A water-soluble pillar[10]arene: synthesis, pH-responsive host–guest complexation, and application in constructing a supra-amphiphile†
Jie
Yang
a,
Xiaodong
Chi
a,
Zhengtao
Li
a,
Guocan
Yu
a,
Jiuming
He
b,
Zeper
Abliz
b,
Ning
Li
a and
Feihe
Huang
*a
aState Key Laboratory of Chemical Engineering, Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: fhuang@zju.edu.cn; Fax: +86-571-8795-3189; Tel: +86-571-8795-3189
bInstitute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, P. R. China
First published on 5th May 2014
Abstract
A water-soluble pillar[10]arene was prepared. Its pH-responsive host–guest complexation with paraquat and application in constructing a supra-amphiphile were investigated.
Supramolecular chemistry has attracted considerable attention in the last decade due to its superiority in the fabrication of artificial molecular machines, supramolecular polymers, supramolecular gels and other functional supramolecular systems.1 Macrocyclic hosts, such as calixarenes,2 crown ethers,3 cyclodextrins,4 and cucurbiturils,5 have played prominent roles in the development of supramolecular chemistry. Therefore, it is of continuing interest to design and synthesize novel macrocyclic hosts. Pillar[n]arenes, as a kind of emerging macrocyclic hosts, have been studied actively since the first paper published in 2008.6a The unique columnar conformation and accessible functionalization of pillararenes gave them an outstanding ability to build various host–guest recognition motifs by selectively binding different kinds of guests and provided a useful platform for the construction of novel supramolecular systems, such as chemosensors,6n transmembrane channels,6q supramolecular polymers6d and drug delivery systems.7f The synthesis and host–guest chemistry of pillar[5,6]arenes have been widely explored. However, limited by the cavity size, research on the host–guest chemistry based on pillar[5,6]arenes was confined to a large extent, so the preparation of advanced pillar[n ≥ 7]arenes plays a significant part in the evolution of pillar[n]arenes.8 Herein, we report the synthesis of the first water-soluble pillar[10]arene (WP10 here) and its pH-responsive binding to paraquat and application in the construction of a supra-amphiphile.
WP10 was synthesized by introducing carboxylate anionic groups on both rims (Scheme 1). Compound 1 was obtained by a one-step method reported by Hou's group.8b By dealkylation of 1, per-hydroxylated pillar[10]arene 2 was obtained, and then methoxycarbonylmethoxy-substituted pillar[10]arene 3 was prepared by etherification of 2. The subsequent acidification after hydrolysis of 3 under basic conditions afforded carboxylic acid-substituted pillar[10]arene 4. By treatment with excessive ammonium hydroxide, WP10 was obtained.
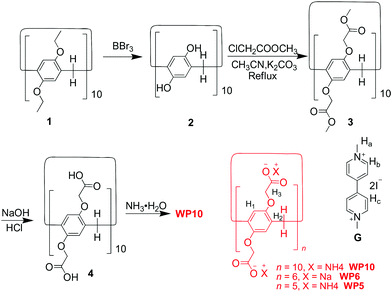 | ||
| Scheme 1 Synthetic route to water-soluble pillar[10]arene WP10 and chemical structures of other compounds studied here. | ||
Due to the existence of twenty carboxylate anionic groups on both rims, WP10 can be a wonderful host for cationic guest molecules. The complexation between paraquat G and WP10 was firstly studied by 1H NMR spectroscopy. As shown in Fig. 1, when equimolar WP10 was added into a solution of G (1.00 mM), the signals related to the protons on paraquat shifted upfield significantly. Additionally, an extensive broadening effect occurred when paraquat interacted with WP10 due to complexation dynamics. The reason is that these protons were located within the cavity of WP10 and were shielded by the electron-rich cyclic structure upon forming a threaded structure between G and WP10. The peak for proton Ha shifted upfield slightly (from 4.42 to 4.15 ppm) compared with protons Hb and Hc on the pyridinium units (Δδ = −0.51 and −0.88 ppm), for the reason that when G penetrated into the cavity of WP10, protons Hb and Hc were located in the electron-rich cavity of WP10, while protons Ha were outside the cavity. On the other hand, protons on WP10 also exhibited slight chemical shift changes due to the interactions with paraquat G.
