Visible-light-initiated photo-oxidative cyclization of phenolic amidines using CBr4 – A metal free approach to 2-aminobenzoxazoles†
Twinkle Keshari,
Vishnu P. Srivastava and
Lal Dhar S. Yadav*
Green Synthesis Lab, Department of Chemistry, University of Allahabad, Allahabad 211 002, India. E-mail: ldsyadav@hotmail.com; Fax: +91 532-246-0533; Tel: +91 532-250-0652
First published on 18th December 2013
Abstract
A mild and efficient one-pot operation for the amination of benzoxazoles has been achieved via ring opening of benzoxazoles followed by visible-light-mediated aerobic photo-oxidative cyclization of the resulting o-phenolic amidines using CBr4 as a mild oxidant. The protocol is economical and environmentally benign as it utilizes visible light and does not require any photosensitizers, additives, heating or inert conditions.
Introduction
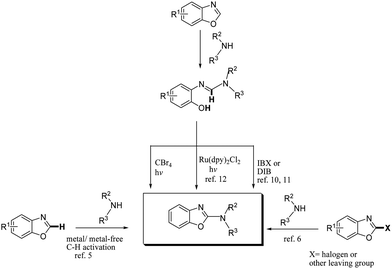
The potential of developing new synthetic methodologies using visible light has recently received much attention from a number of research groups.1 This is because solar energy (visible light) is clean, easy to handle and an unlimited energy source having great prospects for the development of sustainable and eco-friendly protocols for organic synthesis.2 Most visible-light-initiated photochemical organic reactions generally require expensive and potentially toxic ruthenium and iridium complexes as photosensitizers or photocatalysts.3 Thus, the search for novel synthetically useful photochemical reactions without the need for visible-light photosensitization would be a welcome move, especially in terms of economical and environmental issues. The 2-aminobenzoxazole scaffold is a key structural motif in promising pharmaceuticals for the treatment of various disorders, such as Alzheimer's disease, schizophrenia, HIV and inflammatory diseases.4 Fascinated by the medicinal worth of 2-aminobenzoxazoles, great efforts have been made for their synthesis.2-Aminobenzoxazoles have been generally synthesized by direct amination of benzoxazoles with an amine or its surrogates through transition-metal-catalyzed C–H bond activation.5 These methods are quite beneficial and appreciable compared to the traditional C–N bond formation involving nucleophillic displacement of 2-halogenated benzoxazoles or their precursors with amines.6 However, various metal-based established methods have disadvantages of using toxic and/or expensive metal catalysts, even traces of which are to be removed for pharmaceutical applications. Hence, several metal-free methods employing various reagents or catalysts such as TEMPO, TBAI, I2, and NIS have been reported.7 Furthermore, elegant synthetic routes to access 2-aminobenzoxazoles utilising aminophenols as substrates have also been developed.8,9 Besides, Chang and co-workers have recently developed an elegant method for the synthesis of 2-aminobenzoxazoles through a unique ring opening of benzoxazoles with secondary amines followed by an oxidative ring closure of the resulting o-phenolic amidines with iodobenzene diacetate (DIB).10 Later on, Bhanage and co-workers have implemented this strategy using 2-iodobenzoic acid (IBX) as the oxidant.11 These metal-free methods use expensive and potentially explosive oxidants. Very recently, we have developed a convenient synthesis of 2-aminobenzoxazoles from o-phenolic amidines using Ru(dpy)2Cl2 as a visible-light photoredox catalyst.12
In view of the above discussion and our continued efforts for developing efficient heterocyclization reactions,12,13 we envisioned that a metal- and photosensitizer-free visible-light-initiated cyclization of o-phenolic amidines to the corresponding 2-aminobenzoxazoles could be feasible and highly interesting (Scheme 1). For this purpose, we focused on CBr4, which is solid, easy to handle, and absorbs visible light to generate active radical species,14 hence expected to be suitable for our envisaged cyclization reaction.
