Kinetic resolution of 4-substituted-3,4-dihydrocoumarins via Pd-catalyzed asymmetric allylic alkylation reaction: enantioselective synthesis of trans-3,4-disubstituted-3,4-dihydrocoumarins†
Xiao-Hui
Li
a,
Ping
Fang
a,
Di
Chen
a and
Xue-Long
Hou
*ab
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: xlhou@sioc.ac.cn
bShanghai-Hong Kong Joint Laboratory in Chemical Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 25th July 2014
Abstract
The kinetic resolution of 4-substituted-3,4-dihydrocoumarins was realized via Pd-catalyzed allylic substitution reaction using Trost's chiral ligand L12, affording optically active mono- and trans-3,4-disubstituted dihydrocoumarin derivatives in high yields and with high enantioselectivities with an S factor up to 55.
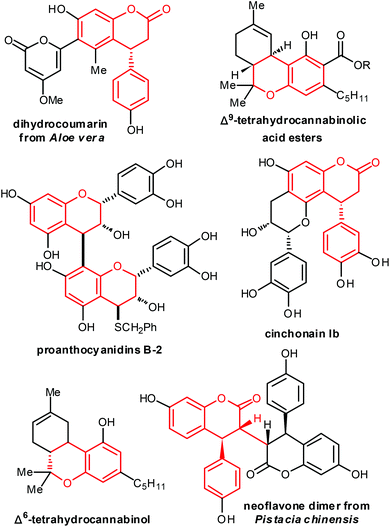
Dihydrocoumarin as a subunit has been found in a wide range of natural products as well as in biologically active molecules (Fig. 1).1 In addition, it is also useful building blocks in the synthesis of some other important compounds.2,5a Therefore, numerous procedures have been developed to approach mono- or disubstituted dihydrocoumarin derivatives,3–6 however, only a few of them5a,e are suitable to synthesise trans-3,4-disubstituted dihydrocoumarin derivatives in an asymmetric and catalytic way, all of which are involved in conjugate additions. The chiral center at the 3-position was installed using an aldol reaction which proceeds very often via the intermediates of conjugate additions.5a,b Thus, the development of new asymmetric and catalytic strategies to access trans-3,4-disubstituted dihydrocoumarins remains to be explored.
In contrast, the palladium-catalyzed asymmetric allylic alkylation (AAA), one of the most powerful tools for the enantioselective formation of carbon–carbon and carbon–heteroatom bonds,7 has found a wide range of applications in organic synthesis. Different kinds of “hard” carbanions, including the ones derived from ketones, amides, acyl silanes, 2-methylpyridine, etc.,8,9 have also been used successfully as nucleophiles in recent achievements. However, only one report appeared using esters as nucleophilic precursors in this reaction,8j in spite of the fact that esters are a very useful class of compounds in organic synthesis.10 Kinetic resolution as a powerful tool has also been used in Pd-catalyzed AAA reactions,11 whereby the nucleophiles, including “hard” carbanions, can also be resolved.12 However, the examples are still rare. We have worked on the Pd-catalyzed AAA reaction for many years, developing high diastereo- and enantioselective reactions with different kinds of “hard” carbanion nucleophiles,9 as well as the resolution of ketones with high efficiencies.12b In light of these results, we envisioned that the esters and lactones could be resolved in the Pd-catalyzed AAA reaction. Herein we would like to report our strategy to approach optically active mono- and trans-3,4-disubstituted dihydrocoumarin derivatives using kinetic resolution of 4-substituted-3,4-dihydrocoumarins via a Pd-catalyzed AAA reaction.
