-F. Chen, M. Shi and W.-L. Duan, J. Am. Chem. Soc., 2010, 132, 5562 CrossRef CAS PubMed; (b) D. Du and W.-L. Duan, Chem. Commun., 2011, 47, 11101 RSC; (c) Y.-R. Chen and W.-L. Duan, Org. Lett., 2011, 13, 5824 CrossRef CAS PubMed; (d) J.-J. Feng, M. Huang, Z.-Q. Lin and W.-L. Duan, Adv. Synth. Catal., 2012, 354, 3122 CrossRef CAS; (e) M. Huang, C. Li, W.-L. Duan and S. Xu, Chem. Commun., 2012, 48, 11148 RSC; (f) D. Du, Z.-Q. Lin, J.-Z. Lu, C. Li and W.-L. Duan, Asian J. Org. Chem., 2013, 2, 392 CrossRef CAS; (g) C. Li, W.-X. Li, S. Xu and W.-L. Duan, Chin. J. Org. Chem., 2013, 33, 799 CrossRef CAS; (h) J. Lu, J. Ye and W.-L. Duan, Org. Lett., 2013, 15, 5016 CrossRef CAS PubMed; (i) J. Lu, J. Ye and W.-L. Duan, Chem. Commun., 2014, 50, 698 RSC; (j) J. Huang, M.-X. Zhao and W.-L. Duan, Tetrahedron Lett., 2014, 55, 629 CrossRef CAS PubMed.
- For synthesis and application of the chiral PCP-PdCl complex, see: (a) J. M. Longmire and X. Zhang, Tetrahedron Lett., 1997, 38, 1725 CrossRef CAS; (b) J. M. Longmire, X. Zhang and M. Shang, Organometallics, 1998, 17, 4374 CrossRef CAS.
- For reviews on pincer Pd catalysts, see: (a) M. Albrecht and G. van Koten, Angew. Chem., Int. Ed., 2001, 40, 3750 CrossRef CAS; (b) N. Selander and K. J. Szabó, Chem. Rev., 2011, 111, 2048 CrossRef CAS PubMed.
- Due to the oxygen sensitivity of phosphines, the 1,4-adducts were converted to the corresponding phosphine borane complexes for analysis.
- For the use of imidazoline pincer catalysts in asymmetric phosphorus addition reactions, see ref. 6k.
- 50 mol% KOAc was used in the reaction to replace the bromide on the palladium atom with acetate. The acetate anion on the palladium atom is very important to achieve high ee. For similar observations in catalysis, see ref. 6k, q and 9a.
- α,β-Unsaturated N-acylpyrrole was first introduced into asymmetric 1,4-addition by Shibasaki et al., see: (a) T. Kinoshita, S. Okada, S.-R. Park, S. Matsunaga and M. Shibasaki, Angew. Chem., Int. Ed., 2003, 42, 4680 CrossRef CAS PubMed; (b) T. Mita, K. Sasaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2005, 127, 514 CrossRef CAS PubMed; (c) N. Yamagiwa, H. Qin, S. Matsunaga and M. Shibasaki, J. Am. Chem. Soc., 2005, 127, 13419 CrossRef CAS PubMed.
- D. A. Evans, G. Borg and K. A. Scheidt, Angew. Chem., Int. Ed., 2002, 41, 3188 CrossRef CAS.
- (a) B. S. Williams, P. Dani, M. Lutz, A. L. Spek and G. von Koten, Helv. Chim. Acta, 2001, 84, 3519 CrossRef CAS; (b) D. Morales-Morales, R. E. Cramer and C. M. Jensen, J. Organomet. Chem., 2002, 654, 44 CrossRef CAS; (c) S. Medici, M. Gagliardo, S. B. Williams, P. A. Chase, S. Gladiali, M. Lutz, A. L. Spek, G. P. M. van Klink and G. van Koten, Helv. Chim. Acta, 2005, 88, 694 CrossRef CAS.
- CCDC 981792, 981793 and 981794 contain the supplementary crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analytical data for all new compounds. CCDC 981792, 981793 and 981794. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qo00017j |
| This journal is © the Partner Organisations 2014 |