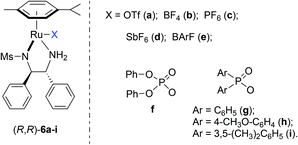
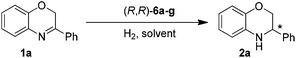
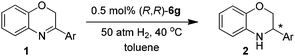
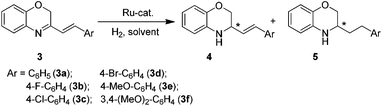
Asymmetric hydrogenation of 3-substituted 2H-1,4-benzoxazines with chiral cationic Ru-MsDPEN catalysts: a remarkable counteranion effect†
Jie
Qin
ab,
Fei
Chen
a,
Yan-Mei
He
a and
Qing-Hua
Fan
*ac
aBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences (CAS), Beijing 100190, P. R. China. E-mail: fanqh@iccas.ac.cn; Fax: (+86)-10-62554449
bTechnical Center, China State Construction Engineering Co., Ltd, No. 15 Linhe Street, Shunyi District, Beijing 101300, P. R. China
cCollaborative Innovation Center of Chemical Science and Engineering, Tianjin 300071, P. R. China
First published on 24th July 2014
Abstract
The enantioselective hydrogenation of 3-aryl and 3-styryl-substituted-2H-1,4-benzoxazines was developed using the chiral cationic Ru(η6-cymene)(MsDPEN)(Ar2PO2) system in high yields with up to 99% ee. The counteranion was found to be critically important for the high enantioselectivity. Furthermore, the regioselectivity could be regulated by the counteranion of the catalyst in the asymmetric hydrogenation of 3-styryl-2H-1,4-benzoxazines.
Optically active 3,4-dihydro-2H-1,4-benzoxazines are ubiquitous structural moieties in biologically interesting natural alkaloids and chiral pharmaceuticals.1,2 Some important representative examples include Obscurinervine, Levofloxacin, WIN-55212-2 and compound A (Fig. 1). Consequently, many research efforts have been devoted to the asymmetric synthesis of these chiral compounds over the past few decades.3–9 Among them, asymmetric hydrogenation represents one of the most convenient and atom-economical approaches,8,9 and the transition-metal-catalyzed reduction of the corresponding readily available benzoxazines has attracted much attention over the past few years.9 In 1998, Satoh and co-workers first reported the Ir-BPPM (N-(t-butoxy-carbonyl)-4-(diphenylphosphino)-2-[(diphenylphosphino)methyl]pyrrolidine) and bismuth(III) iodide system catalyzed hydrogenation of 7,8-difluoro-3-methyl-2H-1,4-benzoxazine affording the product as a key intermediate for the synthesis of Levofloxacin with 90% ee.9a Most recently, a variety of chiral metal catalysts, including Ir, Ru and Fe complexes,9b–f have been developed to successfully catalyze the enantioselective hydrogenation of 3-aryl benzoxazine derivatives with good to excellent enantioselectivities. Despite great progress achieved in this field, all these metallic catalysts contained phosphorus ligands around the metal center which are often air sensitive. Furthermore, in most cases, only the 3-aryl benzoxazines were studied with these reported catalysts.9c–g From the viewpoint of both scientific interest and practical applications, it is desirable to develop more efficient and stable catalysts for the asymmetric hydrogenation of a broad range of 3-substituted benzoxazine derivatives.
