Knoevenagel condensation of diethylmalonate with aldehydes catalyzed by immobilized bovine serum albumin (BSA)
Perla Ramesh,
Basetty Shalini and
Nitin W. Fadnavis*
Natural Products Chemistry Division, Discovery Block, Indian Institute of Chemical Technology, Uppal Road, Hyderabad-500007, India. E-mail: fadnavis@iict.res.in; fadnavisnw@yahoo.com; Fax: +91-40-27160512; Tel: +91-40-27191631
First published on 25th November 2013
Abstract
Knoevenagel condensation between aldehydes and diethylmalonate is catalyzed efficiently by bovine serum albumin (BSA) immobilized covalently on an epoxy-functionalized polymeric support, Immobead IB-350. The reaction is carried out conveniently at room temperature in DMSO in which aliphatic, heterocyclic and aromatic aldehydes react efficiently. After extraction with heptane and treatment with Candida antarctica lipase CAL B, the products are obtained in >95% purity and 85–89% yield. The solvent DMSO, unreacted diethylmalonate and immobilized BSA were easily recovered. Immobilized BSA and recovered DMSO were recycled 5 times without any appreciable loss in yield.
1. Introduction
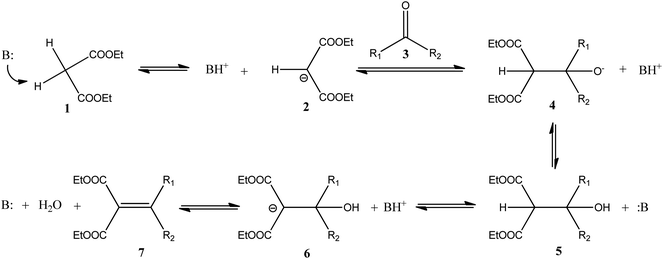
Knoevenagel condensation between aldehydes or ketones and active methylene compounds such as diethyl malonate is a century-old reaction in organic chemistry. The reaction is catalyzed by a mild base which deprotonates the malonate derivative 1 to form carbanion 2 which, in turn attacks the carbonyl group of aldehyde or ketone 3. Proton transfer between the base and condensation product 5 and elimination of a water molecule produces the condensation product 7 (Scheme 1).1,2The reaction is well known and has been used in synthesis of a large variety of intermediates useful in the manufacture of top selling drugs in the world such as Atorvastatin, Pioglitazone, AMG 837, MDL 103371, Pregabalin, Ro 24-5913, Lumefantrine, Entacapone etc.2 The reaction needs a mild base which is sufficiently basic to abstract a proton from the active methylene group but not basic enough to catalyze self-condensation of the carbonyl compound. In the early days, a mild base such as piperidine or pyridine was used and later investigations have lead to the use of a mixture of a secondary amine and a carboxylic acid, or amino acids such as β-alanine,2 L-proline,3 L-histidine and L-arginine,4 for the reactions. Environmental concerns mandate milder reaction conditions; the use of less toxic solvents, recovery and reuse of solvents and catalysts. In this context, biocatalytic routes are being developed for many transformations, especially for intermediates of pharmaceutical interest, as an alternative to inorganic or organo-metallic catalysts because of mild reaction conditions and high specificity.5,6 During these investigations, the protein bovine serum albumin (BSA) has emerged as an unexpected catalyst for a variety of reactions such as stereoselective oxidations and reductions, including epoxidation of electron-deficient alkenes,7 stereoselective thio-Michael addition to chalcones,8 nitroaldol reactions,9 and synthesis of 2-aminothiophenes via the Gewald reaction10 etc. Although the protein does not have any known natural catalytic function, it is available in a pure crystalline state at a reasonable price. Structurally, the protein contains as many as 60 Lys, 41 Asp and 58 Glu residues11 and with an isoelectric point near pH 4.5 (ref. 12) it is mildly basic at neutral pH and is ideally suited as a catalyst for Knoevenagel condensation. Herein we reported successful application of BSA in the condensation of diethyl malonate with a variety of aldehydes with 85–89% isolated yield and high purity (>95%). In view of recyclability, BSA was covalently immobilized on an epoxy functionalized polymeric support Immobead IB-350. The reaction was performed in DMSO at room temperature and the product was extracted with heptane. Although hexane can also be used in place of heptane with equal efficiency, heptane is preferred due to its low toxicity and higher recovery for recycling. DMSO and immobilized BSA were recycled 5 times without any appreciable loss in yield (Scheme 2).
