Ultrasound-promoted synthesis of bi-, tri- and tetrapodal polyhydroquinolines, 1,4-dihydropyridines and the corresponding pyridines†
G. L.
Balaji
,
K.
Rajesh
,
M.
Venkatesh
,
S.
Sarveswari
and
V.
Vijayakumar
*
Centre for Organic and Medicinal Chemistry, School of Advanced Sciences, VIT University, Vellore 632 014, Tamil Nadu, India. E-mail: kvpsvijayakumar@gmail.com; Tel: +91-416 220 2332
First published on 19th November 2013
Abstract
Bi-, tri- and tetrapodal polyhydroquinolines and 1,4-dihydropyridines were synthesised by the reaction of various alkylating agents with polyhydroquinolines and 1,4-dihydropyridines under sonication conditions. These were in turn converted to the corresponding pyridines also using sonication. Sonication produced higher yields in a faster reaction time. All the synthesized compounds were characterized using spectral data.
1. Introduction
In recent years, a great deal of consideration has been given to the synthesis of polyhydroquinoline compounds, which is attributed to their diverse therapeutic and pharmacological significance. Quinolines with a 1,4-dihydropyridine (1,4-DHP) nucleus are of high pharmacological importance. Members of the quinoline family have been widely used as anti-asthmatic, antibacterial, anti-inflammatory, antimalarial and tyrosine kinase inhibiting agents.1 1,4-Dihydropyridine derivatives have been used as drugs for treating cardiovascular diseases.2 Cardiovascular agents such as amlodipine, nicardipine, nifedipine and other similar derivatives are 1,4-DHPs, and have been found to be efficient in the treatment of hypertension.3 Extensive studies on these compounds have revealed various therapeutic applications, including their use as cerebral anti-ischemic agents, chemosensitizers, platelet anti-aggregators and neuroprotectants.4 These examples demonstrate the outstanding potential of 1,4-DHPs as significant drug candidates. In addition, oxidation of these compounds into pyridines has also been extensively studied.5 Hence, the synthesis of this particular heterocyclic nucleus is also of great interest.Thus far, this interest has often manifested in the synthesis of monopodal polyhydroquinoline derivatives, and highly functional ones are infrequently examined. The tripodal derivative 1,3,5-tris(N-alkylaminomethyl)benzene has drawn much attention as an efficient building block for the synthesis of functional moieties such as molecular receptors, since its aryl ring acts as a tiny inflexible platform for receptor synthesis.6 Recent investigations revealed the utility of 1,3,5-tris methyl benzene derivatives as catalysts,7 as well as in the field of analytical chemistry.8 Some of the bi-, tri- and tetrapodal moieties like bis(indolyl)methanes are found in cruciferous plants and are known to promote beneficial estrogen metabolism and induce apoptosis in human cancer cells.9
There are some reports on the synthesis of tripodal 1,4-DHPs, but these are associated with serious limitations such as the use of costly reagents (e.g. tripodal aldehydes) and longer reaction times (6 hours).10
Ultrasonic-assisted organic synthesis has been considered a powerful technique to accelerate reactions using a green chemistry approach, since it affords maximum yield by minimizing the amount of waste by-products.11 Although this technique has been employed in many organic reactions, this is the first ever attempt to synthesize tri- and tetrapodal polyhydroquinolines and 1,4-DHPs under ultrasonic irradiation. The ultrasonic irradiation technique has been employed to synthesize the bi-, tri-, and tetrapodal 1,4-DHPs and their corresponding pyridines with the aim to reduce reaction times. The desired products have been synthesised using cheaper reagents like K2CO3 and 20% HNO3, and as the reaction was accelerated due to ultrasonication, we can say that we succeeded in our endeavor. Due to the technical efficiency of reagent activation under ultrasonic irradiation, this technique has also been adopted to oxidize the synthesized bi-, tri- and tetrapodal polyhydroquinolines and 1,4-DHPs to their corresponding pyridines.12 Compared to conventional thermal heating, ultrasound-assisted reactions require less time to complete, due to the reduction of particle size13 under high frequency sound waves (more than 20 kHz), which leads to an acceleration of the reaction.
2. Results and discussions
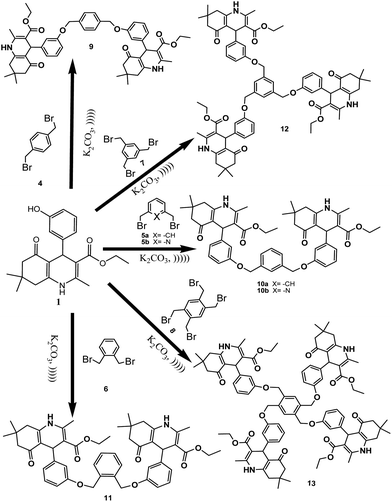
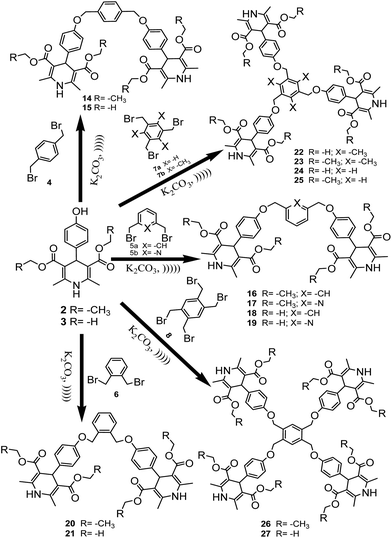
In a continuation of our earlier reports on the synthesis of polyhydroquinolines, 1,4-DHPs14,15 and heterocyclic compounds bearing bi-, tri- and tetrapodal moieties,16 we synthesized various derivatives of polyhydroquinoline and 1,4-DHPs using hydroxy benzaldehydes, ammonium acetate and β-ketoesters. The obtained products were then treated with the alkylating agents bis-, tris- and tetra-bromomethyl benzene to afford bi-, tri- and tetrapodal polyhydroquinolines, respectively (Scheme 1) in the presence of K2CO3 as a mild base (in order to control the reaction). Initially, the reaction was attempted with the strong base NaH, but its strong alkaline nature afforded both N-alkylated and O-alkylated products, and hence, a low yield was observed for the desired product. Consequently, the reaction was attempted with K2CO3, a mild base which afforded the desired product in a high yield since it only favoured O-alkylation. After monitoring the reaction with various solvents like acetonitrile, DMF, DMSO and THF, an optimum yield was observed using DMF and hence, DMF was considered as a suitable solvent. The reaction was carried out at a controlled temperature of 60 °C to yield the corresponding bi-/tri-/tetrapodal polyhydroquinolines.The conventional methodology used required 48 hours for the completion of the reaction due to high steric hindrance, especially for the tri- and tetrapodal derivatives. Hence, the methodology was changed to an ultrasonic-assisted reaction since sonochemical-associated enhancement of the reaction rate is a well known phenomenon in organic reactions. During the ultrasonic irradiation process, formation of cavitation bubbles with a high temperature and pressure takes place. These cavities burst, resulting in the liberation of high amounts of energy. In addition, due to some intermediate conditions, intermolecular reactions are facilitated outside the cavity. These events act to accelerate the reaction rate, and hence, the reaction could be completed within 20 minutes using K2CO3 as a mild base, affording a good yield (Table 1). It has also been suggested that ultrasonication accelerates the rate of reaction by minimizing the particle size of the catalyst. The results here were found to be in agreement with earlier reports that ultrasonication reduces the particle size of the catalyst into the nanometric range.13 After completion of the reaction, a green work up was chosen to extract the product using an aqueous medium. The described molecular structures have been characterized and also confirmed using the corresponding spectral data.
