Regioselective direct ortho C-acylation of phenol and naphthol derivatives catalyzed by modified ZnCl2 on Al2O3 as catalyst under solvent-free and microwave conditions
Hossein
Naeimi
*a,
Atefeh
Amini
a and
Mohsen
Moradian
b
aDepartment of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, 87317, I.R. Iran. E-mail: naeimi@kashanu.ac.ir; Fax: +98-361-5552397; Tel: +98-361-5912388
bInstitute of Nanoscience and Nanotechnology, University of Kashan, Kashan, I.R. Iran
First published on 12th March 2014
Abstract
In this study, we present a new and practical method for the synthesis of some ortho C-acylated mono- and di-hydroxyaromatic moieties. A Friedel–Crafts reaction of phenolic substrates was carried out in the presence of zinc chloride supported on alumina as catalyst and carboxylic acids as acylating agents and leads to regioselectively ortho C-acylated compounds with respect to the phenolic hydroxyl group. The reaction proceeds smoothly under microwave irradiation with a wide range of starting materials. This reaction gives access to a variety of acylated compounds in high yield and in the absence of solvent by the use of the more active and stable solid catalyst. In addition, this reaction occurs with high regioselectivity at the ortho position and is compatible with other reported methods. The obtained hydroxyaryl ketones were characterized and confirmed by physical and spectroscopic data.
Introduction
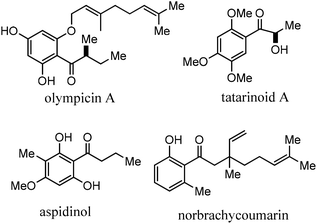
Acylation reactions are widely employed in the fine chemical industry to produce a variety of synthetic fragrances and pharmaceuticals.1ortho-Hydroxyacetophenone (o-HAP) is a key intermediate in the production of 4-hydroxycoumarin and warfarin, which are both used as anticoagulant drugs,2 and it has also been employed for obtaining flavonones.3,4 Phenolic drugs containing acyl groups are attractive targets for prodrug design due to their extensive first pass metabolism5 (Fig. 1).Three major synthetic pathways can be followed for this transformation, (1) the well-known Friedel–Crafts acylation reaction,6,7 (2) copper-catalyzed ortho-acylation of phenols with aryl aldehydes8 and (3) metal-catalyzed C–H activation of ketone moieties in the presence of [bis(trifluoroacetoxy)iodo]benzene as oxidant.9 The Friedel–Crafts acylation of phenols has been generally carried out with Brønsted or Lewis acid catalysts.10,11 The Friedel–Crafts acylation can be achieved by reaction of acid chlorides with a variety of condensing agents such as hydrogen fluoride,12 concentrated sulfuric acid,13 phosphorus pentoxide,14 polyphosphoric acid15 and methanesulfonic acid in alumina.16 In the past few years the possibility of obtaining o- and p-hydroxyacetophenone derivatives using solid catalysts with respect to the more active, stable, easily separated and recycled catalysts has been studied. Strong Brønsted solid acids, such as ionic resins, Nafion® and heteropolyacids exhibited moderate activity for the liquid-phase Fries rearrangement of phenols and form preferentially p-hydroxyacetophenone, however, they are quickly deactivated by waste formation.17
The development of cleaner technologies is a major challenge in green chemistry. Microwave enhanced chemistry represents a fundamental step forward in the capabilities of synthetic chemists. Today, the use of dedicated microwave instrumentation is becoming popular in many undergraduate laboratories, providing students with an in-depth view on the new advancements of the modern synthesis.18 A solvent-free or solid state reaction may be carried out using the reactants alone or incorporating them in clays, zeolites, silica, alumina or other mixtures.19 Adsorption of surfactants at the solid–liquid interface is an important topic in numerous processes ranging from mineral beneficiation to detergency.20,21 Active alumina, due to its high surface area, mechanical strength and thermal stability has found several applications as an adsorbent and catalyst.22 The application of zinc chloride supported on the alumina surface as catalyst was previously reported.23,24 In organic reactions, microwave-assisted solvent-free synthesis25,26 has been of growing interest as an efficient, economic and clean procedure.27
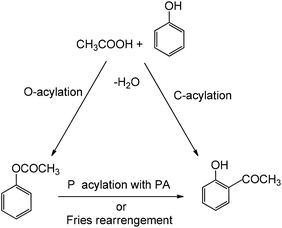
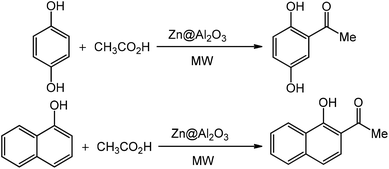
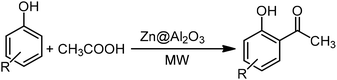
In this research, we examined the ortho-acylation of phenol and naphthol compounds with organic acids as acylating agents catalyzed by alumina supported-Lewis acid as a new catalyst under microwave irradiation and atmospheric pressure conditions. They catalyze two main reaction pathways leading from phenol to o-hydroxyacetophenone, i.e. the direct C-acylation of phenol and the O-acylation of phenol forming the phenyl acetate intermediate, which is consecutively transformed via intermolecular phenol/phenyl acetate C-acylation (Scheme 1). All of the reactions were accomplished in the absence of solvent, to afford the corresponding ortho-acylated hydroxyaryl compounds in high yields.