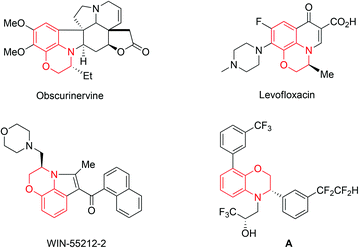 | ||
| Fig. 1 Selected examples of alkaloids and bioactive compounds containing 3,4-dihydro-2H-1,4-benzoxazines frameworks. | ||
In the past few years, we have found that cationic ruthenium complexes of chiral mono-tosylated diamines10 are very efficient catalysts for the asymmetric hydrogenation of various N-containing heterocycles.11,12 This catalytic system was also demonstrated to be highly enantioselective for the asymmetric hydrogenation of a broad range of acyclic ketimines and cyclic imines.13 The achiral counteranions were found to influence the enantioselectivity significantly in the hydrogenation of imines and quinoxalines.12c,13 Interestingly, in the hydrogenation of 2,4-diaryl substituted-3H-1,5-benzodiazepines, the choice of achiral counteranions determined the sense of asymmetric induction.13e Encouraged by these results and as a continuation of ongoing endeavour to prepare chiral N-containing heterocycles and amines, herein, we report the details of enantioselective hydrogenation of a variety of 2H-1,4-benzoxazines using the chiral cationic Ru-MsDPEN complexes (Fig. 2) with excellent enantioselectivities (up to 99% ee). Unexpectedly, it was found that the achiral counteranion regulated both the regioselectivity and enantioselectivity in the hydrogenation of 3-styryl-substituted-2H-1,4-benzoxazines.
Based on our previous work,13b (R,R)-6e was firstly chosen to catalyze the asymmetric hydrogenation of 3-phenyl-2H-1,4-benzoxazine (1a) in different solvents under 50 atm H2 pressure at 20 °C for 12 h. This reaction was found to be sensitive to the solvent and higher enantioselectivity (65% ee) was observed in THF (entry 1 in Tables 1 and S1 in ESI†). Further investigation of a variety of catalysts, (R,R)-6a–g, showed that the counteranion of the catalyst had a significant effect on the stereochemical outcome of the reaction (entries 1–7 in Tables 1 and S2 in ESI†). The catalyst (R,R)-6g, bearing an achiral Ph2PO2 anion, turned out to be optimal in enantioselectivity but with low reactivity (entry 7 in Table 1).
| Entry | Catalyst | Solvent | H2 (atm)/temp (°C) | Conv.b (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol) in solvent (1 mL), catalyst (1.0 mol%), stirred for 12 h. b The conversions were determined by 1H NMR spectroscopy of the crude reaction mixture. c The enantiomeric excesses were determined by chiral HPLC with a chiral OD-H column. d With the catalyst (0.2 mol%) for 24 h. e With the catalyst (0.1 mol%) for 24 h. | |||||
| 1 | (R,R)-6e | THF | 50; 20 | >95 | 65 |
| 2 | (R,R)-6a | THF | 50; 20 | >95 | 8 |
| 3 | (R,R)-6b | THF | 50; 20 | >95 | 13 |
| 4 | (R,R)-6c | THF | 50; 20 | >95 | 47 |
| 5 | (R,R)-6d | THF | 50; 20 | >95 | 55 |
| 6 | (R,R)-6f | THF | 50; 20 | 92 | 83 |
| 7 | (R,R)-6g | THF | 50; 20 | 19 | 92 |
| 8 | (R,R)-6g | CH2Cl2 | 50; 20 | 32 | 82 |
| 9 | (R,R)-6g | CH2ClCH2Cl | 50; 20 | 57 | 88 |
| 10 | (R,R)-6g | Toluene | 50; 20 | >95 | 94 |
| 11 | (R,R)- 6g | Toluene | 50; 40 | >95 | 94 |
| 12 | (R,R)-6g | Toluene | 80; 20 | >95 | 94 |
| 13 | (R,R)-6g | Toluene | 10; 20 | 74 | 94 |
| 14d | (R,R)-6g | Toluene | 50; 40 | >95 | 94 |
| 15e | (R,R)-6g | Toluene | 50; 40 | 40 | 93 |
To further improve the catalytic performance of (R,R)-6g, the influence of solvents was studied once again (entries 7–10 in Tables 1 and S3 in ESI†). To our great delight, full conversion and the highest enantioselectivity (94% ee) were obtained in toluene (entry 10 in Table 1). In addition, the influences of temperature and hydrogen pressure were studied and it was found that the enantioselectivity is insensitive to hydrogen pressure and temperature (entries 10–13 in Tables 1 and S4 in ESI†). Remarkably, good reactivities and identical enantioselectivities were observed when the hydrogenation was carried out at a substrate/catalyst ratio of 500 upon prolonged reaction time (entry 14 in Table 1). A similar enantioselectivity was observed when the reaction proceeded at a lower catalyst loading of 0.1 mol% (entry 15 in Table 1).