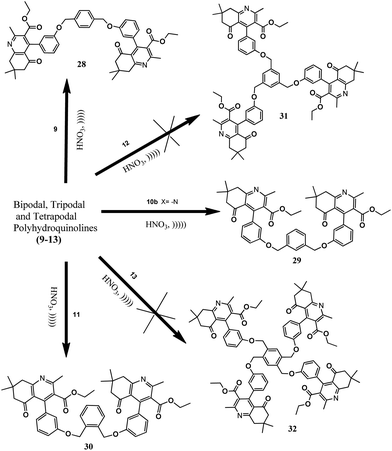
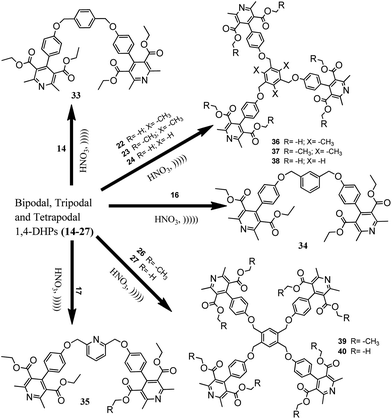
Bipodal polyhydroquinolines were aromatized to their corresponding pyridines (Scheme 2) quantitatively under mild and heterogeneous conditions. In this regard, 20% HNO3 was used as an oxidizing agent. Furthermore, it is an excellent monoprotic acid source and is established as a highly effective reagent for the oxidation of bipodal polyhydroquinolines. However, it failed to convert tri- and tetrapodal polyhydroquinolines. This prompted us to attempt the aromatization of bi-, tri- and tetrapodal 1,4-DHPs to the corresponding pyridines, which was successfully carried out (Schemes 3 and 4). Hence, this synthetic method also proved to be an efficient and economically cheap method for oxidizing bi-, tri- and tetrapodal 1,4-DHPs. Moreover, conducting the reaction under ultrasonic irradiation was sufficient to produce a >99% product yield within 3 minutes (Table 2). The spectral data confirmed the formation of the desired novel bi-, tri- and tetrapodal pyridines.
| Substrate (1,4-DHP) | Alkylating agent | Product | Time | Yield (%) | ||
|---|---|---|---|---|---|---|
| Conv.a (hours) | U.S.b (minutes) | Conv.a | U.S.b | |||
| a Reaction was carried out by a conventional method. b Reaction was carried out by an ultrasound irradiation method. | ||||||
| 1 | 4 | 9 | 48 | 20 | 65 | 81 |
| 1 | 5a | 10a | 48 | 20 | 70 | 75 |
| 1 | 5b | 10b | 48 | 20 | 72 | 82 |
| 1 | 6 | 11 | 48 | 20 | 75 | 89 |
| 1 | 7 | 12 | 48 | 20 | 52 | 73 |
| 1 | 8 | 13 | 48 | 20 | 45 | 65 |
| 2 | 4 | 14 | 48 | 20 | 67 | 79 |
| 2 | 5a | 16 | 48 | 20 | 19 | 45 |
| 2 | 5b | 17 | 48 | 20 | 67 | 85 |
| 2 | 6 | 20 | 48 | 20 | 10 | 40 |
| 2 | 7a | 22 | 48 | 20 | 67 | 75 |
| 2 | 7b | 23 | 48 | 20 | 50 | 68 |
| 2 | 8 | 26 | 48 | 20 | 49 | 77 |
| 3 | 4 | 15 | 48 | 20 | 72 | 74 |
| 3 | 5a | 18 | 48 | 20 | 64 | 81 |
| 3 | 5b | 19 | 48 | 20 | 59 | 74 |
| 3 | 6 | 21 | 48 | 20 | 52 | 60 |
| 3 | 7a | 24 | 48 | 20 | 69 | 76 |
| 3 | 7b | 25 | 48 | 20 | 63 | 70 |
| 3 | 8 | 27 | 48 | 20 | 54 | 62 |
The 1H NMR spectrum for compound 9 exhibits singlets at δ 0.93 ppm and δ 1.06 ppm, which correspond to the methyl groups at C-15, 15′ and C-16, 16′, respectively. A triplet at δ 1.21 ppm corresponds to the protons at C-14, 14′. Multiplets at δ 2.17 ppm and at δ 2.24 ppm correspond to the protons at C-8, 8′ and C-6, 6′ respectively. Singlets at δ 2.31 ppm and δ 2.33 ppm correspond to the protons at C-11 and C-11′, respectively and the quartet at δ 4.08 ppm corresponds to the protons at C-13, 13′.