Results and discussion
Analysis of the catalyst
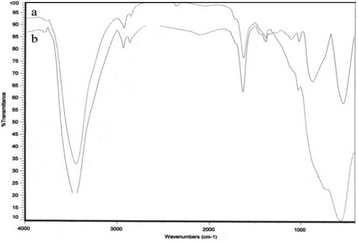
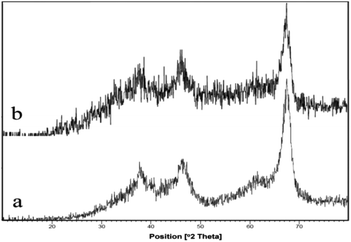
Heterogeneous ZnCl2@Al2O3 as a new and more active Lewis acid catalyst was investigated by powder X-ray diffraction (XRD) and FT-infrared spectroscopy (FT-IR), which indicate successful bonding of zinc ions into the internal surface of solid alumina.A comparison between the IR spectra of the catalyst with that of the initial alumina demonstrates the presence of zinc adsorbed on the surface of the solid alumina.
Fig. 2 shows the FT-IR spectra of alumina (a) and ZnCl2@Al2O3 (b), absorption bands near 3411 cm−1 represent the O–H mode of the alumina. In the modified catalyst, the bands at 611 cm−1 and 735 cm−1 assigned as Zn–O bands are clearly observed.28
The XRD patterns of alumina and ZnCl2@Al2O3 demonstrate typical reflections between 2θ = 20 and 70°. The X-ray diffraction (XRD) pattern of modified alumina with zinc chloride is similar to that of unmodified alumina (Fig. 3), which shows that the crystallinity of the catalyst is retained.
Catalytic studies
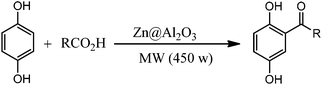
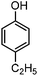
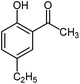
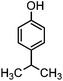
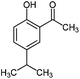
For the first time, we have studied the acylation reaction of hydroquinone (as a phenol moiety) with acetic acid in the presence of alumina supported zinc chloride (ZnCl2@Al2O3) as a new solid catalyst under microwave irradiation and solvent free conditions (Scheme 2). The results show that in the presence of ZnCl2@Al2O3 the ortho-acylated products were obtained in high yields.In continuation of this work, we have used ZnCl2@Al2O3 for the ortho-acylation of various phenol and naphthol derivatives with acetic acid, under microwave conditions (Scheme 3). The corresponding results are shown in Table 1. As shown in Table 1, the reaction is regioselective in where C-acylation occurred. In all cases, particularly those with available para positions, the O-acylated product was obtained in high yields and the para products nearly were not observed.