Under the optimized reaction conditions (entry 11 in Table 1), a variety of 3-aryl-substituted-2H-1,4-benzoxazines were efficiently hydrogenated in the presence of 0.5 mol% (R,R)-6g to afford the corresponding chiral heterocycles with good to excellent enantioselectivities in most cases (82–98% ee, entries 1–10 in Table 2). It was evident that the electronic properties of the substituents at the para position of the phenyl ring had no apparent effect on activity and enantioselectivity (entries 1–7), and 3-(4-MeO-C6H4)-2H-1,4-benzoxazine (1e) gave the highest ee value (98% ee, entry 5). When the substituent is located at the meta position, the enantiomeric excess slightly dropped (entries 8–10). However, for the ortho substituted substrates (1k–l), reaction could not even occur (entries 11–12). This was probably due to the undesirable steric effect of the ortho substituent.
| Entry | Ar | Yieldb (%) | eec (%) |
|---|---|---|---|
| a Reaction conditions: substrate 1a–l (0.2 mmol) in toluene (2 mL), (R,R)-6g (0.5 mol%), H2 (50 atm), stirred at 40 °C for 12 h. b Isolated yield. c Determined by chiral HPLC. | |||
| 1 | C6H5 (1a) | 95 | 94 |
| 2 | 4-F–C6H4 (1b) | 98 | 95 |
| 3 | 4-Cl–C6H4 (1c) | 97 | 96 |
| 4 | 4-Br–C6H4 (1d) | 95 | 96 |
| 5 | 4-MeO–C6H4 (1e) | 96 | 98 |
| 6 | 4-CF3–C6H4 (1f) | 97 | 92 |
| 7 | 4-C6H5–C6H4 (1g) | 95 | 97 |
| 8 | 3-MeO–C6H4 (1h) | 95 | 90 |
| 9 | 3-Cl–C6H4 (1i) | 94 | 82 |
| 10 | 3,4-Cl2–C6H4 (1j) | 98 | 86 |
| 11 | 2-MeO–C6H4 (1k) | <5 | nd |
| 12 | 2-Cl–C6H4 (1l) | <5 | nd |
After establishing the successful catalyst system for the asymmetric hydrogenation of 3-aryl-substituted-2H-1,4-benzoxazines, we further expanded the substrate scope to 3-styryl-substituted-2H-1,4-benzoxazine derivatives (Table 3). Recently, Zhou et al. reported that the [Ir(COD)Cl]2/(S)-SegPhos/I2 system could catalyze this reaction and full conversions were obtained, but with mixtures of partially (4a) and completely hydrogenated products (5a).9b In our initial study, the hydrogenation of 3a in toluene with 1.0 mol% (R,R)-6e also afforded the partially (4a) and completely hydrogenated products (5a) with a ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in 75% and 92% ee, respectively (entry 1 in Table 3). Interestingly, the catalyst screening demonstrated that the counteranion played an important role in both the regioselectivity and enantioselectivity control of this reduction (entries 1–7 in Table 3). Significantly, the catalyst (R,R)-6h exhibited extremely high 1,2-selectivity (4a/5a, 97
2 in 75% and 92% ee, respectively (entry 1 in Table 3). Interestingly, the catalyst screening demonstrated that the counteranion played an important role in both the regioselectivity and enantioselectivity control of this reduction (entries 1–7 in Table 3). Significantly, the catalyst (R,R)-6h exhibited extremely high 1,2-selectivity (4a/5a, 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and afforded 4a in an enantiopure form (entry 6). Next, we optimized the reaction conditions by varying the solvents, hydrogen pressure and reaction time. In contrast to MeOH, weakly polar solvents, such as CH2Cl2, ClCH2CH2Cl and apolar toluene, are suitable to obtain high enantioselectivities and regioselectivities (entry 8 vs. entries 6 and 9–10). Higher regioselectivity was achieved when the reaction was carried out under ascending hydrogen pressure and decreasing reaction temperature (entry 12 in Tables 3 and S6 in ESI†). Notably, the enantioselectivity and regioselectivity were maintained when catalyst loading was reduced to 0.2 mol% (entry 11).