2. Results and discussion
Several catalysts have been investigated for their usefulness in Knoevenagel condensation reactions which include ZnCl2,14 ammonium acetate–basic alumina,15 heterogeneous solid bases16 for instance hydrotalcite,17 hydroxyapatite-encapsulated γ-Fe2O3,18 MCM-41,19 modified silica gel,20 MgO/ZrO2,21 TiCl4,22 MgF2,23 HClO4–SiO2,24 Ni–SiO2,25 surfactants,26 phosphates,27 zeolites,28–32 clays,33 guanidine,34 organic-functionalized molecular sieves and silicate–organic composite materials.35 The reaction has been carried out even in water without any catalyst36 and by microwave irradiation.37,38 Many of these catalytic systems suffer from disadvantages such as use of toxic solvents, high temperatures and inefficient catalyst recycling, and many of the studies are confined to condensation reactions of aromatic aldehydes. A catalytic system which is useful for condensation of both aliphatic and aromatic aldehydes is highly desirable. It is even more desirable to conduct the reaction in a solvent of low toxicity at room temperature. The immobilized bovine serum albumin appears to be an excellent catalyst and DMSO is a solvent of low toxicity. The protein and the polymer are commercially available and it is easy to prepare the polymer-bound protein.2.1. BSA binding
Binding of BSA (1–30 mg mL−1 in 50 mM potassium phosphate buffer, pH 8.0, 1 mL) to the polymer was studied to establish maximum protein loading. The protein solution remained in contact with the polymer for 12 h to reach equilibrium and the decrease in the protein content of the supernatant was measured by the absorbance at 280 nm. At a fixed polymer weight, the amount of protein bound to the polymer increased with increasing amount of protein in the solution and finally reached a saturation limit of 90–100 μg mg−1 (Fig. 1). Such a behaviour is routinely observed and is generally analyzed using a Langmuir adsorption isotherm or its modified form.39 The binding data was found to fit the standard Langmuir adsorption isotherm (eqn (1)),| Y = BmaxX/(Kd + X) | (1) |
 | ||
| Fig. 1 Binding of BSA to Immobead 350. [BSA] = 1 to 30 mg mL−1; wt of polymer = 25 mg; total volume 1 mL in 50 mM potassium phosphate buffer, pH 8.0, temp. 30 °C. | ||
However, such an analysis has been questioned since adsorption isotherms imply reversibility of binding and a uniform distribution of the protein in a monolayer on the polymer surface.40 In the present case, although BSA is irreversibly and covalently bound to the polymer, we do observe that the protein loading can be predicted using the parameters Bmax = 171 μg mg−1 polymer and Kd = 19 mg mL−1 with a fair degree of confidence (±10%) even when the protein concentration is fixed and the amount of polymer is varied. Although the theoretical validity of the Langmuir adsorption isotherm in case of protein immobilization via covalent bond formation is questionable, there is no doubt about its practical utility. For our present purpose, a BSA solution of 30 mg mL−1 was sufficient to achieve 10% (w/w) loading of BSA on Immobead 350 and more importantly, the protein was strongly bound to the polymer and could be reused many times. The freeze dried polymer can be stored in a refrigerator at 5–7 °C for months without any loss of catalytic activity.