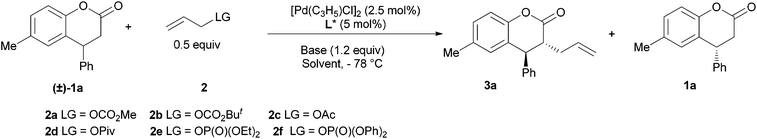
Initially, 6-methyl-4-phenyl-3,4-dihydrocoumarin 1a was subjected to the reaction with allyl carbonate 2a in the presence of catalytic amounts of [Pd(C3H5)Cl]2 and L1, using LiHMDS as a base in THF at −78 °C, providing 3a in 51% yield with 62% ee and 39% recovery of 1a with 66% ee, the S-factor being 8.3 (Table 1, entry 1). These results encouraged us to further study the impact of the reaction parameters on the reaction (Table 1).
| Entry | Base | Solvent | L | 2 | 1a | 3a | S | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Yieldb (%) | eec (%) | Yieldb (%) | eec (%) | drd | ||||||
a Reaction was carried out in THF at −78 °C, molar ratio of 1a/2/[Pd(C3H5)Cl]2/L/base = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120.
b Isolated yield.
c Determined using chiral HPLC.
d Determined using 1H NMR.
e Calculated using the method described by Kagan.13
f Due to a reversed sequence of peaks using HPLC.
g Reaction was carried out at −65 °C. 120.
b Isolated yield.
c Determined using chiral HPLC.
d Determined using 1H NMR.
e Calculated using the method described by Kagan.13
f Due to a reversed sequence of peaks using HPLC.
g Reaction was carried out at −65 °C.
|
||||||||||
| 1 | LiHMDS | THF | L1 | 2a | 39 | 66 | 51 | 62 | >30/1 | 8.3 |
| 2 | NaHMDS | THF | L1 | 2a | 59 | 37 | 36 | 69 | >30/1 | 7.8 |
| 3 | KHMDS | THF | L1 | 2a | 35 | 6 | 57 | 4 | >30/1 | 1.1 |
| 4 | LDA | THF | L1 | 2a | 27 | −1f | 71 | 0 | 7/1 | 1 |
| 5 | KOtBu | THF | L1 | 2a | 24 | −5f | 38 | 2 | 16/1 | 1.1 |
| 6g | LiHMDS | DME | L1 | 2a | 31 | 67 | 55 | 50 | >30/1 | 5.8 |
| 7 | LiHMDS | Et2O | L1 | 2a | 50 | 39 | 50 | 31 | >30/1 | 2.7 |
| 8 | LiHMDS | Toluene | L1 | 2a | 36 | 39 | 48 | 42 | 14/1 | 3.5 |
| 9 | LiHMDS | DCM | L1 | 2a | 35 | 19 | 50 | 15 | 14/1 | 1.6 |
| 10 | LiHMDS | Hexane | L1 | 2a | 33 | 27 | 36 | 39/58 | 1/1 | 2.9 |
| 11 | LiHMDS | THF | L1 | 2b | 36 | 64 | 52 | 55 | >30/1 | 6.5 |
| 12 | LiHMDS | THF | L1 | 2c | 61 | 24 | 28 | 70 | >30/1 | 7.1 |
| 13 | LiHMDS | THF | L1 | 2d | 40 | 34 | 32 | 73 | 6/1 | 8.9 |
| 14 | LiHMDS | THF | L1 | 2e | 49 | 51 | 43 | 67 | 10/1 | 8.3 |
| 15 | LiHMDS | THF | L1 | 2f | 36 | 65 | 54 | 54 | >30/1 | 6.4 |
| 16 | LiHMDS | THF | L2 | 2a | 19 | — | 47 | 0 | >30/1 | — |
| 17 | LiHMDS | THF | L5 | 2a | 30 | 43 | 51 | 31 | >30/1 | 2.8 |
| 18 | LiHMDS | THF | L6 | 2a | 35 | 63 | 47 | 60 | >30/1 | 7.5 |
| 19 | LiHMDS | THF | L7 | 2a | 36 | 58 | 49 | 39 | >30/1 | 3.9 |
| 20 | LiHMDS | THF | L8 | 2a | 36 | 65 | 54 | 58 | >30/1 | 7.2 |
| 21 | LiHMDS | THF | L9 | 2a | 37 | −43f | 55 | −34f | >30/1 | 3.0 |
| 22 | LiHMDS | THF | L10 | 2a | 43 | 61 | 47 | 60 | >30/1 | 7.3 |
| 23 | LiHMDS | THF | L11 | 2a | 43 | 59 | 49 | 66 | >30/1 | 8.7 |
| 24 | LiHMDS | THF | L12 | 2a | 40 | 66 | 45 | 86/81 | 2.5/1 | 26 |
| 25 | LiHMDS | THF | L12 | 2f | 49 | 75 | 46 | 89 | >30/1 | 39 |
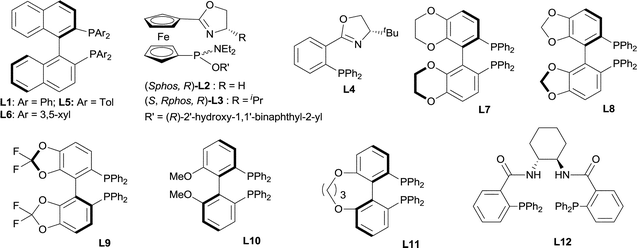