| Entry | Substrate | Product | Power (W) | Time (min) | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction conditions: phenol or naphthol derivatives 0.95 mmol, HOAc 1.2 mmol, [Zn] 0.73 mmol as ZnCl2@Al2O3. b Isolated yield based on the phenol and naphthol substrates. c In the presence of excess clean Al2O3 as catalyst. d In the presence of ZnCl2 (1 mmol) as catalyst. | |||||
| 1 |
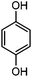
|
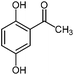
|
450 | 1.3 | 98 |
| 2 |
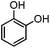
|
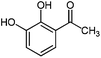
|
450 | 1.5 | 97 |
| 3 |
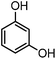
|
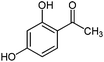
|
450 | 1.8 | 98 |
| 4 |
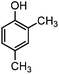
|
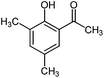
|
600 | 2 | 95 |
| 5 |
|
|
450 | 2 | 83 |
| 6 |
|
|
450 | 2.2 | 85 |
| 7 |
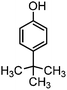
|
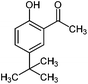
|
450 | 2 | 80 |
| 8 |
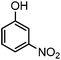
|
— | 900 | 3.0 | 0 |
| 9 |
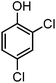
|
— | 900 | 5.0 | 0 |
| 10 |
|
— | 900 | 5.0 | 0 |
| 11 |
|
|
900 | 5.0 | 20 |
| 12 |
|
— | 900 | 3.0 | 0 |
| 13 |
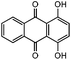
|
— | 900 | 4.0 | 0 |
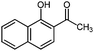
| 14 |
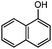
|
|
900 | 1.6 | 98 |
| 15c |
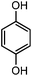
|
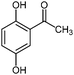
|
900 | 10 | 0 |
| 16d |
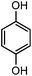
|
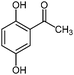
|
900 | 8 | 62 |
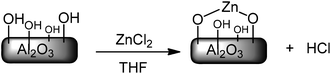
The hydroxyl groups on the surface of the activated alumina reacted with zinc chloride and formed a new composite modified alumina with the zinc ions bonded to oxygen of the surface (Scheme 4). In this case, the zinc ions are supported on the surface and can be applied as a catalyst.
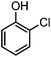
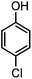
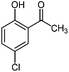
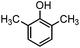
Generally, in this method, due to the low activity of the catalyst, phenol rings with electron withdrawing groups such as halogens and nitro groups hardly reacted and the desired products were obtained in low yields (Table 1, entries 8–11). If both ortho positions of the phenol ring are occupied by some other substituent, the reaction does not occur (Table 1, entry 12). This sequence is consisted with attention to that 2,6-dimethyl phenol did not produce para-acylated product under the reaction conditions. In other cases, ortho-acylated compounds were chemo-selectively produced in high yields (Table 1, entries 1–7 and 14). In Table 1, entry 11, the reaction of 4-chlorophenol with acetic acid in the presence of the ZnCl2@Al2O3 as solid catalyst only produced 20% of the desired product and the initial substituted phenol remained untouched at end of the reaction.
For further investigation of the catalytic activity of modified alumina, the desired reaction was performed in the presence of clean Al2O3 as catalyst and no product was observed. Also, in the presence of ZnCl2 as a neat catalyst, the desired product was formed only with 68% yield (Table 1, entries 15 and 16).
A simplified and possible mechanism for this useful protocol is provided in Scheme 5. Regioselectivity is fundamental for this methodology and achieved from the key step of zinc chelation to both the phenol and carboxylic acid substrates. After chelation, the acid substrate is localized at the nearest position to the phenol substrate. Thereupon, the active phenol ring attacks and then joins onto the carbonyl group of the carboxylic acid from the ortho position (Scheme 5).
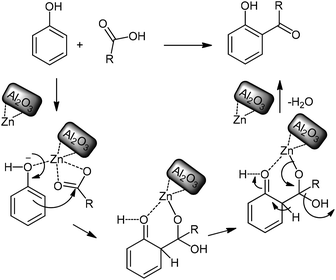 | ||
| Scheme 5 A possible mechanism for the acylation reaction in the presence of modified alumina as catalyst. | ||
For extended application of this solid catalyst in acylation reactions, we also examined the acylation of hydroquinone with other organic acids, such as propanoic, butanoic, and pentanoic acid under free solvent and microwave conditions. The obtained results are summarized in Table 2. As shown in this table, in this reaction, the solid catalyst can catalyze the reaction in the presence of some organic acids and produced ortho-acylated compounds in high yields and short reaction times.
The presence of OH stretching broad bands in the 3100–3500 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching strong bands in 1735–1750 cm−1 IR region, and existence of the broad singlet peak with δ (9.4–11.9) ppm in the 1H NMR data in all of the products, are completely consistent with the ortho-acylated phenols and naphthols.