2.2. Condensation reaction
Knoevenagel condensation is usually performed in the presence of excess diethylmalonate (1.5 to 2 equivalents) to avoid self-condensation of aldehyde. However, on a large scale production this entails separation of diethylmalonate from product by vacuum distillation. On studying the effect of mole ratio of diethylmalonate to aldehyde under a set of uniform conditions, we have discovered that diethylmalonate to aldehyde mole ratio of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
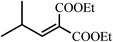
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is enough to achieve complete condensation of the aldehyde. Thus a mixture of aldehyde (25 mmol) and diethylmalonate (30 mmol) in DMSO (7 mL) was shaken with the BSA coated polymer (1 g) on an orbital shaker at room temperature and 200 rpm overnight. The progress of the reaction was followed by TLC. The immobilized BSA catalyzes the condensation reaction for aliphatic, aromatic and heterocyclic aldehydes efficiently and the reactions are complete in 12 h. The condensation between iso-valeraldehyde and diethylmalonate is of special interest since the product is used in the manufacture of Pregabalin, a drug used in treatment of several central nervous system disorders such as epilepsy, neuropathic pain, anxiety and social phobia.13
1 is enough to achieve complete condensation of the aldehyde. Thus a mixture of aldehyde (25 mmol) and diethylmalonate (30 mmol) in DMSO (7 mL) was shaken with the BSA coated polymer (1 g) on an orbital shaker at room temperature and 200 rpm overnight. The progress of the reaction was followed by TLC. The immobilized BSA catalyzes the condensation reaction for aliphatic, aromatic and heterocyclic aldehydes efficiently and the reactions are complete in 12 h. The condensation between iso-valeraldehyde and diethylmalonate is of special interest since the product is used in the manufacture of Pregabalin, a drug used in treatment of several central nervous system disorders such as epilepsy, neuropathic pain, anxiety and social phobia.13
2.3. Product separation
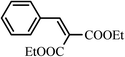
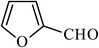
The apolar condensation products were more soluble in heptane than in DMSO, while diethylmalonate is far more soluble in DMSO than in heptane. Taking advantage of these properties, the product was extracted from DMSO solution with heptanes (3 × 15 mL). Evaporation of the heptane extracts gave the Knoevenagel condensation product along with a small quantity of unreacted diethylmalonate. The contaminating diethylmalonate (approx. 0.5 mmol) was removed by its selective hydrolysis with Candida antarctica lipase (CALB) to obtain the Knoevenagel condensation product with a purity of >99% in 85–90% final isolated yields for entries 1–8. In case of nicotinaldehyde (entry 9), the reaction was slow (48 h for complete consumption of aldehyde) and gave a mixture of aldol (50%) and the Knoevenagel condensation product (32%) which were isolated by column chromatography (Table 1). It is apparent that the basic pyridine ring affects elimination of water molecule from the aldol product.2.4. Recycle of DMSO and unreacted diethylmalonate
The DMSO solution after heptane extraction contained about 4.5–5 mmol of unreacted diethylmalonate. This was supplemented with additional diethylmalonate (25 mmol), the recovered polymer beads and the aldehyde (25 mmol) were added and the reaction was repeated. The polymer beads and DMSO were recycled 5 times without loss in product yield or in activity of BSA catalyst.2.5. Stability and recycle of catalyst
The retention of the catalytic activity of BSA coated polymer was studied by conducting the condensation of iso-valeraldehyde with diethylmalonate as described in Section 4.3, and the disappearance of the aldehyde was monitored by assay of an aliquot with Schiff's reagent. As the reaction progresses, the colour of the Schiff's reagent becomes fainter (λ = 550 nm) and finally the reagent remains colourless. The catalytic activity, based on the time required for 50% conversion (t 1/2), was unchanged (200 ± 20 min) under the experimental conditions for at least 5 cycles.3. Conclusion
Knoevenagel condensation of aldehydes with diethylmalonate has been carried out in an environmentally friendly fashion with immobilized bovine serum albumin in DMSO at room temperature. The catalyst, the solvent and unreacted diethylmalonate can be easily recovered and reused. The reaction is useful in general, and the condensation between iso-valeraldehyde and diethylmalonate is of special interest since the product is used in the manufacture of Pregabalin, a drug used in treatment of several central nervous system disorders.4. Experimental