Singlets at δ 5.04 and δ 5.07 ppm correspond to the protons at C-23, 23′ and C-4, 4′, respectively. A broad singlet at δ 6.18 ppm corresponds to the protons of nitrogen and another at δ 6.76 ppm corresponds to the aromatic protons at C-20, 20′. Doublets at δ 6.81 ppm and δ 6.84 ppm correspond to the protons at C-22 and C-22′, respectively, and the triplet at δ 6.99 ppm corresponds to the protons at C-21, 21′. A multiplet at δ 7.14 ppm corresponds to the protons at C-18, 18′. A singlet at δ 7.41 ppm corresponds to the protons at C-25, 25′ and C-26, 26′. The m/z value observed at the 812.4785 (M + 1) peak in the HRMS spectrum also confirms the formation of the target molecule. Furthermore, during the conversion of 9 to its corresponding pyridine 28, a slight change in chemical shift values has been observed, along with the absence of two signals; one is the broad singlet due to the –NH proton and the other is a singlet for a proton at C-4, 4′. This clearly revealed that the compound 9 had been oxidized to 28. The m/z value observed at the 808 (M + 1) peak in the LC-MS spectrum also confirmed the formation of the desired target with high purity.
3. Experimental section
3.1 General
Solvents and reagents were commercially sourced and used without further purification. Melting points were recorded on an Elchem Microprocessor-based DT apparatus in open capillary tubes and corrected with benzoic acid. NMR spectra were recorded either on Bruker 400 or 500 MHz spectrometers using TMS as an internal standard (chemical shifts δ in ppm). Mass spectra were recorded either via HRMS or LC-MS by Agilent 1200 series LC and Micromass ZQ spectrometers. HRMS spectra were recorded using a 1260 Infinity Nano HPLC with Chipcube 6550 and iFunnel Q-TOF system. Positive and negative modes of ionization were recorded using electrospray ionization. Thin-layered chromatography (TLC) was performed on preparative plates of silica gel (SD Fine). Visualization was carried out using an iodine chamber. Column chromatography was performed using silica gel (60–120 mesh).3.2 Ultrasound set-up
The ultrasound system for sonochemical synthesis was generated using an ultrasonic instrument set-up (horn type). The specification and details of the experimental set-up are as follows: Make: E-Chrom Tech Co. Ltd., Taiwan. Operating frequency: 22 kHz. Rated output power: 800 W. Diameter of titanium tip of horn: 1.3 × 10−2 m. Surface area of ultrasound irradiating face: 1.32 × 10−4 m2.3.3 Representative procedure for the synthesis of bi-, tri- and tetrapodal 1,4-DHPs
3.4 Diethyl 4,4′-(3,3′-(1,4-phenylenebis(methylene)bis(oxy)bis(3,1-phenylene))bis(2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate)) (9)
Yellow solid. Mp 130–132 °C. 1H NMR (500 MHz, CDCl3) δH: 0.93 (s, 6H, C-15, 15′), 1.06 (s, 6H, C-16, 16′), 1.21 (t, J = 7.25 Hz, 6H, C-14, 14′), 2.17 (m, 4H, C-8, 8′), 2.24 (m, 4H, C-6, 6′), 2.31 (s, 3H, C-11), 2.33 (s, 3H, C-11′), 4.08 (q, J = 7.24 Hz, 4H, C-13, 13′), 5.04 (s, 4H, C-23, 23′), 5.07 (s, 2H, C-4, 4′), 6.18 (bs, 2H), 6.76 (m, 2H, C-20, 20′), 6.81 (d, J = 8 Hz, 1H, C-22), 6.84 (d, J = 8 Hz, 1H, C-22′), 6.99 (t, J = 8, 2H, C-21, 21′), 7.14 (m, 2H, C-18, 18′), 7.41 (s, 4H, C-25, 25′, 26, 26′). 13C NMR (125 MHz, CDCl3) δc: 14.26 (C-14, 14′), 19.35 (C-11, 11′), 27.18 (C-15, 15′), 29.43 (C-16, 16′), 32.66 (C-7, 7′), 36.36 (C-4, 4′), 40.98 (C-8, 8′), 50.74 (C-6, 6′), 59.81 (C-13, 13′), 70.19 (C-23, 23′), 105.73 (C-3, 3′), 111.99 (C-20, 20′), 112.77 (C-9, 9′), 114.64 (C-18, 18′), 121.28 (C-22, 22′), 127.86 (C-25, 25′), 128.79 (C-26, 26′), 137.19 (C-21, 21′), 143.65 (C-24, 24′), 143.76 (C-17, 17′), 148.15 (C-2, 2′), 148.63 (C-10, 10′), 158.63 (C-19, 19′), 167.41 (C-12, 12′), 195.44 (C-5, 5′). HRMS (m/z): calcd 812.4359 (M+). Found: 812.4785 (M+).3.5 Diethyl 4,4′-(3,3′-(1,3-phenylenebis(methylene)bis(oxy)bis(3,1-phenylene))bis(2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate)) (10a)