First, some bases were examined (Table 1, entries 1–5). It was found that they had a great impact on the enantioselectivity, the ee values of both 1a and 3a were less than 10% when employing KHMDS, LDA and KOt-Bu as the base (entries 3–5), while a comparable S-factor was afforded if NaHMDS was used (entry 2 vs. entry 1) instead of LiHMDS. The screening of some common solvents showed that the S-factor decreased from 8.3 to 5.8 with DME as the solvent (entry 6 vs. entry 1). An even worse S-factor was afforded with Et2O, toluene, DCM or hexane as the solvent (entries 7–10), and also no diastereoselectivity was observed in hexane (entry 10). The influence of the leaving group (LG) on the allyl reagent was also investigated (entries 11–15). The S-factor decreased when carbonate 2b, acetate 2c and phosphate 2f were used (entries 11, 12 and 15). The reactions of carboxylate 2d and phosphate 2e gave similar S-factors but lower dr values than that of 2a (entries 13 and 14 vs. entry 1). A series of chiral ligands with different electronic and steric factors were screened (Fig. 2). Racemic 3a was afforded in 47% yield and 1a was recovered in 19% yield by using L2 (entry 16), and the reaction was sluggish in the presence of L3 or L4 (not shown in Table 1). A worse S-factor was achieved when L5 with an o-tolyl group on the P atom was used (entry 17 vs. entry 1). The reaction using L6 with much more steric hindrance on the P atom, afforded a slightly lower S-factor (entry 18 vs. entry 1), and those using ligands L7, L8, L9 and L10 with a biphenylene backbone, afforded S-factors between 3.0–8.7 (entries 19–23). However, the S-factor increased significantly with Trost's ligand L12 though the diastereoselectivity was low (entry 24). Both the S-factor and the diastereoselectivity were improved if allyl phosphate 2f was used, the S-factor being 39 and dr being 30/1 (entry 25).
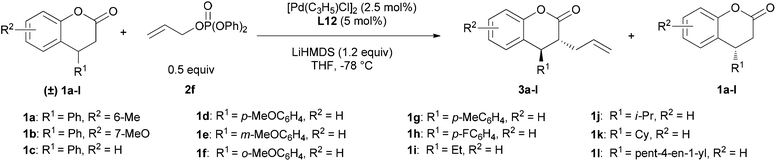
With the optimized conditions, the substrate scope was examined (Table 2). It can be seen that all reactions provided the allylated products 3 in high yields with an excellent trans-stereoselectivity, a dr over 30/1 and high yields of the recovered starting materials 1, the S-factor being 8–55. The substituent on the phenyl ring of the 3,4-dihydrocoumarins’ core had a smaller effect on the enantioselectivity and yield for both the recovered starting materials and allylated products (entries 1–2). The reaction also proceeded well for the substrates with a 4-aryl substituent having either an electron-withdrawing or donating group at different positions of the phenyl ring (entries 3–8). It is worthwhile to note that the dihydrocoumarins with aliphatic substituents at the 4-position were also suitable substrates for this kinetic resolution, affording the corresponding allylated products 3i–k, accompanied by recovered 1i–k, in high efficiency, the S-factor being 13–43 (entries 9–11). Only for substrate 1l with a pent-4-en-1-yl group the S-factor decreased to 8 (entry 12). Hence, when R1 was an alkyl group, the results were uncertain. The S-factor was excellent for the reaction of the dihydrocoumarin with an ethyl group on the 4-position (entry 9), while the S-factor dropped greatly for that with a pent-4-en-1-yl group on the 4-position (entry 12) and comparative S-factors were afforded for the dihydrocoumarins with an isopropyl or cyclohexyl group on the 4-position (entries 10 and 11).