3) and afforded 4a in an enantiopure form (entry 6). Next, we optimized the reaction conditions by varying the solvents, hydrogen pressure and reaction time. In contrast to MeOH, weakly polar solvents, such as CH2Cl2, ClCH2CH2Cl and apolar toluene, are suitable to obtain high enantioselectivities and regioselectivities (entry 8 vs. entries 6 and 9–10). Higher regioselectivity was achieved when the reaction was carried out under ascending hydrogen pressure and decreasing reaction temperature (entry 12 in Tables 3 and S6 in ESI†). Notably, the enantioselectivity and regioselectivity were maintained when catalyst loading was reduced to 0.2 mol% (entry 11).
| Entry | Catalyst | Substrate | Solvent | Conv.b (%) | 4/5c | eed (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 3a (0.1 mmol) in solvent (1 mL), catalyst (1.0 mol%), H2 (50 atm), stirred at 20 °C for 12 h. b Determined by 1H NMR of the crude reaction mixture. c Determined by 1H NMR spectroscopy of the crude reaction mixture. d Determined by chiral HPLC. e 0.2 mol% catalyst was used. f Reaction conditions: substrate 3a–f (0.2 mmol) in toluene (2 mL), (R,R)-6h (0.5 mol%), H2 (80 atm), stirred at 20 °C for 12 h. g Isolated yield. | ||||||
| 1 | (R,R)-6e | (3a) | Toluene | >99 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
75/92 |
| 2 | (R,R)-6c | (3a) | Toluene | >99 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
8/14 |
| 3 | (R,R)-6a | (3a) | Toluene | >99 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
47/10 |
| 4 | (R,R)-6f | (3a) | Toluene | >99 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
75/nd |
| 5 | (R,R)-6g | (3a) | Toluene | >99 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
98/nd |
| 6 | (R,R)-6h | (3a) | Toluene | >99 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
99/nd |
| 7 | (R,R)-6i | (3a) | Toluene | >99 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
98/nd |
| 8 | (R,R)-6h | (3a) | MeOH | >99 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 47 |
28/50 |
| 9 | (R,R)-6h | (3a) | DCM | >99 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
95/nd |
| 10 | (R,R)-6h | (3a) | DCE | >99 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
94/nd |
| 11e | (R,R)-6h | (3a) | Toluene | >99 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98/nd |
| 12 | (R,R)- 6h | (3a) | Toluene | >99 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2
|
99/nd |
| 95 | ||||||
| 13f | (R,R)-6h | (3b) | Toluene | 96g | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
91/nd |
| 14f | (R,R)-6h | (3c) | Toluene | 94g | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
98/nd |
| 15f | (R,R)-6h | (3d) | Toluene | 93g | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98/nd |
| 16f | (R,R)-6h | (3e) | Toluene | 95g | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
97/nd |
| 17f | (R,R)-6h | (3f) | Toluene | 97g | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
98/nd |
Under the optimized reaction conditions (entry 12 in Table 3), several 3-styryl-substituted-2H-1,4-benzoxazine derivatives were reduced at 20 °C for 12 h in toluene under 80 atm of hydrogen using 0.5 mol% (R,R)-6h. Generally, excellent enantioselectivities (91–99% ee) and regioselectivities (95/5–98/2) were achieved, no matter the substrates with either an electron-donating or an electron-withdrawing substituent on the phenyl group (entries 12–17 in Table 3).