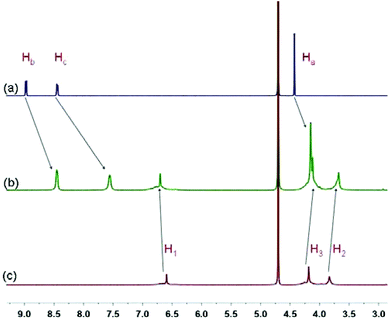 | ||
| Fig. 1 Partial 1H NMR spectra (400 MHz, D2O, 293 K): (a) G (1.00 mM); (b) G (1.00 mM) and WP10 (1.00 mM); (c) WP10 (1.00 mM). Ha–Hc and H1–H3 are the related protons on G and WP10, respectively, displayed in Scheme 1. | ||
Further evidence for the complexation between WP10 and G was obtained from UV-vis absorption spectroscopy and NOESY. When G and WP10 were mixed in water with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, the color of the resulting solution turned red immediately, and the spectrum exhibited a broad absorption above 400 nm, which corresponded to the characteristic absorption of the charge-transfer complex between electron-rich WP10 and electron-deficient G (Fig. S16, ESI†).9 Additionally, a NOESY study of a solution of 5.00 mM WP10 and G was conducted to investigate the relative spatial positions of this host–guest complex. As shown in Fig. S17,† correlations between protons Hb and Hc on G and protons H1 on WP10 were observed, suggesting that paraquat G was threaded into the cavity of WP10.
1 molar ratio, the color of the resulting solution turned red immediately, and the spectrum exhibited a broad absorption above 400 nm, which corresponded to the characteristic absorption of the charge-transfer complex between electron-rich WP10 and electron-deficient G (Fig. S16, ESI†).9 Additionally, a NOESY study of a solution of 5.00 mM WP10 and G was conducted to investigate the relative spatial positions of this host–guest complex. As shown in Fig. S17,† correlations between protons Hb and Hc on G and protons H1 on WP10 were observed, suggesting that paraquat G was threaded into the cavity of WP10.
For the estimation of the binding constant, fluorescence titration experiments of WP10 with G were conducted in aqueous solution at room temperature. As shown in Fig. S13,† the decrease of fluorescence intensity was found upon gradual addition of G. A mole ratio plot (Fig. S14, ESI†) based on the fluorescence titration experiments demonstrated that the complex between WP10 and G has a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Further evidence for the formation of the 1
1 stoichiometry. Further evidence for the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between WP10 and G was obtained by the electrospray ionization mass spectrum. A peak was observed at m/z 1392.3 corresponding to [WP10⊃G − 2I − 7NH4 + 3H]2− (Fig. S21, ESI†). By a non-linear curve-fitting method, the Ka value for WP10⊃G was calculated to be (1.25 ± 0.21) × 107 M−1 (Fig. S15, ESI†), which is higher than that for the complex between WP5 and G (8.20 × 104 M−1),10 but lower than the corresponding Ka value for the complex between WP6 and G (1.02 × 108 M−1).7e A reasonable explanation for this binding constant difference was proposed. WP10 tends to form a much larger cavity than WP5 or WP6 due to the strong electrostatic repulsion generated by the negative anionic groups on both rims in solution. Therefore, the cavity of WP10 is too large for paraquat to form a more stable host–guest complex, resulting in a lower Ka value than that of WP6. While for the relatively stronger electrostatic interaction generated by the twenty carboxylate anionic groups, the Ka value is larger than that of WP5.
1 complex between WP10 and G was obtained by the electrospray ionization mass spectrum. A peak was observed at m/z 1392.3 corresponding to [WP10⊃G − 2I − 7NH4 + 3H]2− (Fig. S21, ESI†). By a non-linear curve-fitting method, the Ka value for WP10⊃G was calculated to be (1.25 ± 0.21) × 107 M−1 (Fig. S15, ESI†), which is higher than that for the complex between WP5 and G (8.20 × 104 M−1),10 but lower than the corresponding Ka value for the complex between WP6 and G (1.02 × 108 M−1).7e A reasonable explanation for this binding constant difference was proposed. WP10 tends to form a much larger cavity than WP5 or WP6 due to the strong electrostatic repulsion generated by the negative anionic groups on both rims in solution. Therefore, the cavity of WP10 is too large for paraquat to form a more stable host–guest complex, resulting in a lower Ka value than that of WP6. While for the relatively stronger electrostatic interaction generated by the twenty carboxylate anionic groups, the Ka value is larger than that of WP5.
To investigate the pH-responsiveness of WP10, 1H NMR spectroscopy was utilized (Fig. S18, ESI†). When the water-soluble pillar[10]arene precipitated from D2O after acidification of the solution by adding DCl, proton signals of WP10 disappeared accompanied by the proton signals of paraquat G returning to the positions before complexation with WP10. These indicated that no interactions existed between WP10 and G after WP10 was acidified. When the solution was made basic again by addition of NaOD, the precipitated host redissolved, the proton signals of WP10 reappeared, and the protons of paraquat G shifted upfield again. These results demonstrated that the host–guest complexation between WP10 and G could be easily controlled by changing the pH of the solution.