| Entry | Recovered substrate | Product | S | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | Yieldb (%) | eec (%) | 3 | Yieldb (%) | eec (%) | drd | ||
a Reaction was carried out in THF at −78 °C, molar ratio of 1/2f/[Pd(C3H5)Cl]2/L12/LiHMDS = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120.
b Isolated yield.
c Determined using chiral HPLC.
d Determined using 1H NMR.
e Calculated using the method described by Kagan.13 120.
b Isolated yield.
c Determined using chiral HPLC.
d Determined using 1H NMR.
e Calculated using the method described by Kagan.13
|
||||||||
| 1 | 1a | 49 | 75 | 3a | 46 | 89 | >30/1 | 39 |
| 2 | 1b | 43 | 76 | 3b | 46 | 92 | >30/1 | 55 |
| 3 | 1c | 45 | 79 | 3c | 50 | 87 | >30/1 | 35 |
| 4 | 1d | 33 | 43 | 3d | 46 | 90 | >30/1 | 29 |
| 5 | 1e | 48 | 76 | 3e | 43 | 87 | >30/1 | 33 |
| 6 | 1f | 42 | 91 | 3f | 51 | 76 | >30/1 | 23 |
| 7 | 1g | 42 | 94 | 3g | 48 | 80 | >30/1 | 31 |
| 8 | 1h | 46 | 90 | 3h | 44 | 82 | >30/1 | 31 |
| 9 | 1i | 51 | 75 | 3i | 47 | 90 | >30/1 | 43 |
| 10 | 1j | 44 | 64 | 3j | 44 | 76 | >30/1 | 14 |
| 11 | 1k | 52 | 67 | 3k | 48 | 74 | >30/1 | 13 |
| 12 | 1l | 44 | 70 | 3l | 50 | 60 | >30/1 | 8 |
The absolute configuration of 1i was determined to be S by comparing its optical rotation with that reported in the literature.14 The NOE data of 3i showed its 3,4-trans-configuration. Thus, the absolute configuration of 3i was assigned to be (3R,4R).
In summary, the present work successfully realized the kinetic resolution of lactones reacting as carbon nucleophiles in the Pd-catalyzed AAA, providing optically active mono- and trans-3,4-disubstituted dihydrocoumarin derivatives in high yields with high ee values, the S-factor being 8–55. It also provided a new approach for applying the Pd-catalyzed AAA in organic synthesis. Studies on the extension of the protocol to other carbon nucleophiles and applications of the aforementioned procedure in organic synthesis are in progress.