Conclusions
In summary, the half-sandwich Ru(II) complexes of monosulfonylated chiral diamines bearing an Ar2PO2 anion have been disclosed to be highly efficient for the asymmetric hydrogenation of a variety of 3-aryl and 3-styryl-substituted-2H-1,4-benzoxazines, providing a convenient access to the corresponding optically active 3,4-dihydro-2H-1,4-benzoxazines with biological importance. The remarkable counteranion effect was found to be critically important for tuning the enantioselectivity and regioselectivity. Extending application of these catalyst systems in the asymmetric hydrogenation of other heteroaromatic compounds and investigation for detailed insight into the nature of this remarkable counteranion effect are in progress.Acknowledgements
Financial support from the National Natural Science Foundation of China (grant no. 21232008 and 21302190), the National Basic Research Program of China (973 Program, no. 2010CB833300), and the Chinese Academy of Sciences (CMS-PY-201303) is greatly acknowledged.Notes and references
- For selected examples of the alkaloids, see: (a) The Alkaloids: Chemistry and Physiology, ed. R. H. F. Manske, Academic Press, New York, 1965, vol. 8 Search PubMed; (b) K. S. Brown and C. Djerassi, J. Am. Chem. Soc., 1964, 86, 2451 CrossRef CAS.
- For selected reviews on the chiral pharmaceuticals, see: (a) Antibiotics and Antiviral Compounds, ed. K. Korhn, H. A. Rirst and H. Maag, VCH, Weinheim, 1993 Search PubMed; (b) Pharmaceutical Substances, ed. A. Kleemann, J. Engel, B. Kutscher and D. Reichert, Thieme, Stuttgart, New York, 4th edn, 2001 Search PubMed; For selected examples of the chiral pharmaceuticals, see: (c) S. Atarashi, S. Yokohama, K. Yamazaki, K. Sakano, M. Imamura and I. Hayakawa, Chem. Pharm. Bull., 1987, 35, 1896 CrossRef CAS; (d) J.-Y. Shim, E. R. Collantes, W. J. Welsh, B. Subramaniam, A. C. Howlett, M. A. Eissenstat and S. J. Ward, J. Med. Chem., 1998, 41, 4521 CrossRef CAS PubMed; (e) S. D. McAllister, G. Rizvi, S. Anavi-Goffer, D. P. Hurst, J. Barnett-Norris, D. L. Lynch, P. H. Reggio and M. E. Abood, J. Med. Chem., 2003, 46, 5139 CrossRef CAS PubMed; (f) P.-F. Jiao, B.-X. Zhao, W.-W. Wang, Q.-X. He, M.-S. Wan, D.-S. Shin and J.-Y. Miao, Bioorg. Med. Chem. Lett., 2006, 16, 2862 CrossRef CAS PubMed; (g) A. Wang, C. P. Prouty, P. D. Pelton, M. Yong, K. T. Demarest, W. V. Murray and G.-H. Kuo, Bioorg. Med. Chem. Lett., 2010, 20, 1432 CrossRef CAS PubMed.
- For recent reviews, see: (a) B. Achari, S. B. Mandal, P. K. Dutta and C. Chowdhury, Synlett, 2004, 2449 CAS; (b) J. Ilaš, P. Š. Anderluh, M. S. Dolenc and D. Kikelj, Tetrahedron, 2005, 61, 7325 CrossRef PubMed.