4.1. General
Bovine serum albumin (fraction V, cat. no. a-7030) and Schiff's reagent were purchased from Sigma-Aldrich, USA. Immobead IB 350 was purchased from Chiral Vision, The Netherlands. Addzyme CALB (lipase from Candida antarctica) was obtained from Advanced Enzyme Technologies Ltd, Thane, India (http://www.enzymeindia.com). IR spectra were recorded on a Perkin-Elmer RX-1 FT-IR system. 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were recorded on a Bruker Avance-300 MHz spectrometer. HPLC analyses were carried out on Shimadzu HPLC Unit LC 20 AD with diode array detector. HPLC columns were obtained from Phenomenex, USA.4.2. Covalent immobilization of BSA on IB 350
The polymer (500 mg) was incubated with tert-BuOH (10 mL) for 2 h in a conical flask. The solvent was decanted and water (10 mL) was added to the polymer, shaken on an orbital shaker at 150 rpm for 15 min and the supernatant was discarded. BSA solution (5 mL, 25 mg mL−1, in 0.1 M sodium carbonate buffer pH 8.0) was added and the content was shaken for 12 h at 5–7 °C in a cold chamber. The polymer was washed repeatedly with cold carbonate buffer (5–7 °C) till the supernatant was free of protein, and finally with distilled water. The BSA coated polymer was freeze dried and stored in a refrigerator for further use. The protein content of the supernatant and combined washings was determined from its absorbance at 280 nm. The difference in the protein content of the control, and that of the supernatant gave the value for adsorbed protein. It was estimated that the polymer has a BSA loading of 100 mg g−1.4.3. Knoevenagel condensation
A typical procedure is exemplified by iso-valeraldehyde condensation with diethylmalonate as described below.A mixture of iso-valeraldehyde (2.15 g, 25 mmol) and diethylmalonate (4.8 g, 30 mmol) in DMSO (7 mL) was taken in a 50 mL conical flask, the BSA coated polymer (1 g) was added and the flask was shaken on an orbital shaker at room temperature and 200 rpm overnight. The progress of the reaction was followed by TLC and consumption of aldehyde was measured with Schiff's reagent. After the aldehyde was completely consumed, the supernatant was decanted from the polymer and the product was extracted from DMSO solution with heptane (3 × 15 mL). The heptane extracts were washed once with saturated sodium chloride solution to remove traces of DMSO and dried over MgSO4. Evaporation of heptane gave the Knoevenagel condensation product along with a small quantity of unreacted diethylmalonate (5.5 g, HPLC purity 94–95%).
4.4. Determination of aldehyde concentration in reaction mixture
An aliquot of the reaction mixture (10 μL) was added to Schiff's reagent (8 mL) and then mixed well. After incubation for 15 min, absorbance was measured at 550 nm (Δε = 465 M−1 cm−1). As the reaction progressed, the absorbance decreased from 1.06 to 0.001 in 12 h indicating that the reaction was complete.4.5. Product purification
The condensation product obtained from heptane extract (5.5 g) was suspended in Tris–HCl buffer (100 mL, 0.1 M containing 1% calcium acetate, pH 7.2) and enzyme Addzyme CALB (Lipase from Candida antarctica) (100 μL) was added. The contents were stirred magnetically and pH was kept near 7.2 by addition of 2 N NaOH. The consumption of NaOH was complete in 10 min. The product was extracted with heptane, dried over anhydrous magnesium sulfate and recovered by evaporation of heptane on rotavapor (4.9 g, yield 86%, >99% pure by HPLC).4.6. HPLC Analysis conditions
Condensation reaction of iso-valeraldehyde with diethylmalonate was followed by reverse phase HPLC analysis with diethyltartrate as an external standard. Aliquots of reaction mixture (25 μL) were mixed with 40% (v/v) acetonitrile–water mixture (1 mL containing 10 mg mL−1 of (+)-diethyl L-tartrate) and the mixture was injected for analysis. Column: Merck Hibar LiChrospher 100 RP-18 (5 μm) 250 × 4.6 mm; flow rate 0.5 mL min−1, detection wavelength 210 nm. Solvent system: acetonitrile–water. Gradient analysis conditions: start at 40% acetonitrile, hold for 15 min at 40%, change from 40 to 80% in 5 min, hold at 80% for 5 min, return to 40% in 5 min. Retention times: DMSO 5.5 min, (+)-diethyl (L)-tartrate 7.1 min, iso-valeraldehyde 10.4 min, diethylmalonate 17.5 min, diethyl 2-(3-methylbutylidene)malonate 34.1 min. Since the aldehyde has very low absorbance at 210 nm, the response was low. The concentration of aldehyde was thus determined from the analysis with Schiff's reagent as described in Section 4.4.Acknowledgements
We thank UGC New Delhi for grant of SRF to P. Ramesh, and CSIR New Delhi for financial support under XII Five Year Plan CSC0108-ORIGIN.Notes and references
- G. Jones, The Knoevenagel Condensation, Org. React., 2011, 204 Search PubMed
.
