Inclusion [2]complexes based on a pillar[5]arene with mono(ethylene oxide) substituents and vinylogous viologens†
Xiaodong
Chi
and
Min
Xue
*
Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: xuemin@zju.edu.cn; Fax: +86-571-8795-3189; Tel: +86-571-8795-3189
First published on 1st November 2013
Abstract
Host–guest complexation between a pillar[5]arene with mono(ethylene oxide) substituents and vinylogous viologens was studied. It was found that the pillar[5]arene formed [2]pseudorotaxanes with these vinylogous viologens in acetonitrile with association constants up to (4.7 ± 0.3) × 104 M−1.
Threaded structures,1 such as pseudorotaxanes and rotaxanes, have been widely applied in the construction of molecular machines,2 mechanically bonded macromolecules3 and functional supramolecular polymers.4 Pseudorotaxanes, which were the fundamental building blocks for the construction of sophisticated mechanically interlocked supramolecular assemblies with intriguing properties, have attracted much attention and been widely studied.5 However, the currently available host–guest recognition motifs which are good for the preparation of inclusion structures are still limited.6 This has impeded the development of research on threaded structures. Therefore, the discovery of new host–guest recognition motifs, especially the ones which have high binding constants will undoubtedly push forward the development of supramolecular chemistry.
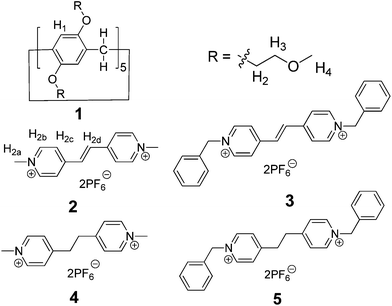
Pillararenes,7,8 as a new class of supramolecular hosts which have gained a wide range of interests since their first synthesis in 2008, are exceptional hosts for organic salts because of their rigid symmetrical pillar structures. Paraquat derivatives (N,N′-dialkyl-4,4′-bipyridinium salts) have been commonly used in the preparation of functional supramolecular systems with many kinds of hosts, including crown ethers,9 cyclodextrins,5a,10 calixarenes11 and cucurbiturils.4c A series of threaded structures, including pseudorotaxanes and supramolecular polymers, have been prepared based on the pillararene/paraquat recognition motif.7a,12 However, as π-extended viologens, vinylogous viologens, which expand the properties and functions of paraquat derivatives, such as photo-responsive isomerization, have been rarely studied as building blocks to construct threaded structures.6b,13 Considering that the design and investigation of new recognition motifs based on pillararenes and vinylogous viologens will undoubtedly promote not only the development of pillararene host–guest chemistry but also the research on threaded structures, we are interested in the study of the host–guest complexation between pillararenes and vinylogous viologens. Herein, we report the formation of [2]pseudorotaxanes between a pillar[5]arene with mono(ethylene oxide) substituents (1 in Fig. 1)14 and vinylogous viologens (2 and 3 in Fig. 1). This is the first investigation of the host–guest chemistry between pillararenes and vinylogous viologens. The crystal structure of the complex between 1 and 2 was also reported. Furthermore, the guest-substituent effect on the host–guest complexation was addressed using similar salt guests 4 and 5 (Fig. 1).
When the pillar[5]arene host 1 was mixed with an equivalent of guest 2 in acetonitrile, a yellow color was observed immediately due to charge transfer between the electron-rich aromatic rings of 1 and the electron-poor pyridinium rings of 2, providing a direct evidence for complexation. The recognition of 1 to vinylogous viologen 2 was first investigated by 1H NMR spectroscopy. Partial proton NMR spectra of 1, 2, and an equimolar solution of 1 and 2 are shown in Fig. 2. Only one set of peaks was found for the solution of 1 and 2, indicating fast-exchange complexation on the proton NMR time scale. After complexation between 1 and 2, peaks corresponding to H2b of guest 2 and H3 and H4 of host 1 shifted downfield by 0.02 ppm, 0.01 ppm and 0.10 ppm, respectively, while H1 and H2 of host 1 and H2a of guest 2 shifted upfield by 0.12 ppm, 0.17 ppm and 0.01 ppm, respectively (Fig. 2). Additionally, the signals of H2c and H2d disappeared after complexation (Fig. 2, spectra b and c), suggesting that these protons were located in the shielding region of the cyclic pillar structure. All these chemical shift changes indicated that the complexation of 1 with 2 took place in solution.