Acknowledgements
This work was financially supported by the Major Basic Research Development Program (2010CB833300), the National Natural Science Foundation of China (21272251, 21121062, 21032007), the Chinese Academy of Sciences, the Technology Commission of Shanghai Municipality, and the Croucher Foundation of Hong Kong.Notes and references
- (a) M. Iinuma, T. Tanaka, M. Mizuno, T. Katsuzaki and H. Ogawa, Chem. Pharm. Bull., 1989, 37, 1813 CrossRef CAS; (b) M. Takechi, Y. Tanaka, M. Takehara, G.-I. Nonaka and I. Nishioka, Phytochemistry, 1985, 24, 2245 CrossRef CAS; (c) T. B. Adams, D. B. Greer, J. Doull, I. C. Munro, P. Newberne, P. S. Portoghese, R. L. Smith, B. M. Wagner, C. S. Weil, L. A. Woods and R. A. Ford, Food Chem. Toxicol., 1998, 36, 249 CrossRef CAS; (d) X.-F. Zhang, L. Xie, Y. Liu, J.-F. Xiang, L. Li and Y.-L. Tang, J. Mol. Struct., 2008, 888, 145 CrossRef CAS PubMed; (e) J. Posakony, M. Hirao, S. Stevens, J. A. Simon and A. Bedalov, J. Med. Chem., 2004, 47, 2635 CrossRef CAS PubMed; (f) A. Kumar, B. K. Singh, R. Tyagi, S. K. Jain, S. K. Sharma, A. K. Prasad, H. G. Raj, R. C. Rastogi, A. C. Watterson and V. S. Parmar, Bioorg. Med. Chem., 2005, 13, 4300 CrossRef CAS PubMed; (g) F. Roelens, K. Huvaere, W. Dhooge, M. Van Cleemput, F. Comhaire and D. De Keukeleire, Eur. J. Med. Chem., 2005, 40, 1042 CrossRef CAS PubMed; (h) M. Iinuma, T. Tanaka and F. Asai, Phytochemistry, 1994, 36, 941 CrossRef CAS; (i) F. L. Hsu, G.-I. Nonaka and I. Nishioka, Chem. Pharm. Bull., 1985, 33, 3142 CrossRef CAS; (j) G. Nonaka, O. Kawahara and I. Nishioka, Chem. Pharm. Bull., 1982, 30, 4277 CrossRef CAS; (k) S. Nishimura, S. Taki, S. Takaishi, Y. Iijima and T. Akiyama, Chem. Pharm. Bull., 2000, 48, 505 CrossRef CAS; (l) D. M. X. Donnelly and G. M. Boland, Nat. Prod. Rep., 1995, 12, 321 RSC; (m) G. Speranza, A. D. Meo, P. Manitto, D. Monti and G. Fontana, J. Agric. Food Chem., 1996, 44, 274 CrossRef CAS; (n) X.-F. Zhang, H.-M. Wang, Y.-L. Song, L.-H. Nie, L.-F. Wang, B. Liu, P.-P. Shen and Y. Liu, Bioorg. Med. Chem. Lett., 2006, 16, 949 CrossRef CAS PubMed; (o) H. Tanaka, R. Takahashi, S. Morimoto and Y. Shoyama, J. Nat. Prod., 1997, 60, 168 CrossRef CAS; (p) W. Tang, H. Hioki, K. Harada, M. Kubo and Y. Fukuyama, J. Nat. Prod., 2007, 70, 2010 CrossRef CAS PubMed.
- (a) M. E. Wall, D. R. Brine, G. A. Brine, C. G. Pitt, R. I. Freudenthal and H. D. Christensen, J. Am. Chem. Soc., 1970, 92, 3466 CrossRef CAS; (b) D.-O. Kim and C. Y. Lee, Crit. Rev. Food Sci. Nutr., 2004, 44, 253 CrossRef CAS PubMed; (c) G. Appendino, S. Gibbons, A. Giana, A. Pagani, G. Gianpaolo, M. Stavri, E. Smith and M. M. Rahman, J. Nat. Prod., 2008, 71, 1427 CrossRef CAS PubMed; (d) S. A. Ahmed, S. A. Ross, D. Slade, M. M. Radwan, F. Zulfiqar and M. A. ElSohly, J. Nat. Prod., 2008, 71, 536 CrossRef CAS PubMed; (e) G. R. Handrick, R. K. Razdan, D. B. Uliss, H. C. Dalzell and E. Boger, J. Org. Chem., 1977, 42, 2563 CrossRef CAS; (f) E. Ballerini, L. Minuti, O. Piermatti and F. Pizzo, J. Org. Chem., 2009, 74, 4311 CrossRef CAS PubMed; (g) J. D. Elliott, M. A. Lago, R. D. Cousins, A. Gao, J. D. Leber, K. F. Erhard, P. Nambi, N. A. Elshourbagy and C. Kumar, J. Med. Chem., 1994, 37, 1553 CrossRef CAS.