White solid. Mp 102–104 °C. 1H NMR (500 MHz, CDCl3) δH: 0.89 (s, 3H, C-15), 0.91 (s, 3H, C-15′), 1.03 (s, 3H, C-16), 1.05 (s, 3H, C-16′), 1.21 (t, J = 7.25 Hz, 6H, C-14, 14′), 2.14 (m, 4H, C-8, 8′), 2.25 (m, 4H, C-6, 6′), 2.31 (s, 6H, C-11, 11′), 4.07 (q, J = 7.16 Hz, 4H, C-13, 13′), 5.04 (s, 2H, C-4, 4′), 5.05 (s, 4H, C-23, 23′), 6.78 (m, 4H, C-20, 20′, 22, 22′), 6.89 (bs, 2H), 6.99 (t, J = 8 Hz, 2H, C-21, 21′), 7.15 (m, 2H, C-18, 18′), 7.366 (s, 3H, C-25, 25′, 26′), 7.437 (s, 1H, C-26). 13C NMR (125 MHz, CDCl3) δC: 14.27(C-14, 14′), 19.16 (C-11, 11′), 27.16 (C-15, 15′), 29.41 (C-16′, 16′), 32.60 (C-7, 7′), 36.34 (C-8, 8′), 40.79 (C-4, 4′), 50.76 (C-6, 6′), 59.81 (C-13, 13′), 70.03 (C-23, 23′), 105.64 (C-3, 3′), 111.76 (C-20, 20′), 112.37 (C-9, 9′), 112.53 (C-18, 18′), 114.52 (C-22, 22′), 121.14 (C-24, 24′), 126.47 (C-22, 22′), 127.14 (C-10, 10′), 128.81 (C-17, 17′), 129.03 (C-26′), 137.37 (19, 19′), 144.09 (C-26), 148.81 (C-2, 2′), 158.50 (C-24, 24′), 167.46 (C-12, 12′), 196.01 (C-5, 5′). HRMS (m/z): calcd 812.5142 (M+). Found: m/z 812.4785 (M+).3.6 Diethyl 4,4′-(3,3′-(pyridine-2,6-diylbis(methylene)bis(oxy)bis(3,1-phenylene))bis(2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate)) (10b)
Yellow solid. Mp 114–116 °C. 1H NMR (500 MHz, CDCl3) δH: 0.87 (s, 3H, C-15), 0.89 (s, 3H, C-15′), 0.97 (s, 3H, C-16), 1.01 (s, 3H, C-16′), 1.17 (t, J = 12 Hz, 6H, C-14, 14′), 1.78 (m, 4H, C-8, 8′), 2.17 (m, 4H, C-6, 6′), 2.27 (s, 6H, C-11, 11′), 4.06 (q, J = 7 Hz, 4H, C-13, 13′), 5.03 (s, 2H, C-4, 4′), 5.12 (s, 4H, C-23, 23′), 6.47 (bs, 2H), 6.77 (d, J = 5.2 Hz, 2H, C-22, 22′), 6.83 (d, J = 8 Hz, 2H, C-20, 20′), 6.98 (t, J = 7.2 Hz, 2H, C-21, 21′), 7.13 (m, 2H, C-18, 18′), 7.39 (d, J = 8 Hz, 2H, C-25, 25′), 7.67 (t, J = 8.2, 1H, C-26). 13C NMR (125 MHz, CDCl3) δC: 14.31 (C-14, 14′), 19.08 (C-11, 11′), 27.16 (C-15, 15′), 29.48 (C-16, 16′), 32.64 (C-7, 7′), 36.61 (C-8, 8′), 40.66 (C-4, 4′), 50.74 (C-6, 6′), 59.80 (C-13, 13′), 70.09 (C-23, 23′), 105.66 (C-3, 3′), 111.82 (C-20, 20′), 112.96 (C-9, 9′), 113.92 (C-18, 18′), 120.74 (C-22, 22′), 121.42 (C-25, 23), 128.87 (C-22, 22′), 137.59 (C-10, 10′), 143.75 (C-17, 17′), 148.39 (C-26), 148.79 (C-2, 2′), 157.01 (C-24, 24′), 158.05 (C-19, 19′), 167.43 (C-12, 12′), 196.01 (C-5, 5′). HRMS (m/z): calcd 813.1614 (M+). Found: 813.1427 (M+).3.7 Diethyl 4,4′-(((1,2-phenylenebis(methylene))bis(oxy))bis(3,1-phenylene))bis(2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate) (11)
White solid. Mp 86–88 °C. 1H NMR (500 MHz, CDCl3) δH: 0.87 (s, 3H, C-15), 0.88 (s, 3H, C-15′), 0.97 (s, 3H, C-16), 1.01 (s, 3H, C-16′), 1.17 (t, J = 7 Hz, 6H, C-14, 14′), 2.05 (m, 4H, C-6, 6′), 2.17 (s, 6H, C-11, 11′), 2.29 (m, 4H, C-8, 8′), 4.05 (q, J = 7.25 Hz, 4H, C-13, 13′), 5.03 (s, 4H, C-23, 23′), 5.07 (s, 2H, C-4, 4′), 6.74 (bs, 2H), 6.94 (m, 4H, C-20, 20′, 22, 22′), 7.11 (m, 4H, C-25, 25′, 26, 26′), 7.30 (s, 1H, C-18), 7.45 (s, 1H, C-18′), 7.68 (t, J = 6.4 Hz, 2H, C-21, 21′). 13C NMR (125 MHz, CDCl3) δC: 14.31 (C-14, 14′), 19.08 (C-11, 11′), 27.16 (C-15, 15′), 29.48 (C-16, 16′), 32.64 (C-7, 7′), 36.61 (C-4, 4′), 40.60 (C-8, 8′), 50.83 (C-6, 6′), 59.84 (C-13, 13′), 67.43 (C-23, 23′), 105.80 (C-3, 3′), 111.28 (C-20, 20′), 112.28 (C-9, 9′), 113.91 (C-18, 18′), 121.08 (C-22, 22′), 128.93 (C-25, 25′), 130.79 (C-26, 26′), 135.05 (C-21, 21′), 144.34 (C-24, 24′), 144.34 (C-17, 17′), 148.93 (C-2, 2′), 149.79 (C-10, 10′), 158.52 (C-19, 19′), 167.58 (C-12, 12′), 196.01 (C-5, 5′). HRMS (m/z): calcd 812.4359 (M+). Found: 812.4785 (M+).3.8 Triethyl 4,4′,4′′-(3,3′,3′′-(2,4,6-trimethylbenzene-1,3,5-triyl)tris(methylene)tris(oxy)tris(benzene-3,1-diyl))tris(2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate) (12)