O stretching strong bands in 1735–1750 cm−1 IR region, and existence of the broad singlet peak with δ (9.4–11.9) ppm in the 1H NMR data in all of the products, are completely consistent with the ortho-acylated phenols and naphthols.
Experimental section
Materials
Chemicals were purchased from the Merck and Fluka Chemical Companies in high purity. All of the materials were of commercial reagent grade. The phenols and naphthols were purified by standard procedures and purity determined by thin layer chromatography (TLC) and gas chromatography (GC).Apparatus
IR spectra were recorded as KBr pellets on a Perkin-Elmer 781 spectrophotometer and an Impact 400 Nickolet FTIR spectrophotometer. 1H NMR and 13C NMR spectra were recorded in CDCl3 with (400 MHz) spectrometer using TMS as an internal reference. Microwave irradiations were carried out in microwave oven specially designed for organic synthesis (Milestone LAVIS 1000 Basic Microwave) from Milestone Company. Melting points were obtained with a Yanagimoto micro melting point apparatus are uncorrected. The purity determination of the substrates and reaction monitoring were accomplished by TLC on silica-gel polygram SILG/UV 254 plates.Preparation of supported zinc chloride on alumina
A suspension of Al2O3 was activated at 300 °C during 5 h (3.0 g) and then ZnCl2 (1.0 g) in 40 ml of THF was added and the mixture refluxed at 80 °C for 15 h. After cooling the resulting masses to room temperature, the solids were filtered, washed successively with acetone and absolute ethanol and were dried under vacuum. The amount of the zinc ion on the surface of solid modified catalyst was measured by atomic absorption spectroscopy (1.45 mmol g−1 Al2O3). The solid modified catalyst was also characterized using FT-IR and XRD spectrophotometers.Typical procedure for the synthesis of phenol and naphthol derivatives using alumina supported-Lewis acid
The procedures for the ortho-acylation reaction are very simple. In a typical reaction, hydroquinone (0.95 mmol), ZnCl2@Al2O3 (0.50 g) and acetic acid (1.2 mmol) were combined for 80 s under microwave irradiation (450 W) and under atmospheric pressure. The reaction-mixture temperatures reached about 40 °C during microwave irradiation. After cooling to room temperature, the reaction mixture was dissolved in ethyl acetate (15 ml) and H2O (about 30 ml). After extracting the organic layer, it was washed with aqueous NaHCO3 (20 ml), dried with CaCl2, filtered and evaporated to give a crude product. Then crude products were chromatographed on silica gel using petroleum ether as the eluent. The products were confirmed by spectroscopic data and physical methods and were consistent with previously reported data.29–312,5-Dihydroxyacetophenone (a): mp 194–196 °C (lit.31 mp 198–200 °C); IR (KBr)/ν (cm−1): 3100–3500, 1620, 1500–1580, 1200, 1290; 1H NMR/DMSO-d6/δ ppm: 2.4 (s, 3 H), 6.5–6.9 (m, 3 H), 8.6 (s, 1 H), 9.4(s, 1 H).
2,6-Dihydroxyacetophenone (b): mp 158–162 °C (lit.30 mp 156–158 °C); IR (KBr)/ν (cm−1): 3100–3500, 1630, 1515, 1586, 1297; 1H NMR/CDCl3/δ ppm: 2.6 (s, 3 H), 6.4 (d, 2H, J = 5.8 Hz), 7.2 (m, 1 H), 11.8 (s, 2 H).
2,4-Dihydroxyacetophenone (c): mp 142–145 °C (lit.31 mp 144–146 °C); IR (KBr)/ν (cm−1): 3000–3500, 1620,1570; 1H NMR/CDCl3/δ ppm: 2.7 (s, 3 H), 6.4 (s, 1 H), 7.3–7.7 (m, 3 H), 12.8 (s, 1 H).
2-Hydroxy-3,5-dimethylacetophenone (d): oil, b.p. 230 °C (lit.29 b.p. 227 °C); IR (KBr)/ν (cm−1): 3100–3450, 1770, 1650, 1190, 1250; 1H NMR/CDCl3/δ ppm: 1.9 (s, 3 H), 2.4 (s, 3 H), 2.8 (s, 3 H), 6.8 (s, 1 H), 7.3 (s, 1 H), 12.6 (s, 1 H).