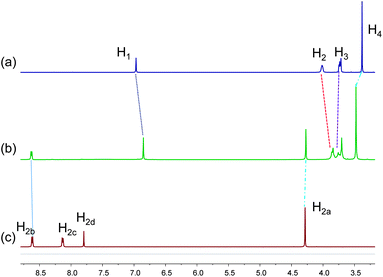 | ||
| Fig. 2 Partial 1H NMR spectra (400 MHz, CD3CN, 22 °C) of: (a) 1.00 mM 1; (b) 1.00 mM 1 and vinylogous viologen 2; (c) 1.00 mM vinylogous viologen 2. | ||
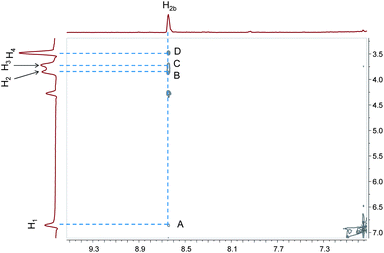
To establish the binding mode between 1 and 2, a 2D NOESY experiment was carried out. The NOESY spectrum (Fig. 3) of an equimolar solution of 1 and 2 shows cross peaks A, B, C and D representing the correlations between the signal of protons H2b of 2 and those of protons H1, H2, H3, and H4 of 1. These correlations indicated the existence of the host–guest complexation between pillararene 1 and methyl vinylogous viologen 2.
Additionally, the electrospray ionization mass spectrum of an equimolar solution of 1 and 2 exhibited a peak at m/z = 1545.9 (Fig. S5, ESI†), corresponding to [1⊃2 – PF6]+, which also confirmed the complexation between 1 and 2. Furthermore, the complex-related fragment peaks were also found for other host–guest complexes of pillararene 1 with other guests 3–5 (Fig. S6–S8, ESI†).
Further evidence of the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
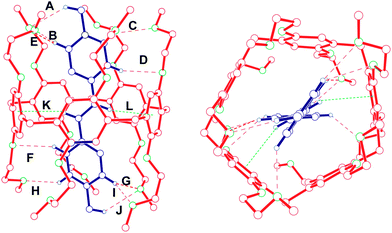
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex between 1 and 2 was from X-ray analysis of a single crystal grown by slow diffusion of isopropyl ether into an equimolar acetonitrile solution of 1 and 2. The crystals of 1⊃2 have a red color due to the charge transfer between the electron-rich aromatic rings of 1 and the electron-poor pyridinium rings of 2. The crystal structure of 1⊃2 shows that vinylogous viologen 2 threads through the cavity of host 1 to form an inclusion complex in the solid state, which is stabilized by C–H⋯O hydrogen bonding interactions and C–H⋯π interactions (Fig. 4). The inclusion of guest 2 into the cavity of host 1 in the solid state is in accordance with the above-mentioned disappearance of NMR signals of protons of H2c and H2d of 2 after the complexation between 1 and 2 in solution. Ten C–H⋯O hydrogen bonds (A–J) are formed between eight hydrogen atoms of guest 2 and eight oxygen atoms on mono(ethylene oxide) substituents of pillar[5]arene 1 (Fig. 4). Not only six pyridinium hydrogen atoms but also two N-methylene hydrogen atoms are involved in these hydrogen bonding interactions. The C–H⋯π-plane distances of two hydrogen atoms of guest 2 (K and L) are 2.96 Å and 2.91 Å, respectively, shorter than 3.05 Å, implying the existence of C–H⋯π interactions.12a What is more interesting is that guest 2 is threaded symmetrically into the cavity of host 1. The H⋯O distances of F and D are the same and the H⋯O distances of H and C are almost the same (Fig. 4).