White solid. Mp 124–126 °C. 1H NMR (500 MHz, CDCl3) δH: 0.92 (s, 9H, C-15, 15′, 15′′), 1.05 (s, 9H, C-16, 16′, 16′′), 1.24 (t, J = 7 Hz, 9H, C-14, 14′, 14′′), 2.19 (m, 9H, C-26, 26′, 26′′), 2.32 (m, 21H, C-6, 6′, 6′′, 8, 8′, 8′′, 11, 11′, 11′′), 4.09 (q, J = 6.25 Hz, 6H, C-13, 13′, 13′′, 13′′′), 5.02 (s, 3H, C-4, 4′, 4′′), 5.06 (s, 6H, C-23, 23′, 23′′), 6.65 (bs, 3H), 6.80 (d, J = 8 Hz, 3H, C-20, 20′, 20′′), 6.90 (s, 3H, C-18, 18′, 18′′), 7.00 (d, J = 7.5 Hz, 3H, C-22, 22′, 22′′), 7.15 (t, J = 6.4 Hz, 3H, C-21, 21′, 21′′). 13C NMR (125 MHz, CDCl3) δC: 14.28 (C-14, 14′, 14′′), 19.08 (C-11, 11′, 11′′), 26.14 (C-26, 26′, 26′′), 27.09 (C-15, 15′, 15′′), 29.40 (C-16, 16′, 16′′), 32.66 (C-7, 7′, 7′′), 36.39 (C-4, 4′, 4′′), 40.90 (C-8, 8′, 8′′), 50.75 (C-6, 6′, 6′′), 59.81 (C-13, 13′, 13′′), 65.28 (C-23, 23′, 23′′), 105.77 (C-3, 3′, 3′′), 111.89 (C-20, 20′, 20′′), 112.01 (C-9, 9′, 9′′), 115.14 (C-18, 18′, 18′′), 121.23 (C-22, 22′, 22′′), 128.71 (C-25, 25′, 25′′), 138.78 (C-21, 21′, 21′′), 139.05 (C-24, 24′, 24′′), 143.86 (C-17, 17′, 17′′), 148.58 (C-2, 2′, 2′′), 149.12 (C-10, 10′, 10′′), 159.02 (C-19, 19′, 19′′), 167.49 (C-12, 12′, 12′′), 195.71 (C-5, 5′, 5′′). HRMS (m/z): calcd 1221.1178 (M+). Found: 1221.1200 (M+).3.9 Tetraethyl 4,4′,4′′,4′′′-(((benzene-1,2,4,5-tetrayltetrakis (methylene))tetrakis(oxy))tetrakis(benzene-3,1-diyl))tetrakis(2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate) (13)
White solid. Mp 100–102 °C. 1H NMR (500 MHz, CDCl3) δH: 0.93 (s, 6H, C-15, 15′), 0.96 (s, 6H, C-15′′, 15′′′), 1.00 (s, 6H, C-16, 16′), 1.04 (s, 6H, C-16′′, 16′′′), 1.19 (t, J = 7 Hz, 12H, C-14, 14′, 14′′, 14′′′), 2.14 (s, 12H, C-11, 11′, 11′′, 11′′′), 2.32 (s, 8H, C-6, 6′, 6′′, 6′′′), 2.61 (s, 8H, C-8, 8′, 8′′, 8′′′), 4.04 (q, J = 7.25 Hz, 8H, C-13, 13′, 13′′, 13′′′), 5.02 (s, 8H, C-23, 23′, 23′′, 23′′′), 5.09 (s, 4H, C-4, 4′, 4′′, 4′′′), 6.77 (bs, 2H), 6.82 (bs, 2H), 6.93 (d, J = 8 Hz, 4H, C-20, 20′, 20′′, 20′′′), 7.11 (d, J = 8 Hz, 4H, C-22, 22′, 22′′, 22′′′), 7.33 (m, 6H, C-21, 21′, 21′′, 21′′′, 25, 25′), 7.51 (s, 4H, C-18, 18′, 18′′, 18′′′). 13C NMR (125 MHz, CDCl3) δC: 14.28 (C-14, 14′, 14′′, 14′′′), 19.12 (C-11, 11′, 11′′, 11′′′), 27.22 (C-15, 15′, 15′′, 15′′′), 28.41 (C-16, 16′, 16′′, 16′′′), 32.69 (C-7, 7′, 7′′, 7′′′), 36.73 (C-4, 4′, 4′′, 4′′′), 40.78 (C-8, 8′, 8′′, 8′′′), 50.85 (C-6, 6′, 6′′, 6′′′), 59.79 (C-13, 13′, 13′′, 13′′′), 67.44 (C-23, 23′, 23′′, 23′′′), 105.16 (C-3, 3′, 3′′, 3′′′), 111.63 (C-20, 20′, 20′′, 20′′′), 113.19 (C-9, 9′, 9′′, 9′′′), 113.62 (C-18, 18′, 18′′, 18′′′), 120.89 (C-22, 22′, 22′′, 22′′′), 128.45 (C-25, 25′, 25′′, 25′′′), 135.14 (C-21, 21′, 21′′, 21′′′), 139.05 (C-24, 24′, 24′′, 24′′′), 143.98 (C-17, 17′, 17′′, 17′′′), 144.21 (C-2, 2′, 2′′, 2′′′), 149.52 (C-10, 10′, 10′′, 10′′′), 158.50 (C-19, 19′, 19′′, 19′′′), 167.55 (C-12, 12′, 12′′, 12′′′), 195.85 (C-5, 5′, 5′′, 5′′′). HRMS (m/z): calcd 1547.4263 (M+). Found: 1547.4030 (M+).3.10 Oxidation of bi-, tri- and tetrapodal 1,4-DHPs into the corresponding bi-, tri- and tetrapodal pyridines
A suspension of 1.0 equiv. of bi-, tri- and tetrapodal 1,4-DHPs in 4 equiv. volumes of 20% HNO3 were kept under ultrasonic irradiation for 3 minutes at 60 °C. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was filtered and poured into 500 ml of water, neutralized with Na2CO3, then dried over anhydrous Na2SO4 (50 mg), which was added to the filtrate. Bi-, tri- and tetrapodal pyridines were obtained through simple filtration and evaporation of the solvent.3.11 Diethyl 4,4′-(((1,4-phenylenebis(methylene))bis(oxy))bis(3,1-phenylene))bis(2,7,7-trimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-3-carboxylate) (28)
White solid. Mp 134–136 °C. 1H NMR (500 MHz, CDCl3) δH: 0.96 (t, J = 9 Hz, 6H, C-14, 14′), 1.14 (s, 12H, C-15, 15′, 16, 16′), 2.51 (s, 4H, C-6, 6′), 2.71 (s, 6H, C-11, 11′), 3.22 (s, 4H, C-8, 8′), 4.02 (q, J = 9.0 Hz, 4H, C-13, 13′), 5.06 (s, 4H, C-23, 23′), 6.76 (m, 4H, C-20, 20′, 22, 22′), 7.02 (d, J = 9.4 Hz, 2H, C-18, 18′), 7.31 (d, J = 9.4 Hz, 2H, C-21, 21′), 7.45 (s, 4H, C-25, 25′, 26, 26′). LCMS: m/z 808 (M + 1); HRMS (m/z): calcd 808.1291 (M+). Found: m/z 808.1300 (M+).3.12 Diethyl 4,4′-(((pyridine-2,6-diylbis(methylene))bis(oxy))bis(3,1-phenylene))bis(2,7,7-trimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-3-carboxylate) (29)