- For the methods for the synthesis of 4-monosubstituted or trans-3,4-disubstituted dihydrocoumarin derivatives in a racemic way, see selected reports: (a) A. Bojilova and C. Ivanov, Synthesis, 1976, 267 CrossRef CAS; (b) K. Li, L. N. Foresee and J. A. Tunge, J. Org. Chem., 2005, 70, 2881 CrossRef CAS PubMed; (c) J.-M. Lee, T.-H. Tseng and Y.-J. Lee, Synthesis, 2001, 2247 CrossRef CAS PubMed; (d) J. Barluenga, F. Andina and F. Aznar, Org. Lett., 2006, 8, 2703 CrossRef CAS PubMed; (e) A. R. Jagdale and A. Sudalai, Tetrahedron Lett., 2007, 48, 4895 CrossRef CAS PubMed; (f) Z. Zhang, Y. Ma and Y. Zhao, Synlett, 2008, 1091 CAS; (g) C.-R. Piao, Y.-L. Zhao, X.-D. Han and Q. Liu, J. Org. Chem., 2008, 73, 2264 CAS; (h) K. Ohkata, Y. G. Lee, Y. Utsumi, K. Ishimaru and K. Akiba, J. Org. Chem., 1991, 56, 5052 CrossRef CAS; (i) G. Speranza, C. F. Morelli and P. Manitto, Synthesis, 2000, 123 CrossRef CAS PubMed; (j) S. Duan, R. Jana and J. A. Tunge, J. Org. Chem., 2009, 74, 4612 CrossRef CAS PubMed; (k) K. Chattopadhyay, R. Jana, V. W. Day, J. T. Douglas and J. A. Tunge, Org. Lett., 2010, 12, 3042 CrossRef CAS PubMed; (l) E. Fillion, A. M. Dumas, B. A. Kuropatwa, N. R. Malhotra and T. C. Sitler, J. Org. Chem., 2006, 71, 409 CrossRef CAS PubMed; (m) W. H. dos Santos and L. C. da Silva-Filho, Synthesis, 2012, 3361 CAS.
- For the asymmetric catalytic methods for the synthesis of 4-monosubstituted dihydrocoumarin derivatives, see selected reports: (a) R. Noyori, in Asymmetric Catalysis in Organic Synthesis, John Wiley and Sons, New York, 1994 Search PubMed; (b) M. A. McGuire, S. C. Shilcrat and E. Sorenson, Tetrahedron Lett., 1999, 40, 3293 CrossRef CAS; (c) Y. Luo and A. J. Cornell, Angew. Chem., Int. Ed., 2010, 49, 2750 CrossRef CAS PubMed; (d) T. Korenaga, R. Maenishi, K. Osaki and T. Sakai, Heterocycles, 2010, 80, 157 CrossRef CAS PubMed; (e) I. Singh, A. K. Prasad, A. K. Sharma, R. K. Saxena, C. E. Olsen, A. L. Cholli, L. A. Samuelson, J. Kumar, A. C. Watterson and V. S. Parmar, Bioorg. Med. Chem., 2003, 11, 529 CrossRef CAS; (f) C. B. Jacobsen, Ł. Albrecht, J. Udmark and K. A. Jørgensen, Org. Lett., 2012, 14, 5526 CrossRef CAS PubMed.