1 inclusion complex between 1 and 2 was from X-ray analysis of a single crystal grown by slow diffusion of isopropyl ether into an equimolar acetonitrile solution of 1 and 2. The crystals of 1⊃2 have a red color due to the charge transfer between the electron-rich aromatic rings of 1 and the electron-poor pyridinium rings of 2. The crystal structure of 1⊃2 shows that vinylogous viologen 2 threads through the cavity of host 1 to form an inclusion complex in the solid state, which is stabilized by C–H⋯O hydrogen bonding interactions and C–H⋯π interactions (Fig. 4). The inclusion of guest 2 into the cavity of host 1 in the solid state is in accordance with the above-mentioned disappearance of NMR signals of protons of H2c and H2d of 2 after the complexation between 1 and 2 in solution. Ten C–H⋯O hydrogen bonds (A–J) are formed between eight hydrogen atoms of guest 2 and eight oxygen atoms on mono(ethylene oxide) substituents of pillar[5]arene 1 (Fig. 4). Not only six pyridinium hydrogen atoms but also two N-methylene hydrogen atoms are involved in these hydrogen bonding interactions. The C–H⋯π-plane distances of two hydrogen atoms of guest 2 (K and L) are 2.96 Å and 2.91 Å, respectively, shorter than 3.05 Å, implying the existence of C–H⋯π interactions.12a What is more interesting is that guest 2 is threaded symmetrically into the cavity of host 1. The H⋯O distances of F and D are the same and the H⋯O distances of H and C are almost the same (Fig. 4).
We further explored the difference of the host–guest binding properties of pillar[5]arene 1 with salt guests 2–5. This was carried out by isothermal titration calorimetry (ITC), which is a powerful tool for studying the host–guest complexes because it not only gives the complexation association constants (Ka) but also yields their thermodynamic parameters (enthalpy and entropy changes). The stoichiometries of the complexations between 1 and 2–5 were all determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in solution (Fig. S1–S4, ESI†). The association constants (Ka) were determined in acetonitrile to be (4.7 ± 0.3) × 104 M−1 for 1⊃2, (1.2 ± 0.2) × 104 M−1 for 1⊃3, (2.0 ± 0.5) × 103 M−1 for 1⊃4, and (1.5 ± 0.5) × 103 M−1 for 1⊃5 (Fig. S1–S4, ESI†). It is found that the ethylene oxide substituted groups play a crucial role in the present strong host–guest binding. The difference in Ka values indicates that the substituent groups on guests have the different influence on the host–guest complexation in solution. The association constants of 1⊃2 and 1⊃3 were much higher than those of 1⊃4 and 1⊃5. This could be attributed to the rigid double bond, which can help the guest to stay in the cavity of the pillar[5]arene not only by the preorganization of the guest for the host–guest complexation but also by additional C–H⋯π interactions. Besides, the association constant of 1⊃2 (or 1⊃4) was higher than that of 1⊃3 (or 1⊃5). This demonstrated that the end phenyl groups also influenced the host–guest complexation. Because of the existence of these phenyl groups, the guest threads more difficultly through the cavity of the pillar[5]arene.
1 in solution (Fig. S1–S4, ESI†). The association constants (Ka) were determined in acetonitrile to be (4.7 ± 0.3) × 104 M−1 for 1⊃2, (1.2 ± 0.2) × 104 M−1 for 1⊃3, (2.0 ± 0.5) × 103 M−1 for 1⊃4, and (1.5 ± 0.5) × 103 M−1 for 1⊃5 (Fig. S1–S4, ESI†). It is found that the ethylene oxide substituted groups play a crucial role in the present strong host–guest binding. The difference in Ka values indicates that the substituent groups on guests have the different influence on the host–guest complexation in solution. The association constants of 1⊃2 and 1⊃3 were much higher than those of 1⊃4 and 1⊃5. This could be attributed to the rigid double bond, which can help the guest to stay in the cavity of the pillar[5]arene not only by the preorganization of the guest for the host–guest complexation but also by additional C–H⋯π interactions. Besides, the association constant of 1⊃2 (or 1⊃4) was higher than that of 1⊃3 (or 1⊃5). This demonstrated that the end phenyl groups also influenced the host–guest complexation. Because of the existence of these phenyl groups, the guest threads more difficultly through the cavity of the pillar[5]arene.
In summary, we studied the host–guest complexation between pillar[5]arene 1 with mono(ethylene oxide) substituents and vinylogous viologens 2 and 3. 1H NMR, 2D NOESY and single crystal X-ray analysis indicated that pillar[5]arene 1 forms a stable inclusion complex with vinylogous viologen 2 both in solution and in the solid state. The good binding ability of pillar[5]arene 1 to vinylogous viologens, which largely resulted from the introduction of the ethylene oxide substituents to provide multiple hydrogen bonds between the host and guest, makes this newly established host–guest recognition motif applicable in the design and preparation of new functional supramolecular systems.
This work was supported by the National Natural Science Foundation of China (31002701).