Yellow solid. Mp 126–128 °C. 1H NMR (500 MHz, CDCl3) δH: 0.96 (t, J = 9 Hz, 6H, C-14, 14′), 1.12 (s, 6H, C-15, 15′), 2.19 (s, 6H, C-16, 16′), 2.47 (s, 4H, C-6, 6′), 2.62 (s, 6H, C-11, 11′), 3.10 (s, 4H, C-8, 8′), 4.01 (q, J = 9.0 Hz, 4H, C-13, 13′), 5.19 (s, 4H, C-23, 23′), 6.77 (m, 4H, C-20, 20′, 22, 22′), 6.99 (m, 2H, C-20, 20′), 7.31 (d, J = 9.0 Hz, 2H, C-18, 18′), 7.46 (d, J = 8.6 Hz, 2H, C-25, 25′), 7.76 (t, J = 8.4 Hz, 4H, C-26). LCMS (m/z): calcd 808 (M + 1). Found: 808 (M + 1).3.13 Tetramethyl 4,4′-(((1,4-phenylenebis(methylene))bis(oxy))bis(4,1-phenylene))bis(2,6-dimethylpyridine-3,5-dicarboxylate) (33)
White solid. Mp 150–152 °C. 1H NMR (500 MHz, CDCl3) δH: 1.60 (s, 12 H-7, 7′), 2.60 (s, 6 H-9), 3.59 (s, 6 H-9′), 5.11 (s, 4 H-15, 15′), 7.00 (d, J = 7.5 Hz, 4 arom. H-13, 13′), 7.20 (d, J = 7.5 Hz, 4 arom. H-12, 12′), 7.49 (s, 4 arom. H-17, 17′). 13C NMR (125 MHz, CDCl3) δC: 22.30 (C-7, 7′), 52.39 (C-9, 9′), 69.70 (C-15, 15′), 104.17 (C-3, 5), 114.76 (C-13, 13′), 127.51 (C-17, 17′), 127.84 (C-12, 12′), 128.60 (C-16, 16′), 136.52 (C-11), 155.08 (C-2, 6), 159.02 (C-4, 14), 168.10 (C-8, 8′). HRMS (m/z): calcd 730.6514 (M+). Found: 730.6116 (M+).3.14 Tetramethyl 4,4′-(((1,3-phenylenebis(methylene))bis(oxy))bis(4,1-phenylene))bis(2,6-dimethylpyridine-3,5-dicarboxylate) (34a)
White solid. Mp 100–102 °C. 1H NMR (500 MHz, CDCl3) δH: 2.60 (s, 12H, C-7, 7′), 3.59 (s, 12H, C-9, 9′), 4.93 (s, 2H, C-4), 5.12 (s, 4H, C-15, 15′), 7.01 (d, J = 6.9 Hz, 4H, C-13, 13′), 7.20 (d, J = 8.4 Hz, 4H, C-12, 12′), 7.44 (s, 3H, C-16, 18, 18′), 7.56 (s, 1H, C-19). HRMS (m/z): calcd 730.0176 (M+). Found: 730.0000 (M+).3.15 Tetramethyl 4,4′-(((pyridine-2,6-diylbis(methylene))bis(oxy))bis(4,1-phenylene))bis(2,6-dimethylpyridine-3,5-dicarboxylate) (34b)
White solid. Mp 106–108 °C. 1H NMR (500 MHz, CDCl3) δH: 2.31 (s, 12H, C-7, 7′), 3.63 (s, 12H, C-9, 9′), 5.13 (s, 4H, C-15, 15′), 6.81 (d, J = 8.4 Hz, 4H, C-13, 13′), 7.16 (d, J = 8.1 Hz, 4H, C-12, 12′), 7.42 (d, J = 7.8 Hz, 2H, C-18, 18′), 7.70 (t, J = 7.5 Hz, 1H, C-19). 13C NMR (125 MHz, CDCl3) δC: 22.93 (C-7, 7′), 52.24 (C-9, 9′), 70.58 (C-15, 15′), 114.63 (C-3, 5), 120.51 (C-13, 13′), 126.95 (C-18, 18′), 129.20 (C-12), 129.29 (C-12′), 137.65 (C-11, 11′), 145.69 (C-19), 155.44 (C-2, 6), 156.49 (C-17, 17′), 158.52 (C-4, 14), 168.58 (C-8, 8′). HRMS (m/z): calcd 731.8956 (M+). Found: m/z 731.9000 (M+).3.16 Tetramethyl 4,4′-(((1,2-phenylenebis(methylene))bis(oxy))bis(4,1-phenylene))bis(2,6-dimethylpyridine-3,5-dicarboxylate) (35)
Yellow solid. Mp 88–90 °C. 1H NMR (500 MHz, CDCl3) δH: 2.53 (s, 12H, C-7, 7′), 3.24 (s, 12H, C-9, 9′), 5.38 (s, 4H, C-15, 15′), 6.81 (d, J = 7.4 Hz, 4H, C-13, 13′), 7.17 (d, J = 8.0 Hz, 4H, C-12, 12′), 7.34–7.49 (m, 4H, C-17, 17′, 18, 18′). 13C NMR (125 MHz, CDCl3) δC: 22.35 (C-7, 7′), 52.38 (C-9, 9′), 67.91 (C-15, 15′), 114.74 (C-3, 5), 127.46 (C-13, 13′), 128.57 (C-18, 18′), 128.77 (C-12, 12′), 129.00 (C-17, 17′), 129.24 (C-16, 16′), 134.70 (C-11, 11′), 155.09 (C-2, 6), 158.86 (C-4, 14), 168.08 (C-8, 8′). HRMS (m/z): calcd 730.1498 (M+). Found: m/z 730.1442 (M+).3.17 Hexamethyl 4,4′,4′′-(4,4′,4′′-(2,4,6-trimethylbenzene-1,3,5-triyl)tris(methylene)tris(oxy)tris(benzene-4,1-diyl))tris(2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate) (36)
White solid. Mp 94–96 °C. 1H NMR (400 MHz, CDCl3) δH: 2.46 (s, 9H, C-18, 18′, 18′′), 2.57 (s, 18H, C-7, 7′), 3.62 (s, 18H, C-9, 9′), 5.12 (s, 6H, C-15, 15′, 15′′), 7.02 (d, J = 8.2 Hz, 6H, C-13, 13′), 7.22 (d, J = 8 Hz, 6H, C-12, 12′). 13C NMR (100 MHz, CDCl3) δC: 16.17 (C-18, 18′, 18′′), 23.07 (C-7, 7′), 52.43 (C-9, 9′), 65.13 (C-15, 15′, 15′′), 114.59 (C-3, 5), 127.16 (C-13, 13′), 128.98 (C-12, 12′), 129.42 (C-17, 17′, 17′′), 131.77 (C-16, 16′, 16′′), 139.59 (C-11), 145.86 (C-4), 155.49 (C-2, 6), 159.42 (C-14), 168.81 (C-8, 8′). HRMS (m/z): calcd 1101.1521 (M+). Found: m/z 1101.1537 (M+).3.18 Hexaethyl 4,4′,4′′-(((2,4,6-trimethylbenzene-1,3,5-triyltris(methylene))tris(oxy))tris(benzene-4,1-diyl))tris(2,6-dimethylpyridine-3,5-dicarboxylate) (37)