- For the asymmetric catalytic methods for the synthesis of trans-3,4-disubstituted dihydrocoumarin derivatives: (a) H. Kima and J. Yuna, Adv. Synth. Catal., 2010, 352, 1881 CrossRef PubMed; (b) J. F. Teichert and B. L. Feringa, Chem. Commun., 2011, 47, 2679 RSC; (c) Y.-T. Lee, U. Das, Y.-R. Chen, C.-J. Lee, C.-H. Chen, M.-C. Yang and W. Lin, Adv. Synth. Catal., 2013, 355, 3154 CrossRef CAS PubMed; (d) X. Tang, A. J. Blake, W. Lewis and S. Woodward, Tetrahedron: Asymmetry, 2009, 20, 1881 CrossRef CAS PubMed; (e) E. Alden-Danforth, M. T. Scerba and T. Lectka, Org. Lett., 2008, 10, 4951 CrossRef CAS PubMed; For the asymmetric catalytic methods for synthesis of cis-3,4-disubstituted dihydrocoumarin derivatives: (f) Z. Jin, R. Yang, Y. Du, B. Tiwari, R. Ganguly and Y. R. Chi, Org. Lett., 2012, 14, 3226 CrossRef CAS PubMed; (g) D. Enders, C. Wang, X. Yang and G. Raabe, Adv. Synth. Catal., 2010, 352, 2869 CrossRef CAS PubMed; (h) D. Enders, X. Yang, C. Wang, G. Raabe and J. Runsik, Chem. – Asian J., 2011, 6, 2255 CrossRef CAS PubMed.
- For the asymmetric methods for the synthesis of trans-3,4-disubstituted dihydrocoumarin derivatives with equimolar chiral reagents: (a) G. Blay, M. C. Muñoz, J. R. Pedro and A. Sanz-Marco, Adv. Synth. Catal., 2013, 355, 1071 CrossRef CAS PubMed; (b) D. J. Dixon, S. V. Ley and F. Rodríguez, Angew. Chem., Int. Ed., 2001, 40, 4763 CrossRef CAS; (c) D. Enders, H. Saeidian, Z. Mirjafary, D. Iffland, G. Raabe and J. Runsink, Synlett, 2009, 2872 CrossRef CAS PubMed.
- For reviews, see: (a) A. Pfaltz and M. Lautens, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, New York, 1999, vol. 2, p. 833 Search PubMed; (b) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed; (c) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed; (d) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed.
- (a) B. M. Trost and G. M. Schroeder, J. Am. Chem. Soc., 1999, 121, 6759 CrossRef CAS; (b) M. Braun, F. Laicher and T. Meier, Angew. Chem., Int. Ed., 2000, 39, 3494 CrossRef CAS; (c) T. D. Weiss, G. Helmchen and U. Kazmaier, Chem. Commun., 2002, 1270 RSC; (d) M. Braun and T. Meier, Synlett, 2005, 2968 CrossRef CAS PubMed; (e) B. M. Trost and J. Xu, J. Am. Chem. Soc., 2005, 127, 17180 CrossRef CAS PubMed; (f) B. M. Trost, J. Xu and M. Reichle, J. Am. Chem. Soc., 2007, 129, 282 CrossRef CAS PubMed; (g) É. Bélanger, K. Cantin, O. Messe, M. Tremblay and J.-F. Paquin, J. Am. Chem. Soc., 2007, 129, 1034 CrossRef PubMed; (h) J. Deska and U. Kazmaier, Angew. Chem., Int. Ed., 2007, 46, 4570 CrossRef CAS PubMed; (i) B. M. Trost and D. A. Thaisrivongs, J. Am. Chem. Soc., 2009, 131, 12056 CrossRef CAS PubMed; (j) P. Meletis, M. Patil, W. Thiel, W. Frank and M. Braun, Chem. – Eur. J., 2011, 17, 11243 CrossRef CAS PubMed.
- (a) X. X. Yan, C. G. Liang, Y. Zhang, W. Hong, B. X. Cao, L.-X. Dai and X.-L. Hou, Angew. Chem., Int. Ed., 2005, 44, 6544 CrossRef CAS PubMed; (b) W. H. Zheng, B. H. Zheng, Y. Zhang and X.-L. Hou, J. Am. Chem. Soc., 2007, 129, 7718 CrossRef CAS PubMed; (c) K. Zhang, Q. Peng, X.-L. Hou and Y. D. Wu, Angew. Chem., Int. Ed., 2008, 47, 1741 CrossRef CAS PubMed; (d) W. Liu, D. Chen, X.-Z. Zhu, X.-L. Wan and X.-L. Hou, J. Am. Chem. Soc., 2009, 131, 8734 CrossRef CAS PubMed; (e) J.-P. Chen, C.-H. Ding, W. Liu, X.-L. Hou and L.-X. Dai, J. Am. Chem. Soc., 2010, 132, 15493 CrossRef CAS PubMed.