Notes and references
- (a) S. J. Loeb, Chem. Soc. Rev., 2007, 36, 226–235 RSC; (b) C. Zhang, S. Li, J. Zhang, K. Zhu, N. Li and F. Huang, Org. Lett., 2007, 9, 5553–5556 CrossRef CAS PubMed; (c) A.-P. Pederson, E. W. Ward, D. V. Schoonover, C. Slebodnick and H. W. Gibson, J. Org. Chem., 2008, 73, 9094–9101 CrossRef CAS PubMed; (d) L. M. Klivansky, G. Koshkakaryan, D. Cap and Y. Liu, Angew. Chem., Int. Ed., 2009, 48, 4185–4189 CrossRef CAS PubMed; (e) W. Jiang, A. Schäfer, P. C. Mohr and C. A. Schalley, J. Am. Chem. Soc., 2010, 132, 2309–2320 CrossRef CAS PubMed; (f) B. Zheng, F. Wang, S. Dong and F. Huang, Chem. Soc. Rev., 2012, 41, 1621–1636 RSC; (g) X. Ji, Y. Yao, J. Li, X. Yan and F. Huang, J. Am. Chem. Soc., 2013, 135, 74–77 CrossRef CAS PubMed; (h) H. Zhang, B. Zhou, H. Li, D.-H. Qu and H. Tian, J. Org. Chem., 2013, 78, 2091–2098 CrossRef CAS PubMed; (i) X. Yan, S. Li, T. R. Cook, X. Ji, Y. Yao, J. B. Pollock, G. Yu, J. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 14036–14039 CrossRef CAS PubMed.
- (a) J. D. Badjić, V. Balzani, A. Credi, S. Silvi and J. F. Stoddart, Science, 2004, 303, 1845–1849 CrossRef PubMed; (b) X. Ma and H. Tian, Chem. Soc. Rev., 2010, 39, 70–80 RSC.
- (a) F. Huang and H. W. Gibson, Prog. Polym. Sci., 2005, 30, 982–1018 CrossRef CAS PubMed; (b) Z. Niu and H. W. Gibson, Chem. Rev., 2009, 109, 6024–6046 CrossRef CAS PubMed.
- (a) F. Wang, C. Han, C. He, Q. Zhou, J. Zhang, C. Wang, N. Li and F. Huang, J. Am. Chem. Soc., 2008, 130, 11254–11255 CrossRef CAS PubMed; (b) F. Wang, J. Zhang, X. Ding, S. Dong, M. Liu, B. Zheng, S. Li, L. Wu, Y. Yu, H. W. Gibson and F. Huang, Angew. Chem., Int. Ed., 2010, 49, 1090–1094 CrossRef CAS PubMed; (c) Z. Niu, F. Huang and H. W. Gibson, J. Am. Chem. Soc., 2011, 133, 133–136 CrossRef PubMed; (d) Y. Liu, Y. Yu, J. Gao, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6576–6579 CrossRef CAS PubMed; (e) X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362–369 CrossRef CAS PubMed; (f) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC; (g) S. Dong, B. Zheng, D. Xu, X. Yan, M. Zhang and F. Huang, Adv. Mater., 2012, 24, 3191–3195 CrossRef CAS PubMed; (h) M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Angew. Chem., Int. Ed., 2012, 51, 7011–7015 CrossRef CAS PubMed; (i) L. Chen, Y. Tian, Y. Ding, Y. Tian and F. Wang, Macromolecules, 2012, 45, 8412–8419 CrossRef CAS; (j) Q. Zhang, D.-H. Qu, J. Wu, X. Ma, Q. Wang and H. Tian, Langmuir, 2013, 29, 5345–5350 CrossRef CAS PubMed; (k) Y. Tian, L. Chen, X.-Y. Tian, X. Wang and F. Wang, Polym. Chem., 2013, 4, 453–457 RSC; (l) D.-S. Guo, T.-X. Zhang, Y.-X. Wang and Y. Liu, Chem. Commun., 2013, 49, 6779–6781 RSC; (m) X. Chi, X. Yan, J. Chen, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 2767–2772 RSC; (n) X. Yan, S. Li, J. B. Pollock, T. R. Cook, J. Chen, Y. Zhang, X. Ji, Y. Yu, F. Huang and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15585–15590 CrossRef CAS PubMed.