Yellow solid. Mp 106–108 °C. 1H NMR (500 MHz, CDCl3) δH: 1.03 (t, J = 7.25 Hz, 18H, C-10, 10′), 2.46 (s, 9H, C-18, 18′, 18′′), 2.61 (s, 18H, C-7, 7′), 4.10 (q, 16H, C-9, 9′), 5.13 (s, 6H, C-15, 15′, 15′′), 7.03 (d, J = 8.5 Hz, 6H, C-13, 13′), 7.25 (d, J = 8.5 Hz, 6H, C-12, 12′). 13C NMR (125 MHz, CDCl3) δC: 15.62 (C-10, 10′), 15.99 (C-18, 18′, 18′′), 21.69 (C-7, 7′), 60.25 (C-9, 9′), 69.29 (C-15, 15′, 15′′), 104.13 (C-3, 5), 114.39 (C-13, 13′), 129.78 (C-12, 12′), 134.65 (C-17, 17′, 17′′), 137.28 (C-16, 16′, 16′′), 141.32 (C-11), 145.60 (C-2, 6), 159.77 (C-4, 14), 168.29 (C-8, 8′). HRMS (m/z): calcd 1183.0061 (M+). Found: m/z 1183.0000 (M+).3.19 Hexamethyl 4,4′,4′′-(((benzene-1,3,5-triyltris(methylene)) tris(oxy))tris(benzene-4,1-diyl))tris(2,6-dimethylpyridine-3,5-dicarboxylate) (38)
Yellow solid. Mp 100–102 °C. 1H-NMR (500 MHz, CDCl3) δH: 2.60 (s, 18H, C-7, 7′), 3.59 (s, 18H, C-9, 9′), 5.19 (s, 6H, C-15, 15′, 15′′), 7.02 (d, J = 8.1 Hz, 6H, C-13, 13′), 7.22 (d, J = 8.1 Hz, 6H, C-12, 12′), 7.54 (s, 3H, C-17, 17′, 17′′). 13C NMR (125 MHz, CDCl3) δC: 22.93 (C-7, 7′), 52.24 (C-9, 9′), 69.65 (C-15, 15′, 15′′), 114.59 (C-3, 5), 126.37 (C-13, 13′), 126.97 (C-17, 17′, 17′′), 129.10 (C-12, 12′), 129.29 (C-11), 137.63 (C-16, 16′, 16′′), 145.69 (C-2, 6), 155.43 (C-4), 158.79 (C-14), 168.60 (C-8, 8′). HRMS (m/z): calcd 1059.2498 (M+). Found: m/z 1059.2540 (M+).3.20 Octaethyl 4,4′,4′′,4′′′-(4,4′,4′′,4′′′-(benzene-1,2,4,5-tetrayltetrakis(methylene))tetrakis(oxy)tetrakis(benzene-4,1-diyl))tetrakis(2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate) (39)
White solid. Mp 252–254 °C. 1H NMR (500 MHz, CDCl3) δH: 0.98 (t, J = 6 Hz, 24H, C-10, 10′), 2.61 (s, 24H, C-7, 7′), 4.07 (q, 16H, C-9, 9′), 5.07 (s, 8H, C-15, 15′, 15′′, 15′′′), 6.79 (d, J = 6 Hz, 8H, C-13, 13′), 7.24 (d, J = 7.2 Hz, 8H, C-12, 12′), 7.75 (s, 2H, C-17, 17′). 13C NMR (125 MHz, CDCl3) δC: 13.74 (C-10, 10′), 22.80 (C-7, 7′), 61.32 (C-9, 9′), 67.52 (C-15, 15′, 15′′, 15′′′), 104.14 (C-3, 5), 114.35 (C-13, 13′), 127.24 (C-17, 17′), 129.67 (C-12, 12′), 136.92 (C-11), 140.68 (C-16, 16′, 16′′, 16′′′), 143.81 (C-2, 6), 155.21 (C-4, 14), 167.92 (C-8, 8′). HRMS (m/z): calcd 1490.2116 (M+). Found: m/z 1490.2146 (M+).3.21 Octamethyl 4,4′,4′′,4′′′-(4,4′,4′′,4′′′-(benzene-1,2,4,5-tetrayltetrakis(methylene))tetrakis(oxy)tetrakis(benzene-4,1-diyl))tetrakis(2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate) (40)
White solid. Mp 234–236 °C. 1H NMR (500 MHz, CDCl3) δH: 2.56 (s, 24H, C-7, 7′), 3.55 (s, 24H, C-9, 9′), 5.20 (s, 8H, C-15, 15′, 15′′, 15′′′), 6.98 (d, J = 8 Hz, 8H, C-13, 13′), 7.19 (d, J = 8 Hz, 8H, C-12, 12′), 7.76 (s, 2H, C-17, 17′). HRMS (m/z): calcd 1602.1456 (M+). Found: m/z 1602.1463 (M+).4. Conclusion
In conclusion, polyhydroquinolines and 1,4-DHPs with bi-, tri- and tetrapodal moieties, and the corresponding pyridines, have been synthesized using cheap reagents such as K2CO3 and 20% HNO3, under ultrasonic irradiation, with a faster reaction time. This protocol is an economically attractive method to synthesize similar types of bi-, tri- and tetrapodal compounds with other heterocycles.Acknowledgements
The authors are thankful to VIT University administration in Vellore, India for providing facilities to carry out research work, and also thankful to SAIF, IIT-Madras and VIT-TBI for providing NMR, Mass and IR spectral facilities, respectively. The author G. L. Balaji is thankful to VIT University for providing a Research Associateship.References
- (a) F. Bossert, H. Meyer and E. Wehinger, Angew. Chem., Int. Ed. Engl., 1981, 20, 762–769 CrossRef; (b) H. Nakayama and Y. Kasoaka, Heterocycles, 1996, 42, 901–909 CrossRef CAS.