- R. Menegatti, Green Chemistry – Aspects for the Knoevenagel Reaction, Green Chemistry – Environmentally Benign Approaches, ed. M. Kidwai, InTech, 2012, pp. 13–32, http://www.intechopen.com/books/green-chemistry-environmentally-benign-approaches/green-chemistry-aspects-for-knoevenagel-reaction Search PubMed
.
- L. Thijs, US Patent, 2009/0312560 A1, 2009
.
- A. Rahmati and K. Vakili, Amino Acids, 2010, 39, 911–916 CrossRef CAS PubMed
.
- K. Faber, Biotransformations in Organic Chemistry, Springer-Verlag, Berlin, 4th edn, 2011 Search PubMed
.
- C. C. C. R. de Carvalho and M. M. R. da Fonseca, in Comprehensive Biotechnology, Elsevier, 2nd edn, 2011, vol. 2, pp. 451–460 Search PubMed
.
- S. V. Dzyuba and A. M. Klibanov, Tetrahedron: Asymmetry, 2004, 15, 2771–2777 CrossRef CAS PubMed
.
- N. Gaggero, D. C. M. Albanese, G. Celentano, S. Banfi and A. Aresi, Tetrahedron: Asymmetry, 2011, 22, 1231–1233 CrossRef CAS PubMed
.
- E. Busto, V. Gotor-Fernández and V. Gotor, Org. Process Res. Dev., 2011, 15, 236–240 CrossRef CAS
.
- D.-D. Zhao, L. Li, F. Xu, Q. Wu and X.-F. Lin, J. Mol. Catal. B: Enzym., 2013, 95, 29–35 CrossRef CAS PubMed
.
- K. Hirayama, S. Akashi, M. Furuya and K. I. Fukuhara, Biochem. Biophys. Res. Commun., 1990, 173, 639–646 CrossRef CAS
.
- C. Tanford, S. A. Swanson and W. S. Shore, J. Am. Chem. Soc., 1955, 77, 6414–6421 CrossRef CAS
.
- C. A. Martinez, S. Hu, Y. Dumond, J. Tao, P. Kelleher and L. Tully, Org. Process Res. Dev., 2008, 12, 392–398 CrossRef CAS
.
- P. Shanthan Rao and R. V. Venkataratnam, Tetrahedron Lett., 1991, 32, 5821–5822 CrossRef
.
- S. Balalaie and N. Nemati, Synth. Commun., 2000, 30, 869–875 CrossRef CAS
.
- Y. Ono, J. Catal., 2003, 216, 406–415 CrossRef CAS
.
- A. Corma, S. Iborra, J. Primo and F. Rey, Appl. Catal., A, 1994, 114, 215–225 CrossRef CAS
.
- Y. Zhang and C. Xia, Appl. Catal., A, 2009, 366, 141–147 CrossRef CAS PubMed
.