- For selected examples of kinetic resolution, see: (a) A. Miyadera and A. Imura, Tetrahedron: Asymmetry, 1999, 10, 119 CrossRef CAS; (b) V. N. Charushin, V. P. Krasnov, G. L. Levit, M. A. Korolyova, M. I. Kodess, O. N. Chupakhin, M. H. Kim, H. S. Lee, Y. J. Park and K.-C. Kim, Tetrahedron: Asymmetry, 1999, 10, 2691 CrossRef CAS; (c) V. P. Krasnov, G. L. Levit, I. M. Bukrina, I. N. Andreeva, L. S. Sadretdinova, M. A. Korolyova, M. I. Kodess, V. N. Charushin and O. N. Chupakhin, Tetrahedron: Asymmetry, 2003, 14, 1985 CrossRef CAS; (d) V. P. Krasnov, G. L. Levit, M. I. Kodess, V. N. Charushin and O. N. Chupakhin, Tetrahedron: Asymmetry, 2004, 15, 859 CrossRef CAS PubMed; (e) V. A. Potemkin, V. P. Krasnov, G. L. Levit, E. V. Bartashevich, I. N. Andreeva, M. B. Kuzminsky, N. A. Anikin, V. N. Charushin and O. N. Chupakhin, Mendeleev Commun., 2004, 14, 69 CrossRef PubMed.
- For selected examples of stoichiometric synthesis, see: (a) S. Atarashi, H. Tsurumi, T. Fujiwara and I. Hayakawa, J. Heterocycl. Chem., 1991, 28, 329 CrossRef CAS; (b) L. Xie, Chin. Chem. Lett., 1995, 6, 857 CAS; (c) S. B. Kang, E. J. Ahn, Y. Kim and Y. H. Kim, Tetrahedron Lett., 1996, 37, 9317 CrossRef CAS; (d) J. Adrio, J. C. Carretero, J. L. G. Ruano, A. Pallarés and M. Vicioso, Heterocycles, 1999, 51, 1563 CrossRef CAS; (e) J. F. Bower, P. Szeto and T. Gallagher, Org. Lett., 2007, 9, 3283 CrossRef CAS PubMed; (f) M. K. Parai and G. Panda, Tetrahedron Lett., 2009, 50, 4703 CrossRef CAS PubMed; (g) J. C. Kim, H. G. Choi, M. S. Kim, H.-J. Ha and W. K. Lee, Tetrahedron, 2010, 66, 8108 CrossRef PubMed and ref. 2c.
- For examples of the hydrosilylation of benzoxazines, see: (a) A. V. Malkov, K. Vranková, S. Stončius and P. Kočovský, J. Org. Chem., 2009, 74, 5839 CrossRef CAS PubMed; (b) Y. Jiang, L.-X. Liu, W.-C. Yuan and X.-M. Zhang, Synlett, 2012, 1797 CAS; (c) X.-W. Liu, C. Wang, Y. Yan, Y.-Q. Wang and J. Sun, J. Org. Chem., 2013, 78, 6276 CrossRef CAS PubMed.
- For examples of the organocatalytic asymmetric transfer hydrogenation of benzoxazines, see: (a) M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem., Int. Ed., 2006, 45, 6751 CrossRef CAS PubMed; (b) M. Rueping, F. Tato and F. R. Schoepke, Chem. – Eur. J., 2010, 16, 2688 CrossRef CAS PubMed; (c) M. Rueping, E. Sugiono, A. Steck and T. Theissmann, Adv. Synth. Catal., 2010, 352, 281 CrossRef CAS; (d) M. Rueping, M. Stoeckel, E. Sugiono and T. Theissmann, Tetrahedron, 2010, 66, 6565 CrossRef CAS PubMed; (e) M. Rueping and T. Theissmann, Chem. Sci., 2010, 1, 473 RSC; (f) C. Bleschke, J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, Adv. Synth. Catal., 2011, 353, 3101 CrossRef CAS; (g) D. S. Kundu, J. Schmidt, C. Bleschke, A. Thomas and S. Blechert, Angew. Chem., Int. Ed., 2012, 51, 5456 CrossRef CAS PubMed.
- (a) R. Noyori, Acta Chem. Scand., 1996, 50, 380 CrossRef CAS PubMed; (b) Y.-G. Zhou, P.-Y. Yang and X.-W. Han, J. Org. Chem., 2005, 70, 1679 CrossRef CAS PubMed; (c) J. Xie and Q. Zhou, Acta Chim. Sin., 2012, 70, 1427 CrossRef CAS.