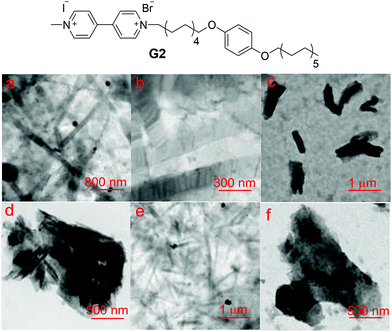
After the investigation of this new molecular recognition motif, we constructed a supra-amphiphile based on the host–guest complexation between WP10 and an amphiphilic guest G2 derived from the 4,4′-bipyridinium unit (Fig. 2). By using the concentration-dependent conductivity, the critical aggregation concentration (CAC) of G2 was determined to be 8.87 × 10−6 M (Fig. S19†). As revealed by TEM, the typical amphiphilic G2 itself self-assembled in water to form nanoribbons (Fig. 2a). Upon addition of WP10, the CAC value increased to 4.06 × 10−5 M (Fig. S20†), which was about 4 times higher than that of G2, confirming the host–guest complexation between WP10 and G2. From the TEM images, nanosheets were observed (Fig. 2c), drastically different from the nanoribbons formed by G2 alone.
A mechanism was proposed to explain the change in the shape of G2 aggregates which transformed from nanoribbons to nanosheets after complexation with WP10. The micro-assembled structure of the aggregates formed by amphiphiles is determined by the curvature of the membrane.11 The hydrophobic parts of amphiphiles favor aggregation while the hydrophilic parts are prone to stay in water, so G2 self-assembled in solution with the hydrophobic alkyl chains in the interlayer of the nanoribbons. After complexation with WP10, the anionic hosts were introduced into the hydrophilic membrane of nanoribbons; because of the steric hindrance and electrostatic repulsion generated upon insertion of WP10 molecules, the formation of a nanosheet structure with lower curvature was spontaneous.
The above discussed pH-responsiveness of the WP10⊃G complex was utilized to control the reversible aggregation nanostructure transition by changing the solution pH. As shown in Fig. 2e, nanoribbons appeared again by adjusting the solution pH to 6.0. As expected, when the pH was adjusted to 7.4, the nanosheets could be regained (Fig. 2f). As demonstrated above, WP10 precipitated when the solution pH decreased, leading to the destruction of WP10⊃G2, so nanoribbons were observed. Therefore, self-assembly of this host–guest system can be easily controlled by changing the solution pH.
In conclusion, we reported the synthesis of the first water-soluble pillar[10]arene and its pH-responsive host–guest complexation with paraquat. Furthermore, a supra-amphiphile based on this new host–guest recognition motif was constructed. Compared to the nanoribbon aggregates built by amphiphilic molecule G2, the host–gust complex WP10⊃G2 self-assembled into nanosheets instead. By changing the solution pH, the reversible transformation from nanoribbons to nanosheets could be controlled easily. This novel recognition motif based on the water soluble pillar[10]arene in aqueous media will be helpful for the fabrication of functional architectures and definitely bring about many promising applications, such as supramolecular polymers, nanoelectronics, drug-delivery and controlled release.
This work was supported by the National Basic Research Program (2013CB834502) and the National Natural Science Foundation of China (21125417).
Notes and references
- (a) J. M. Lehn, Science, 1985, 227, 849–856 CAS; (b) P. Jonkheijm and E. W. Meijer, Science, 2006, 313, 80–83 CrossRef CAS PubMed; (c) Y. Han, Y.-K. Gu, J.-F. Xiang and C.-F. Chen, Chem. Commun., 2012, 48, 11076–11078 RSC; (d) Y. Ding, P. Wang, Y.-K. Tian, Y.-J. Tian and F. Wang, Chem. Commun., 2013, 49, 5951–5953 RSC; (e) G.-Z. Zhao, L.-J. Chen, W. Wang, J. Zhang, G. Yang, D.-X. Wang, Y. Yu and H.-B. Yang, Chem. – Eur. J., 2013, 19, 10094–10100 CrossRef CAS PubMed; (f) B. Jiang, L.-J. Chen, L. Xu, S.-Y. Liu and H.-B. Yang, Chem. Commun., 2013, 49, 6977–6979 RSC; (g) H.-Q. Peng, J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Commun., 2014, 50, 1334–1337 RSC.