- (a) G. J. E. Davidson, S. Sharma and S. J. Loeb, Angew. Chem., Int. Ed., 2010, 49, 4938–4942 CrossRef CAS PubMed; (b) Z. Niu, C. Slebodnick and H. W. Gibson, Org. Lett., 2011, 13, 4616–4619 CrossRef CAS PubMed; (c) Y. Liu, K. Liu, Z. Wang and X. Zhang, Chem.–Eur. J., 2011, 17, 9930–9935 CrossRef CAS PubMed; (d) V. N. Vukotic, K. J. Harris, K. Zhu, R. W. Schurko and S. J. Loeb, Nat. Chem., 2012, 4, 456–460 CrossRef CAS PubMed.
- (a) A. R. Pease, J. O. Jeppesen, J. F. Stoddart, Y. Luo, C. P. Collier and J. R. Heath, Acc. Chem. Res., 2001, 34, 433–444 CrossRef CAS PubMed; (b) X. Yan, P. Wei, M. Zhang, X. Chi, J. Liu and F. Huang, Org. Lett., 2011, 13, 6370–6373 CrossRef CAS PubMed; (c) M.-X. Wang, Acc. Chem. Res., 2012, 45, 182–195 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed; (b) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed; (c) W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen, J.-L. Hou and Z.-T. Li, Angew. Chem., Int. Ed., 2011, 50, 12564–12568 CrossRef CAS PubMed; (d) C. Li, L. Zhao, J. Li, X. Ding, S. Chen, Q. Zhang, Y. Yu and X. Jia, Chem. Commun., 2010, 46, 9016–9018 RSC; (e) C. Li, S. Chen, J. Li, K. Han, M. Xu, B. Hu, Y. Yu and X. Jia, Chem. Commun., 2011, 47, 11294–11296 RSC; (f) Y. Ma, X. Chi, X. Yan, J. Liu, Y. Yao, W. Chen, F. Huang and J.-L. Hou, Org. Lett., 2012, 14, 1532–1535 CrossRef CAS PubMed; (g) M. Holler, N. Allenbach, J. Sonet and J.-F. Nierengarten, Chem. Commun., 2012, 48, 2576–2578 RSC; (h) C. Li, K. Han, J. Li, H. Zhang, J. Ma, X. Shu, Z. Chen, L. Weng and X. Jia, Org. Lett., 2012, 14, 42–45 CrossRef CAS PubMed; (i) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed; (j) X.-B. Hu, Z. Chen, G. Tang, J.-L. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384–8387 CrossRef CAS PubMed; (k) Q. Duan, W. Xia, X. Hu, M. Ni, J. Jiang, C. Lin, Y. Pan and L. Wang, Chem. Commun., 2012, 48, 8532–8534 RSC; (l) X.-Y. Hu, X. Wu, Q. Duan, T. Xiao, C. Lin and L. Wang, Org. Lett., 2012, 14, 4826–4829 CrossRef CAS PubMed; (m) K. Wang, L.-L. Tan, D.-X. Chen, N. Song, G. Xi, S. X.-A. Zhang, C. Li and Y.-W. Yang, Org. Biomol. Chem., 2012, 10, 9405–9409 RSC; (n) W. Xia, X. Hu, Y. Chen, C. Lin and L. Wang, Chem. Commun., 2013, 49, 5085–5087 RSC; (o) Y.-L. Sun, Y.-W. Yang, D.-X. Chen, G. Wang, Y. Zhou, C.-Y. Wang and J. F. Stoddart, Small, 2013, 9, 3224–3229 CAS; (p) X. Chi, M. Xue, Y. Ma, X. Yan and F. Huang, Chem. Commun., 2013, 49, 8175–8177 RSC; (q) Y. Yao, M. Xue, Z. Zhang, M. Zhang, Y. Wang and F. Huang, Chem. Sci., 2013, 4, 3667–3672 RSC; (r) G. Yu, Y. Ma, C. Han, Y. Yao, G. Tang, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2013, 135, 10310–10313 CrossRef CAS PubMed; (s) C. Li, K. Han, J. Li, Y. Zhang, W. Chen, Y. Yu and X. Jia, Chem.–Eur. J., 2013, 19, 11892–11897 CrossRef CAS PubMed; (t) C. Li, J. Ma, L. Zhao, Y. Zhang, Y. Yu, X. Shu and X. Jia, Chem. Commun., 2013, 49, 1924–1926 RSC.