- (a) K. Satoh, M. Inenaga and K. Kanai, Tetrahedron: Asymmetry, 1998, 9, 2657 CrossRef CAS; (b) K. Gao, C.-B. Yu, D.-S. Wang and Y.-G. Zhou, Adv. Synth. Catal., 2012, 354, 483 CrossRef CAS; (c) J. Hu, D. Wang, Z. Zheng and X. Hu, Chin. J. Chem., 2012, 30, 2664 CAS; (d) J. L. Núñez-Rico and A. Vidal-Ferran, Org. Lett., 2013, 15, 2066 CrossRef PubMed; (e) S. Fleischer, S. Zhou, S. Werkmeister, K. Junge and M. Beller, Chem. – Eur. J., 2013, 19, 4997 CrossRef CAS PubMed; (f) N. Arai, Y. Saruwatari, K. Isobe and T. Ohkuma, Adv. Synth. Catal., 2013, 355, 2769 CrossRef CAS; (g) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442 CrossRef CAS PubMed.
- (a) R. Noyori and S. Hashiguchi, Acc. Chem. Res., 1997, 30, 97 CrossRef CAS; (b) T. Ikariya and A. J. Blacker, Acc. Chem. Res., 2007, 40, 1300 CrossRef CAS PubMed; (c) S. Hashiguchi, A. Fujii, J. Takehara, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1995, 117, 7562 CrossRef CAS; (d) T. Ohkuma, N. Utsumi, K. Tsutsumi, K. Murata, C. Sandoval and R. Noyori, J. Am. Chem. Soc., 2006, 128, 8724 CrossRef CAS PubMed.
- For recent reviews on asymmetric hydrogenation of heteroaromatic compounds, see: (a) D.-S. Wang, Q.-A. Chen, S.-M. Lu and Y.-G. Zhou, Chem. Rev., 2012, 112, 2557 CrossRef CAS PubMed; (b) Y.-M. He, F.-T. Song and Q.-H. Fan, Top. Curr. Chem., 2014, 343, 145 CrossRef.
- (a) H. Zhou, Z. Li, Z. Wang, T. Wang, L. Xu, Y. He, Q.-H. Fan, J. Pan, L. Gu and A. S. C. Chan, Angew. Chem., Int. Ed., 2008, 47, 8464 CrossRef CAS PubMed; (b) T. Wang, L. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q.-H. Fan, J. Xiang, Z.-X. Yu and A. S. C. Chan, J. Am. Chem. Soc., 2011, 133, 9878 CrossRef CAS PubMed; (c) J. Qin, F. Chen, Z. Ding, Y. He, L. Xu and Q.-H. Fan, Org. Lett., 2011, 13, 6568 CrossRef CAS PubMed; (d) T. Wang, F. Chen, J. Qin, Y.-M. He and Q.-H. Fan, Angew. Chem., Int. Ed., 2013, 52, 7172 CrossRef CAS PubMed.
- (a) F. Chen, T. Wang, Y. He, Z. Ding, Z. Li, L. Xu and Q.-H. Fan, Chem. – Eur. J., 2011, 17, 1109 CrossRef CAS PubMed; (b) F. Chen, Z. Ding, J. Qin, T. Wang, Y. He and Q.-H. Fan, Org. Lett., 2011, 13, 4348 CrossRef CAS PubMed; (c) F. Chen, Z. Li, Y. He and Q.-H. Fan, Chin. J. Chem., 2010, 28, 1529 CrossRef CAS; (d) Z.-Y. Ding, T. Wang, Y.-M. He, F. Chen, H.-F. Zhou, Q.-H. Fan, Q. Guo and A. S. C. Chan, Adv. Synth. Catal., 2013, 355, 3727 CrossRef CAS; (e) Z.-Y. Ding, F. Chen, J. Qin, Y.-M. He and Q.-H. Fan, Angew. Chem., Int. Ed., 2012, 51, 5706 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00188e |
| This journal is © the Partner Organisations 2014 |