2,3-Dihydroxyacetophenone (e): mp 96–97 °C (lit.29 mp 97–98 °C); IR (KBr)/ν (cm−1): 3100–3600, 1724, 1450; 1H NMR/CDCl3/δ ppm: 2.6 (s, 3 H), 6.8 (t, 1H, J = 5.2 Hz), 7.1 (d, 1H, J = 5.3 Hz), 7.6 (d, 1H, J = 5.4 Hz), 5.8 (s, 1 H), 12.4 (s, 1 H).
2-Acetyl-1-naphthol (f): mp 96–98 °C (lit.29 mp 98 °C); IR (KBr)/ν (cm−1) 3100–3500, 1632, 1573, 1599; 1H NMR/CDCl3/δ ppm: 2.7 (s, 3 H), 6.85 (d, 1H, J = 6.4 Hz), 7.3 (m, 2 H), 7.62 (t, 2H, J = 5.5 Hz), 6.49 (d, 1H, J = 5.6 Hz), 14.2 (s, 1 H).
Acylation of hydroquinone with various organic acids in the presence of ZnCl2@Al2O3 catalyst
0.1 g (0.95 mmol) of hydroquinone, 0.1 ml (1.3 mmol) of propanoic acid and ZnCl2@Al2O3 catalyst (prepared by the method described above), were reacted together for 110 s under microwave irradiation (450 W). Extraction and identification of the products were carried out by the same procedures that were mentioned in the previous section.1-(2,5-Dihydroxyphenyl)-1-propanone (g): mp 96–99 °C (lit.31 mp 95–99 °C) IR (KBr)/ν (cm−1): 3100–3500, 1735, 1440, 1520, 1180; 1H NMR/CDCl3/δ ppm: 1.1 (t, 3 H, J = 6.1 Hz), 2.5(m, 2 H), 6.5 (s, 1 H), 6.7 (d, 1 H, J = 5.8 Hz), 6.9 (d, 1 H, J = 5.8 Hz), 8.6 (s, 1 H), 9.4(s, 1 H).
1-(2,5-Dihydroxyphenyl)-1-butanone (h): bp 346.5 °C; IR (KBr)/ν (cm−1): 3250–3500, 1750, 1600, 1650, 1180; 1H NMR/CDCl3/δ ppm: 1.1 (t, 3 H, J = 5.5 Hz), 1.8 (m, 2 H), 2.5 (m, 2 H), 6.8 (d, 1 H, J = 7.1 Hz), 6.9 (d, 1H, J = 7.1 Hz), 7.1 (s, 1H), 5.5 (s, 1H), 11.9 (s, 1 H).
1-(2,5-Dihydroxyphenyl)-1-pentanone (i): bp 357.5 °C; IR (KBr)/ν (cm−1): 3160–3400, 1740, 1510, 1190; 1H NMR/CDCl3/δ ppm: 0.9 (m, 3 H), 1.4 (m, 2 H), 1.6 (m, 2 H), 2.5 (t, 2 H, J = 6.0 Hz), 6.5(s, 1 H), 6.7 (d, 1 H, J = 5.5 Hz), 6.8 (d, 1 H, J = 5.3 Hz), 8.6 (s, 1 H), 9.4(s, 1 H).
Conclusions
This new solid catalyst can be used for the acylation of mono- and di-hydroxybenzene moieties, to form ortho-hydroxyaryl ketones in high yields. This reaction using a solid acid has advantages such as; reduced pollution, low cost, easy process and workup with high efficiency. The reactions can also take place in solvent-free conditions under microwave irradiation with respect to the development of cleaner technologies in green chemistry. Also we reported a method for the preparation of new compounds with different chemical characteristics that achieve optimal conditions for the preparation of this material.Acknowledgements
The authors are grateful to University of Kashan for supporting this work by grant no. 159148/15.Notes and references
-
H. K. Kouwenhoven and H. Van Bekkum, Handbook of Heterogeneous catalysis, VCH, Weinheim, Germany, 1997 Search PubMed
.
-
K. Krohn and N. Böker, 1,2-, 1,4-, 2,9- and 9,10-anthraquinones and higher fused polycyclic quinone derivatives, Thieme Verlag, Stuttgart, 2006 Search PubMed
.