- (a) Z. Zhang, B. Xia, C. Han, Y. Yu and F. Huang, Org. Lett., 2010, 12, 3285–3287 CrossRef CAS PubMed; (b) X.-B. Hu, L. Chen, W. Si, Y. Yu and J.-L. Hou, Chem. Commun., 2010, 46, 9016–9018 RSC; (c) N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668–5671 CrossRef CAS PubMed; (d) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS PubMed; (e) Z. Zhang, Y. Luo, B. Xia, C. Han, Y. Yu, X. Chen and F. Huang, Chem. Commun., 2011, 47, 2417–2419 RSC; (f) Y. Ma, X. Ji, F. Xiang, X. Chi, C. Han, J. He, Z. Abliz, W. Chen and F. Huang, Chem. Commun., 2011, 47, 12340–12342 RSC; (g) X. Shu, J. Li, X. Wang, W. Chen, X. Jia and C. Li, Org. Biomol. Chem., 2012, 10, 3393–3397 RSC; (h) X.-Y. Hu, P. Zhang, X. Wu, W. Xia, T. Xiao, J. Jiang, C. Lin and L. Wang, Polym. Chem., 2012, 3, 3060–3063 RSC; (i) Y. Yao, M. Xue, X. Chi, Y. Ma, J. He, Z. Abliz and F. Huang, Chem. Commun., 2012, 48, 6505–6507 RSC; (j) Y. Guan, M. Ni, X. Hu, T. Xiao, S. Xiong, C. Lin and L. Wang, Chem. Commun., 2012, 48, 8529–8531 RSC; (k) Z. Zhang, C. Han, G. Yu and F. Huang, Chem. Sci., 2012, 3, 3026–3031 RSC; (l) G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711–8717 CrossRef CAS PubMed; (m) G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS PubMed; (n) Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712–15715 CrossRef CAS PubMed; (o) C. Han, Z. Zhang, G. Yu and F. Huang, Chem. Commun., 2012, 48, 9876–9878 RSC; (p) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248–13251 CrossRef CAS PubMed; (q) H. Li, D.-X. Chen, Y.-L. Sun, Y. Zheng, L.-L. Tan, P. S. Weiss and Y.-W. Yang, J. Am. Chem. Soc., 2013, 135, 1570–1576 CrossRef CAS PubMed; (r) M. Sonawane, J. Jacobs, J. Thomas, L. V. Meervelt and W. Dehaen, Chem. Commun., 2013, 49, 6310–6312 RSC; (s) H. Zhang, X. Ma, J. Guo, K. T. Nguyen, Q. Zhang, X.-J. Wang, H. Yan, L. Zhu and Y. Zhao, RSC Adv., 2013, 3, 368–371 RSC; (t) J. Fan, H. Deng, J. Li, X. Jia and C. Li, Chem. Commun., 2013, 49, 6343–6345 RSC.
- (a) J.-M. Zhao, Q.-S. Zong, T. Han, J.-F. Xiang and C.-F. Chen, J. Org. Chem., 2008, 73, 6800–6806 CrossRef CAS PubMed; (b) S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905–1909 CrossRef CAS PubMed.
- Y. Liu, X.-Y. Li, H.-Y. Zhang, C.-J. Li and F. Ding, J. Org. Chem., 2007, 72, 3640–3645 CrossRef CAS PubMed.
- (a) D.-S. Guo, L.-H. Wang and Y. Liu, J. Org. Chem., 2007, 72, 7775–7778 CrossRef CAS PubMed; (b) C. Talotta, C. Gaeta, Z. Qi, C. A. Schalley and P. Neri, Angew. Chem., Int. Ed., 2013, 52, 7437–7441 CrossRef CAS PubMed.
- (a) C. Li, Q. Xu, J. Li, F. Yao and X. Jia, Org. Biomol. Chem., 2010, 8, 1568–1576 RSC; (b) X. Chi, M. Xue, Y. Yao and F. Huang, Org. Lett., 2013, 15, 4722–4725 CrossRef CAS PubMed.
- (a) K. Kim, D. Kim, J. W. Lee, Y. H. Ko and K. Kim, Chem. Commun., 2004, 848–849 RSC; (b) X. Yan, M. Zhang, P. Wei, B. Zheng, X. Chi, X. Ji and F. Huang, Chem. Commun., 2011, 47, 9840–9842 RSC.
- T. Ogoshi, R. Shiga and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 4577–4580 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterizations. See DOI: 10.1039/c3ra45169k |
| This journal is © The Royal Society of Chemistry 2014 |