- (a) F. R. Buhler and W. Kiowski, J. Hypertens., 1987, 5, S3–S10 CrossRef CAS PubMed; (b) J. L. Reid, P. A. Meredith and F. Pasanisi, J. Cardiovasc. Pharmacol., 1985, 7, S18–S20 CrossRef PubMed.
- (a) Y. L. Chen, K. C. Fang, J. Y. Sheu, S. L. Hsu and C. C. Tzeng, J. Med. Chem., 2001, 44, 2374–2377 CrossRef CAS PubMed; (b) G. Roma, M. D. Braccio, G. Grossi and M. Chia, Eur. J. Med. Chem., 2000, 35, 1021–1035 CrossRef CAS; (c) M. P. Maguire, K. R. Sheets, K. McVety, A. P. Spada and A. Zilberstein, J. Med. Chem., 1994, 37, 2129–2137 CrossRef CAS.
- (a) V. Klusa, Drugs Future, 1995, 20, 135–138 Search PubMed; (b) R. G. Bretzel, C. C. Bollen, E. Maeser and K. F. Federlin, Am. J. Kidney Dis., 1993, 21, 53–64 CAS; (c) R. G. Bretzel, C. C. Bollen, E. Maeser and K. F. Federlin, Drugs Future, 1992, 17, 465–468 Search PubMed; (d) R. Boer and V. Gekeler, Drugs Future, 1995, 20, 499–509 Search PubMed.
- (a) D. Zhang, L. Z. Wu, L. Zhou, X. Han, Q. Z. Yang, L. P. Zhang and C. H. Tung, J. Am. Chem. Soc., 2004, 126, 3440 CrossRef CAS PubMed; (b) J. J. V. Eynde, F. Delfosse, A. Mayence and Y. V. Haverbeke, Tetrahedron, 1995, 51, 6511–6516 CrossRef; (c) M. Anniyappan, D. Muralidharan and P. T. Perumal, Tetrahedron, 2002, 58, 5069–5073 CrossRef CAS; (d) M. M. Heravi, F. K. Behbahani, H. A. Oskooie and R. H. Shoar, Tetrahedron Lett., 2005, 46, 2775–2777 CrossRef CAS PubMed; (e) M. N. Esfahani, M. Moghadam, S. Tangestaninejad, V. Mirkhani and A. R. Momeni, Bioorg. Med. Chem., 2006, 14, 2720–2724 CrossRef PubMed.
- (a) G. Hennrich and E. V. Anslyn, Chem.–Eur. J., 2002, 8, 2219–2224 Search PubMed; (b) H. L. Ruiz, M. C. Hernandez, S. R. Lima and H. Hopflz, J. Mex. Chem. Soc., 2011, 55, 168–175 Search PubMed.
- J. M. Lehn, Supramolecular Reactivity and Catalysis of Phosphoryl Transfer, in Bioorganic Chemistry in Healthcare and Technology, ed. U. K.Pandit and F. C. Alderweireldt, Plenum Press, New York, 1991 Search PubMed.
- (a) L. R. Snyder, J. L. Glajch and J. J. Kirkland, Practical HPLC Method Development, Wiley & Sons, New York, 1988 Search PubMed; (b) F. P. Schmidtchen, Nachr. Chem., Tech. Lab., 1988, 36, 8–12 CAS.
- (a) A. Grohmanni, H. Lanig, W. Bauer, S. Schmidt and F. W. Heinemann, J. Mol. Model., 2000, 6, 119–125 CrossRef; (b) R. Ghorbani, H. Veisi, H. Keypour and A. Ali Dehgani-Firouzabadi, Mol. Diversity, 2010, 14, 87–96 CrossRef PubMed.
- M. A. Zolfigol, E. Kolvari, A. Abdoli and M. Shiri, Mol. Diversity, 2010, 14, 809–813 CrossRef CAS PubMed.
- H. Xu, W. M. Liao and H. F. Li, Ultrason. Sonochem., 2007, 14, 779–782 CrossRef CAS PubMed.
- T. J. Mason and J. L. Luche, in Chemistry Under Extreme or Non-Classical Conditions, ed. R. Van Eldik and C. D. Hubband, Wiley and Spectrum, New York and Heidelberg, 1997, p. 317 Search PubMed.
- K. Rajesh, B. Palakshi Reddy and V. Vijayakumar, Ultrason. Sonochem., 2012, 19, 522–531 CrossRef CAS PubMed.
- K. Rajesh, V. Vijayakumar, T. Narasimhamurthy, J. Suresh and E. R. T. Tiekink, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, o985 CAS.
- R. S. Rathore, B. Palakshi Reddy, V. Vijayakumar, R. Venkat Ragavan and T. Narasimhamurthy, Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 375 CAS.
- K. Rajesh, B. Palakshi Reddy and V. Vijayakumar, Can. J. Chem., 2011, 89, 1236–1244 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45138k |
| This journal is © The Royal Society of Chemistry 2014 |