- M. J. Climent, A. Corma, S. Iborra and J. Primo, J. Catal., 1995, 151, 60–66 CrossRef CAS
.
- M. T. Drexler and M. D. Amiridis, J. Catal., 2003, 214, 136–145 CrossRef CAS
.
- C. Santos, M. L. Mateus, A. P. dos Santos, R. Moreira, E. de Oliveira and P. Gomes, Bioorg. Med. Chem., 2005, 15, 1595–1598 CrossRef CAS PubMed
.
-
Z. Rappoport, The chemistry of phenols, Wiley-Interscience, 2003 Search PubMed
.
-
J. H. P. Tyman, Synthetic and natural phenols, Elsevier Science, 1996 Search PubMed
.
- J. Hu, E. A. Adogla, Y. Ju, D. Fan and Q. Wang, Chem. Commun., 2012, 48, 11256–11258 RSC
.
- F. Mo, L. J. Trzepkowski and G. Dong, Angew. Chem., Int. Ed., 2012, 124, 13252–13256 CrossRef PubMed
.
- I. Neves, F. Jayat, P. Magnoux, G. Perot, F. Ribeiro, M. Gubelmann and M. Guisnet, J. Mol. Catal. A: Chem., 1994, 93, 169–179 CrossRef CAS
.
- G. Y. Popova, A. A. Davydov and T. V. Andrushkevich, React. Kinet. Catal. Lett., 1990, 41, 33–38 CrossRef CAS
.
- L. F. Fieser and E. B. Hershberg, J. Am. Chem. Soc., 1940, 62, 1640–1645 CrossRef CAS
.
- R. D. Haworth, J. Chem. Soc., 1932, 2717–2720 RSC
.
- J. W. Cook, J. Chem. Soc., 1933, 1592–1597 RSC
.
- J. Koo, J. Org. Chem., 1963, 28, 1134–1135 CrossRef CAS
.
- H. Sharghi and B. Kaboudin, J. Chem. Res. (S), 1998, 628–629 RSC
.
- M. J. Climent, A. Corma, S. Iborra and A. Velty, J. Catal., 2004, 221, 474–482 CrossRef CAS PubMed
.
- R. S. Varma and V. V. Namboodiri, Pure Appl. Chem., 2001, 73, 1309–1313 CAS
.
- G. Nagendrappa, Resonance, 2002, 7, 59–68 CrossRef CAS
.
- W. Wang and J. C. T. Kwak, Colloids Surf., A, 1999, 156, 95–110 CrossRef CAS
.
- L. Huang, C. Maltesh and P. Somasundaran, J. Colloid Interface Sci., 1996, 177, 222–228 CrossRef CAS PubMed
.
- M. Trueba and S. P. Trasatti, Eur. J. Inorg. Chem., 2005, 2005, 3393–3403 CrossRef PubMed
.
- N. Bukhanko, A. Samikannu, W. Larsson, A. Shchukarev, A.-R. Leino, K. Kordás, J. Wärnå and J.-P. Mikkola, ACS Sustainable Chem. Eng., 2013, 1, 883–893 CrossRef CAS
.
- Z. Fu, Y. Yu, D. Yin, Y. Xu, H. Liu, H. Liao, Q. Xu, F. Tan and J. Wang, J. Mol. Catal. A: Chem., 2005, 232, 69–75 CrossRef CAS PubMed
.
- D. Habibi, M. Nasrollahzadeh and A. T. Kamali, Green Chem., 2011, 13, 3499–3504 RSC
.
- A. Loupy, A. Petit, J. Hamelin, F. Texier-Boullet, P. Jacquault and D. Mathe, Synthesis, 2000, 1213–1234 Search PubMed
.
- R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, Tetrahedron Lett., 1986, 27, 279–282 CrossRef CAS
.
- K. B. Kumar and P. Raji, Recent Res. Sci. Technol., 2011, 3, 48–52 Search PubMed
.
- R. W. Stoughton, J. Am. Chem. Soc., 1935, 57, 202–204 CrossRef CAS
.
- J. J. Gorman, B. L. Ferguson and T. B. Nguyen, Rapid Commun. Mass Spectrom., 1996, 10, 529–536 CrossRef CAS
.
- S. Paul, P. Nanda, R. Gupta and A. Loupy, Synthesis, 2003, 2877–2881 CAS
.
| This journal is © the Partner Organisations 2014 |