Vermiculite as a natural silicate crystal for hydrogen generation from photocatalytic splitting of water under visible light†
Jian
Zhang
,
Tianyu
Liu
,
Rui
Chen
and
Xiaoheng
Liu
*
Key Laboratory for Soft Chemistry and Functional Materials of Ministry of Education, Key Laboratory of Jiangsu Province for Chemical Pollution Control and Resources Reuse, Nanjing University of Science and Technology, Nanjing, 210094, P.R. China. E-mail: xhliu@mail.njust.edu.cn; Fax: +86-25-84315054; Tel: +86-25-84315943
First published on 24th October 2013
Abstract
Vermiculite with the typical composition of (Mg,Fe,Al)3(Si,Al)4O10(OH)2·4H2O, which is both a type of low cost silicate clay mineral and a high quality natural nanosized material showing paper-like morphology, has wide ranging industrial applications. Herein, we have successfully used this material to effectively split water for hydrogen generation under visible light.
Photocatalytic water splitting using semiconductor particles has been regarded as one of the most promising means of hydrogen production from renewable resources.1 During the past decade, many research studies have been focused on the development of visible-light responsive photocatalysts, because UV light only accounts for about 4% of solar radiation energy, while visible light contributes about 43%. With a view to developing photocatalytic applications using visible-light irradiation, several common approaches have been adopted in order to make photocatalysts visible-light active for water splitting into hydrogen: metallic and non-metallic doping,2–4 semiconductor combinations5–8 and dye sensitization.9–11 Dye sensitization has been identified as a successful way to expand the absorption in visible light region.12–14 The brominated fluorescein-based dye Eosin Y (EY) is one of xanthene dyes, which have been used as sensitizers of TiO2 and other matrixes for the photocatalytic hydrogen evolution.15–17
In contrast to numerous reports synthetizing the photocatalysts activity, number of reports employing natural materials as photocatalytic is still very scarce. Herein, we report for the first time that vermiculite18–20 (VMT) as a free-standing natural phyllosilicate clay and a high quality natural nanosized material can actively achieve water splitting with quantum efficiency (QE) up to 22.8% after sensitized by organic dyes (EY) under visible light. The typical composition of VMT is (Mg, Fe, Al)3(Al, Si)4O10(OH)2·4H2O. Actually, based on the results of ICP (Fe: 4.7%, Al: 14.8%, Mg: 6.1%), we established five different structures with two Fe atoms in one super crystal cell ((Mg7/9,Fe1/9,Al1/9)3(Si10/12,Al2/12)4O10(OH)2·4H2O, Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg estimated at 1
Mg estimated at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 in atomic ratio). Compared to the total energy (by using VASP code), we found that the most stable structure as shown in Fig. S1 (see ESI† for details).
8 in atomic ratio). Compared to the total energy (by using VASP code), we found that the most stable structure as shown in Fig. S1 (see ESI† for details).
The typical FESEM image, as shown in Fig. 1a, revealing that VMT platelet crystals are detected, which display soft surfaces, with small protuberances. Fig. 1b presents a typical TEM image of VMT nanosheets. The typical TEM image of Pt/VMT (Pt3) nanocomposites (Fig. 1c) illustrates that the Pt nanoparticles (NPs) with uniform size decorated on the basal planes, and no free Pt NPs are formed outside the VMT sheets. HRTEM image of Pt NPs which loaded on the surface of VMT (Fig. 1d) shows oriented and ordered lattice fringes for the Pt NPs. The d-spacing value of 0.217 nm coincides with that of face centered, cubic (fcc) Pt (111) (JCPDS no. 65-2868, 0.22655 nm).21
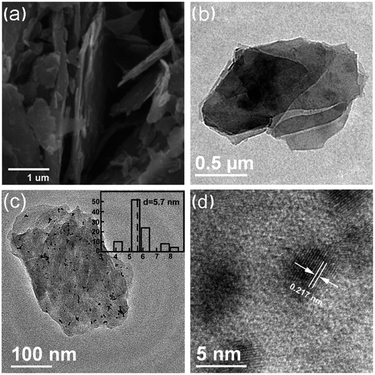 | ||
| Fig. 1 FESEM and TEM images of the bare VMT (a and b), TEM and HRTEM images of Pt/VMT (Pt3) (c and d). The insets in (c) shows size distribution histograms. | ||
The characterization of photocatalyst was determined using X-ray diffraction (Fig. S4, ESI†), BET (Fig. S5, ESI†), and UV-vis absorption spectra (Fig. S6, ESI†), in combination with EDX (Fig. S7, ESI†), which is also described in the ESI.† The inset of Fig. S6† shows a Tauc equation plot for bare VMT sample (Fig. S6†, black curve). The best fit was obtained for a plot of (αhν)2versus photon energy (hν), where α is the absorption coefficient. The extrapolation of the linear regions in the Tauc plot suggests one-regime optical absorption by VMT (marked with dotted line), where the associated direct optical band gap is ca. 3.5 eV.