- (a) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907–5921 RSC; (b) L.-X. Wang, L. Zhao, D.-X. Wang and M.-X. Wang, Chem. Commun., 2011, 47, 9690–9692 RSC; (c) D.-X. Wang, S.-X. Fa, Y. Liu, B.-Y. Hou and M.-X. Wang, Chem. Commun., 2012, 48, 11458–11460 RSC.
- (a) G. J. E. Davidson and S. J. Loeb, Angew. Chem., Int. Ed., 2003, 42, 74–77 CrossRef CAS; (b) F. Huang, F. R. Fronczek and H. W. Gibson, J. Am. Chem. Soc., 2003, 125, 9272–9273 CrossRef CAS PubMed; (c) F. Huang and H. W. Gibson, J. Am. Chem. Soc., 2004, 126, 14738–14739 CrossRef CAS PubMed; (d) F. Huang, D. S. Nagvekar, C. Slebodnick and H. W. Gibson, J. Am. Chem. Soc., 2005, 127, 484–485 CrossRef CAS PubMed; (e) W. Jiang, A. Schäfer, P. C. Mohr and C. A. Schalley, J. Am. Chem. Soc., 2010, 132, 2309–2320 CrossRef CAS PubMed; (f) M. Liu, S. Li, M. Hu, F. Wang and F. Huang, Org. Lett., 2010, 12, 760–763 CrossRef CAS PubMed; (g) M. Liu, X. Yan, M. Hu, X. Chen, M. Zhang, B. Zheng, X. Hu, S. Shao and F. Huang, Org. Lett., 2010, 12, 2558–2661 CrossRef CAS PubMed; (h) S. Li, J. Chen, B. Zheng, S. Dong, Z. Ma, H. W. Gibson and F. Huang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4067–4073 CrossRef CAS; (i) X. Yan, M. Zhang, P. Wei, B. Zheng, X. Chi, X. Ji and F. Huang, Chem. Commun., 2011, 47, 9840–9842 RSC.
- (a) A. Harada, Acc. Chem. Res., 2001, 34, 456–464 CrossRef CAS PubMed; (b) Q.-C. Wang, D.-H. Qu, J. Ren, K. Chen and H. Tian, Angew. Chem., Int. Ed., 2004, 43, 2661–2665 CrossRef CAS; (c) X. Liao, G. Chen, X. Liu, W. Chen, F. Chen and M. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4409–4413 CrossRef CAS PubMed; (d) T. Liu, X. J. Li, Y. F. Qian, X. L. Hu and S. Y. Liu, Biomaterials, 2012, 33, 2521–2531 CrossRef CAS PubMed.
- (a) W.-H. Huang, P. Y. Zavalij and L. Isaacs, Angew. Chem., Int. Ed., 2007, 46, 7425–7427 CrossRef CAS PubMed; (b) Y. Lan, X. J. Loh, J. Geng, Z. Walsh and O. A. Scherman, Chem. Commun., 2012, 48, 8757–8759 RSC.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed; (b) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed; (c) C. Li, L. Zhao, J. Li, X. Ding, S. Chen, Q. Zhang, Y. Yu and X. Jia, Chem. Commun., 2010, 46, 9016–9018 RSC; (d) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS PubMed; (e) Z. Zhang, G. Yu, C. Han, J. Liu, X. Ding, Y. Yu and F. Huang, Org. Lett., 2011, 13, 4818–4821 CrossRef CAS PubMed; (f) C. Li, X. Shu, J. Li, S. Chen, K. Han, M. Xu, B. Hu, Y. Yu and X. Jia, J. Org. Chem., 2011, 76, 8458–8465 CrossRef CAS PubMed; (g) W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen, J.-L. Hou and Z.-T. Li, Angew. Chem., Int. Ed., 2011, 50, 12564–12568 CrossRef CAS PubMed; (h) Z. Zhang, C. Han, G. Yu and F. Huang, Chem. Sci., 2012, 3, 3026–3031 RSC; (i) C. Han, G. Yu, B. Zheng and F. Huang, Org. Lett., 2012, 14, 1712–1715 CrossRef CAS PubMed; (j) M. Holler, N. Allenbach, J. Sonet and J.-F. Nierengarten, Chem. Commun., 2012, 48, 2576–2578 RSC; (k) X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Chem. Commun., 2012, 48, 2967–2969 RSC; (l) I. Nierengarten, S. Guerra, M. Holler, J.