- A. Corma, S. Iborra, I. Rodriguez and F. Sanchez, J. Catal., 2002, 211, 208–215 CAS
.
- P. M. Price, J. H. Clark and D. J. Macquarrle, J. Chem. Soc., Dalton Trans., 2000, 101–110 RSC
.
- M. B. Gawande and R. V. Jayaram, Catal. Commun., 2006, 7, 931–935 CrossRef CAS PubMed
.
- B. Green, R. I. Crane, I. S. Khaidem, R. S. Leighton, S. S. Newaz and T. E. Smyser, J. Org. Chem., 1985, 50, 640–644 CrossRef CAS
.
- R. M. Kumbhare and M. Sridhar, Catal. Commun., 2008, 9, 403–405 CrossRef CAS PubMed
.
- G. Bartoli, R. Beleggia, S. Giuli, A. Giuliani, E. Marcantoni, M. Massaccesi and M. Poletti, Tetrahedron Lett., 2006, 47, 6501–6504 CrossRef CAS PubMed
.
- V. S. R. R. Pullabhotla, A. Rahman and S. B. Jonnalagadda, Catal. Commun., 2009, 10, 365–369 CrossRef CAS PubMed
.
- D. S. Bose and A. V. J. Narsaiah, J. Chem. Res., 2001, 36–38 CrossRef CAS
.
- J. Bennazha, M. Zahouilly, A. Boukhari and E. A. Holt, J. Mol. Catal. A: Chem., 2003, 202, 247–252 CrossRef CAS
.
- S. Saravanamurugan, M. Palanichamy, M. Hartmann and V. Murugesan, Appl. Catal., A, 2006, 298, 8–15 CrossRef CAS PubMed
.
- T. I. Reddy and R. S. Verma, Tetrahedron Lett., 1997, 38, 1721–1724 CrossRef CAS
.
- U. D. Joshi, P. N. Joshi, S. S. Tamhankar, V. V. Joshi, C. V. Rode and V. P. Shiralkar, Appl. Catal., A, 2003, 239, 209–220 CrossRef CAS
.
- A. Corma, V. Fornes, R. M. Martin-Aranda, H. Garcia and J. Primo, Appl. Catal., 1990, 59, 237–248 CrossRef CAS
.
- A. Corma and R. M. Martin-Aranda, Appl. Catal., A, 1993, 105, 271–279 CrossRef CAS
.
- F. Bigi, L. Chesini, R. Maggi and G. Sartori, J. Org. Chem., 1999, 64, 1033–1035 CrossRef CAS
.
- J. Han, Y. Xu, Y. Su, X. She and X. Pan, Catal. Commun., 2008, 9, 2077–2079 CrossRef CAS PubMed
.
- Y. Kubota, Y. Nishizaki, H. Ikeya, M. Saeki, T. Hida, S. Kawazu, M. Yoshida, H. Fujii and Y. Sugi, Microporous Mesoporous Mater., 2004, 70, 135–149 CrossRef CAS PubMed
.
- F. Bigi, M. L. Conforti, R. Maggi, A. Piccinno and G. Sartori, Green Chem., 2000, 2, 101–103 RSC
.
- S. Kantevari, R. Bantu and L. Nagarapu, J. Mol. Catal. A: Chem., 2007, 269, 53–57 CrossRef CAS PubMed
.
- J. S. Yadav, B. V. Subba Reddy, A. K. Basak, B. Visali, A. V. Narsaiah and K. Nagaiah, Eur. J. Org. Chem., 2004, 546–551 CrossRef CAS
.
- J. H. Kim and J. Y. Yoon, in Encyclopedia of Surface and Colloid Science, Marcel Dekker, New York, 2002, pp. 4373–4381 Search PubMed
.
- K. Nakanishi, T. Sakiyama and K. Imamura, J. Biosci. Bioeng., 2001, 91, 233–244 CAS
.
| This journal is © The Royal Society of Chemistry 2014 |