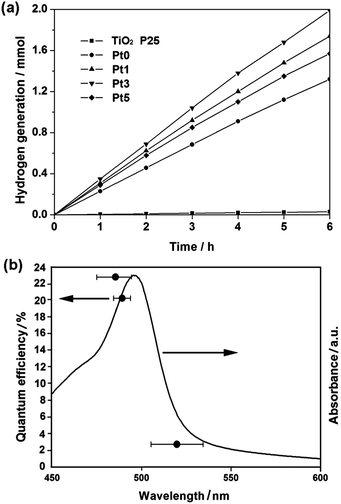
For five samples sensitized by EY in Fig. 2a, all Pt/VMT samples as well as bare VMT have better splitting ability than TiO2 P25 (Degussa) (see ESI† for details). In the presence of 0.1 g VMT in solution as photocatalyst (sample Pt0), this value of hydrogen generation rate exceeds that of TiO2 by a factor of 45. The highest hydrogen production rate, obtained for sample Pt3, is 0.33 mmol h−1. We interpret this finding as caused by the more favourable hydrogen elimination from Pt–H surface bonds,22 as compared with the covalent elimination from a hydrogenated VMT surface. No hydrogen can be detected in the absence of VMT, suggesting that VMT would be active for photocatalytic hydrogen production under the experimental conditions studied. Throughout the testing cycle (Fig. S8, ESI†), VMT exhibited persistent high hydrogen production capability.
Fig. 2b shows the QE of water splitting by EY sensitized photocatalyst (sample Pt3) as a function of the wavelength of incident light. Trend of QE matches well with that of absorption in the optical spectra, and QE reached to highest value of 22.8% at 483 nm. A control experiment showed that indeed no reaction took place in the dark. Although it is very difficult to compare the present results with other reported studies since photocatalytic QE appear to vary according to the reaction conditions, with a view to know the current state of this study in the field of photocatalysis for hydrogen production, here we tried to list several research results of QE for hydrogen evolution according to other reports published elsewhere in Table S1.†
So far, some Fe-containing compounds which have the capability of effectively split water for hydrogen generation in visible light irradiation has been demonstrated.23–25 Metal oxide-based minerals naturally contain doped transition metal ions isomorphically substituted into the structure that can alter the structural as well as electronic properties.26 Of particular interest is doping with transition metals ions such as Fe3+ and form –O–Fe–O– structure that is isostructural with –O–Mg–O– structure. In this study, the substitutions of Mg2+ by Fe3+ in octahedral platelet predominate of VMT27 showed a great effect on the photocatalytic activity for water splitting.
To obtain further insight into the high photocatalytic activity of VMT, transient photoresponse of VMT electrodes was recorded for several on–off cycles of solar irradiation. Fig. 3 shows a comparison of photoresponse for the front and back of bare VMT sample with typical on–off cycles of intermittent solar irradiation. As shown from the figure, an apparently boosted photocurrent response appears from both front and back side of the electrode. The photocurrent densities rise steeply up to approximately 0.4 mA cm−2 and 0.2 mA cm−2 respectively at 2 VRHE. This indicates that most of photogenerated electrons are transported to the back contact across the sample to produce photocurrent under solar irradiation.28
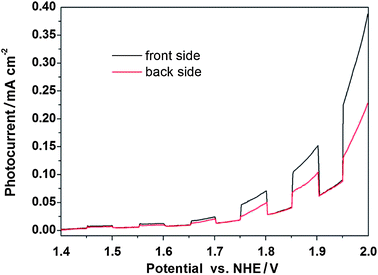 | ||
| Fig. 3 Photoresponse of bare VMT photoanodes in 1 M NaOH under solar light. From both the front side (black) and back side of the electrode (red). | ||
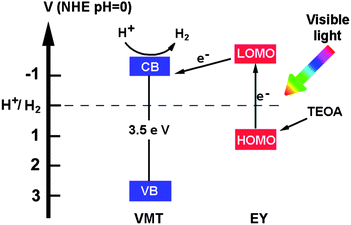
The valence band (VB) of VMT was also measured by VB XPS (Fig. S9†). This is 0.5 eV smaller than anatase TiO2,29,30 suggesting that VMT has a higher VB than anatase TiO2 by ca. 0.5 eV relative to a normal hydrogen electrode (NHE). By comparison with the VB of anatase TiO2, the electronic potentials of the VMT could be determined (Fig. S10†). Clearly, the CB of VMT is more negative than the redox potentials for H+/H2. On the basis of the above results, a proposed mechanism for the EY-sensitized photocatalyst for hydrogen evolution using VMT is shown schematically in Fig. 4.
In summary, we presented here VMT as a natural silicate crystal and a high quality natural nanosized photocatalyst that is able to generate hydrogen from water even in the absence of noble metals. In this way, no (or very small) artificial treatments including chemical synthesis, doping and nanoparticle morphology control are needed at all. Although the estimated QE of the VMT catalysts are still not very high, the easily scalable and abundant reserve of natural clays motivate our continued efforts.
Acknowledgements
This project is supported financially by the Natural Science Foundation of China (NSFC, no. 51272107), and the Natural Science Foundation of Jiangsu Province, China (no. BK2011024).Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS PubMed.