- For reviews, see: (a) H. Pielartzik, B. Irmisch-Pielartzik and T. Eicher, in Houben-Weyl, ed. J. Falbe, Thieme, Stuttgart, 4th edn, 1985, vol. E5, part 1, p. 715 Search PubMed; (b) G. V. Boyd, in The Chemistry of Acid Derivatives, The Chemistry of Functional Groups, ed. S. Patai, Wiley, Chichester, 1979, p. 492; 1992, p. 548 Search PubMed; (c) J. Mulzer, in Comprehensive Organic Synthesis, ed. B. M. Trost, I. Fleming and E. Winterfeldt, Pergamon, Oxford, 1991, vol. 6, p. 323 Search PubMed; (d) I. Collins, J. Chem. Soc., Perkin Trans. 1, 1998, 1869 RSC and references cited therein.
- (a) T. Hayashi, A. Yamamoto and Y. Ito, J. Chem. Soc., Chem. Commun., 1986, 1090 RSC; (b) Y. K. Choi, J. H. Suh, D. Lee, I. T. Lim, J. Y. Jung and M. J. Kim, J. Org. Chem., 1999, 64, 8423 CrossRef CAS; (c) H.-J. Gais, N. Spalthoff, T. Jagusch, M. Frank and G. Raabe, Tetrahedron Lett., 2000, 41, 3809 CrossRef CAS; (d) M. T. Reetz and S. Sostmann, J. Organomet. Chem., 2000, 603, 105 CrossRef CAS; (e) J. M. Longmire, B. Wang and X. Zhang, Tetrahedron Lett., 2000, 41, 5435 CrossRef CAS; (f) S. R. Gilbertson and P. Lan, Org. Lett., 2001, 3, 2237 CrossRef CAS PubMed; (g) D. L. Hughes, M. Palucki, N. Yasuda, R. A. Reamer and P. J. Reider, J. Org. Chem., 2002, 67, 2762 CrossRef CAS PubMed; (h) H.-J. Gais, T. Jagusch, N. Spalthoff, F. Gerhards, M. Frank and G. Raabe, Chem. – Eur. J., 2003, 9, 4202 CrossRef CAS PubMed; (i) B. J. Lussem and H. J. Gais, J. Am. Chem. Soc., 2003, 125, 6066 CrossRef PubMed; (j) J. W. Faller, J. C. Wilt and J. Parr, Org. Lett., 2004, 6, 1301 CrossRef CAS PubMed; (k) C. Fischer, C. Defieber, T. Suzuki and E. M. Carreira, J. Am. Chem. Soc., 2004, 126, 1628 CrossRef CAS PubMed; (l) K. Onitsuka, Y. Matsushima and S. Takahashi, Organometallics, 2005, 24, 6472 CrossRef CAS; (m) X.-B. Jiang, P. Van Leeuwen and J. Reek, Chem. Commun., 2007, 2287 RSC.
- (a) B. H. Zheng and X.-L. Hou, Org. Lett., 2009, 11, 1789 CrossRef PubMed; (b) B.-L. Lei, C.-H. Ding, X.-F. Yang, X.-L. Wan and X.-L. Hou, J. Am. Chem. Soc., 2009, 131, 18250 CrossRef CAS PubMed.
- H. B. Kagan and J. C. Fiaud, in Topics in Stereochemistry, ed. E. L. Eliel and S. H. Wilen, Interscience, New York, 1988, vol. 18, p. 249 Search PubMed.
- J. F. Teichert and B. L. Feringa, Chem. Commun., 2011, 47, 2679 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and analysis data for new compounds, 1H, 13C NMR and HPLC spectra of compounds 1a–1l, 3a–3l. See DOI: 10.1039/c4qo00178h |
| This journal is © the Partner Organisations 2014 |