-F. Nierengarten and R. Deschenaux, Chem. Commun., 2012, 48, 8072–8074 RSC; (m) Y. Guan, M. Ni, X. Hu, T. Xiao, S. Xiong, C. Lin and L. Wang, Chem. Commun., 2012, 48, 8529–8531 RSC; (n) G. Yu, Z. Zhang, C. Han, M. Xue, Q. Zhou and F. Huang, Chem. Commun., 2012, 48, 2958–2960 RSC; (o) P. Wei, X. Yan, J. Li, Y. Ma, Y. Yao and F. Huang, Tetrahedron, 2012, 68, 9179–9185 CrossRef CAS PubMed; (p) X.-Y. Hu, X. Wu, Q. Duan, T. Xiao, C. Lin and L. Wang, Org. Lett., 2012, 14, 4826–4829 CrossRef CAS PubMed; (q) X.-B. Hu, Z. Chen, G. Tang, J.-L. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384–8387 CrossRef CAS PubMed; (r) Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712–15715 CrossRef CAS PubMed; (s) H. Zhang, X. Ma, J. Guo, K. T. Nguyen, Q. Zhang, X.-J. Wang, H. Yan, L. Zhu and Y. Zhao, RSC Adv., 2013, 3, 368–371 RSC; (t) D.-X. Chen, Y.-L. Sun, Y. Zhang, J.-Y. Cui, F.-Z. Shen and Y.-W. Yang, RSC Adv., 2013, 3, 5765–5768 RSC; (u) Y. Fang, L. Wu, J. Liao, L. Chen, Y. Yang, N. Liu, L. He, S. Zou, W. Feng and L. Yuan, RSC Adv., 2013, 3, 12376–12383 RSC; (v) Y.-L. Sun, Y.-W. Yang, D.-X. Chen, G. Wang, Y. Zhou, C.-Y. Wang and J. F. Stoddart, Small, 2013, 9, 3224–3229 CAS; (w) J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Org. Lett., 2013, 15, 6148–6151 CrossRef CAS PubMed; (x) G. Yu, Y. Ma, C. Han, Y. Yao, G. Tang, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2013, 135, 10310–10313 CrossRef CAS PubMed; (y) H. Li, D.-X. Chen, Y.-L. Sun, Y. Zheng, L.-L. Tan, P. S. Weiss and Y.-W. Yang, J. Am. Chem. Soc., 2013, 135, 1570–1576 CrossRef CAS PubMed; (z) S. Dong, B. Zheng, Y. Yao, C. Han, J. Yuan, M. Antonietti and F. Huang, Adv. Mater., 2013, 25, 6864–6867 CrossRef CAS PubMed.
- (a) Y. Ma, Z. Zhang, X. Ji, C. Han, J. He, A. Zeper, W. Chen and F. Huang, Eur. J. Org. Chem., 2011, 5331–5335 CrossRef CAS; (b) Y. Ma, X. Chi, X. Yan, J. Liu, Y. Yao, W. Chen, F. Huang and J.-L. Hou, Org. Lett., 2012, 14, 1532–1535 CrossRef CAS PubMed; (c) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248–13251 CrossRef CAS PubMed; (d) G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711–8717 CrossRef CAS PubMed; (e) G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS PubMed; (f) Q. Duan, C. Yu, L. Yan, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542–10549 CrossRef CAS PubMed; (g) H. Zhang and Y. Zhao, Chem. – Eur. J., 2013, 19, 16862–16879 CrossRef CAS PubMed; (h) D. Xia, G. Yu, J. Li and F. Huang, Chem. Commun., 2014, 50, 3606–3608 RSC.
- (a) Y. Chen, H. Q. Tao, Y. H. Kou, H. Meier, J. L. Fu and D. R. Cao, Chin. Chem. Lett., 2012, 23, 509–511 CrossRef CAS PubMed; (b) X.-B. Hu, Z. Chen, L. Chen, L. Zhang, J.-L. Hou and Z.-T. Li, Chem. Commun., 2012, 48, 10999–11001 RSC; (c) C. Han, Z. Zhang, X. Chi, M. Zhang, G. Yu and F. Huang, Acta Chim. Sin., 2012, 70, 1775–1778 CAS.
- Y. Shimazaki and M. Yamamoto, Langmuir, 1997, 13, 1385–1387 CrossRef CAS.
- T. Ogoshi, M. Hashizume, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 3708–3710 RSC.
- C. Wang, S. Yin, S. Chen, H. Xu, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2008, 47, 9049–9052 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization, stoichiometry and association constant determination, UV-vis data and other materials. See DOI: 10.1039/c4qo00086b |
| This journal is © the Partner Organisations 2014 |