- K. M. Parida, S. Martha, D. P. Das and N. Biswal, J. Mater. Chem., 2010, 20, 7144 RSC.
- H. Yu, S. Yan, Z. Li, T. Yu and Z. Zou, Int. J. Hydrogen Energy, 2012, 37, 12120 CrossRef CAS PubMed.
- B. Klahr, S. Gimenez, F. Fabregat-Santiago, J. Bisquert and T. W. Hamann, J. Am. Chem. Soc., 2012, 134, 16693 CrossRef CAS PubMed.
- Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575 CrossRef CAS PubMed.
- Z. Han, F. Qiu, R. Eisenberg, P. L. Holland and T. D. Krauss, Science, 2012, 338, 1321 CrossRef CAS PubMed.
- X. Wang, G. Liu, L. Wang, J. Pan, G. Q. Lu and H.-M. Cheng, J. Mater. Chem., 2011, 21, 869 RSC.
- J. Xu, Y. Li, S. Peng, G. Lu and S. Li, Phys. Chem. Chem. Phys., 2013, 15, 7657 RSC.
- T. Sreethawong, C. Junbua and S. Chavadej, J. Power Sources, 2009, 190, 513 CrossRef CAS PubMed.
- W. J. Youngblood, S. A. Lee, K. Maeda and T. E. Mallouk, Acc. Chem. Res., 2009, 42, 1966 CrossRef CAS PubMed.
- T. M. McCormick, B. D. Calitree, A. Orchard, N. D. Kraut, F. V. Bright, M. R. Detty and R. Eisenberg, J. Am. Chem. Soc., 2010, 132, 15480 CrossRef CAS PubMed.
- J. Lee, J. Kwak, K. C. Ko, J. H. Park, J. H. Ko, N. Park, E. Kim, D. H. Ryu, T. K. Ahn, J. Y. Lee and S. U. Son, Chem. Commun., 2012, 48, 11431 RSC.
- E. Reisener, D. J. Powell, C. Cavazza, J. C. Fontecilla-Camps and F. A. Armstrong, J. Am. Chem. Soc., 2009, 131, 18457 CrossRef PubMed.
- T. T. Le, M. S. Akhtar, D. M. Park, J. C. Lee and O. Yang, Appl. Catal., B, 2012, 57, 397 CrossRef PubMed.
- N. Nuraje, X. Deng, J. Qi, M. A. Allen, Y. Lei and A. M. Belcher, Adv. Mater., 2012, 24, 2885 CrossRef CAS PubMed.
- F. Labat, I. Ciofini, H. P. Hratchian, M. Frisch, K. Raghavchari and C. Adamo, J. Am. Chem. Soc., 2009, 131, 14290 CrossRef CAS PubMed.
- F. Kanamaru and V. Vand, Am. Mineral., 1970, 55, 1550 CAS.
- V. T. Shmuradko, F. I. Panteleenko, O. P. Reut, E. F. Panteleenko and N. V. Kirshina, Refract. Ind. Ceram., 2012, 53, 254 CrossRef.
- G. S. Martynková, M. Valášková, P. Capková and V. Matejka, J. Colloid Interface Sci., 2007, 313, 281 CrossRef PubMed.
- C. Wang, H. Daimon, T. Onodera, T. Koda and S. H. Sun, Angew. Chem., Int. Ed., 2008, 47, 3588 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76 CrossRef CAS PubMed.
- F. L. Formal, N. Tetreault, M. Cornuz, T. Moehl, M. Grätzel and K. Sivula, Chem. Sci., 2011, 2, 737 RSC.
- Y. Hou, F. Zuo, A. Dagg and P. Feng, Nano Lett., 2012, 12, 6464 CrossRef CAS PubMed.
- K. A. Brown, M. B. Wilker, M. Boehm, G. Dukovic and P. W. King, J. Am. Chem. Soc., 2012, 134, 5627 CrossRef CAS PubMed.
- J. Baltrusaitis, C. Hatch and R. Orlando, J. Phys. Chem. C, 2012, 116, 18847 CAS.
- M. M. Ruck, J. Pironon, D. Guiliaume and M. Cathelineau, Clay Miner., 2003, 38, 303 CrossRef PubMed.
- A. Hagfeldt, H. Lindstrom, S. Sodergren and S. Lindquist, J. Electroanal. Chem., 1995, 381, 39 CrossRef.
- G. Liu, L. Wang, C. Sun, X. Yan, X. Wang, Z. Chen, S. C. Smith, H. Cheng and G. Q. Lu, Chem. Mater., 2009, 21, 1266 CrossRef CAS.
- G. Liu, P. Niu, L. Yin and H. Cheng, J. Am. Chem. Soc., 2012, 134, 9070 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, structure of VTM, XRD, BET, UV-vis, EDX, VB XPS, cycling measurements of hydrogen generation and electronic potential diagram. See DOI: 10.1039/c3ra45301d |
| This journal is © The Royal Society of Chemistry 2014 |