Results and discussion
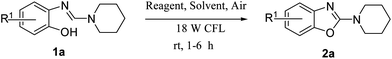
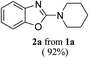
In order to realize our goal, we commenced the study with a model reaction of 2-(piperidin-1-yl-methyleneamino)phenol 1a using a stoichiometric amount of CBr4 in acetonitrile as a solvent under visible light irradiation with an 18 W CFL for 1 h in the presence of air at rt. To our delight, 2-aminobenzoxazole 2a was obtained in 92% yield after 1 h. To ensure the necessity of the reaction parameters such as CBr4, air and visible light for the present photo-oxidative cyclization, a series of control experiments were performed which revealed that in the absence of any one of the parameters, a little or no product formation was observed (Table 1, entries 10–12). On performing the cyclization under inert argon atmosphere, a decrease in the yield was observed (Table 1, entry 11). Next, optimization of the solvent was carried out which revealed CH3CN to be the best solvent for the reaction (Table 1, entry 1). However, several other solvents such as THF, dioxane, DCM, CH3NO2, and toluene were also explored which did not give satisfactory results (Table 1, entries 2–6). Notably, a radical generating bromine source was also found to be one of the major parameters necessary for the initiation of the cyclization reaction. Thus, various bromine sources were tested and it was found that CBr4 was the best in terms of the yield and reaction time (Table 1, entry 1). However, in the case of tetrabutyl ammonium bromide (TBNBr) no cyclization of o-phenolic amidine 1a was observed which might be due to inability of TBNBr to give bromine radical under the given reaction conditions (Table 1, entry 9).| Entry | Reagent | Solvent | Time (h) | Yield (%)e |
|---|---|---|---|---|
| a Reaction conditions: o-phenolic amidine (1 mmol), reagent (1 mmol), solvent (3 mL), under 18 W CFL (Compact fluorescent lamp; Philips, 6500 k, 1010 1 m, 85 mA) irradiation under an air atmosphere at rt.b In dark.c Under inert Ar atmosphere.d Absence of bromine source.e Isolated yield of 2a after flash chromatography. | ||||
| 1 | CBr4 | CH3CN | 1 | 92 |
| 2 | CBr4 | THF | 6 | 25 |
| 3 | CBr4 | Dioxane | 6 | 30 |
| 4 | CBr4 | DCM | 6 | 35 |
| 5 | CBr4 | CH3NO2 | 6 | 20 |
| 6 | CBr4 | Toluene | 6 | 15 |
| 7 | Br2 | CH3CN | 1 | 71 |
| 8 | NBS | CH3CN | 3 | 67 |
| 9 | TBNBr | CH3CN | 6 | 0 |
| 10b | CBr4 | CH3CN | 6 | 0 |
| 11c | CBr4 | CH3CN | 6 | 12 |
| 12d | — | CH3CN | 6 | 0 |
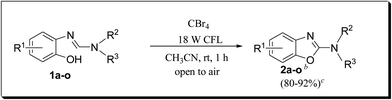
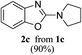
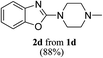
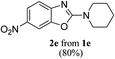
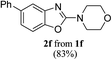
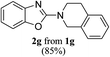
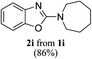
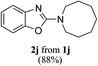
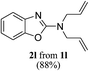
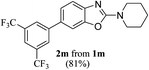
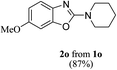
Keeping in view the potential of CBr4 as an efficient reagent for visible-light-initiated photo-oxidative cyclization of o-phenolic amidines in CH3CN (Table 1, entry 1), we switched to investigate the substrate scope of the reaction. As depicted in Table 2, phenolic amidines bearing electron-neutral, electron-donating or electron-withdrawing substituents generally afforded 2-aminobenzoxazoles in excellent yields (80–92%). This shows little electronic effect of the substituents on the oxidative cyclization reaction. Interestingly, amidines with various functionalites such as CH3, OCH3, NO2, Cl, Ph and CF3 were easily tolerated to give aminobenzoxazoles in excellent yields and high purity. However, on the part of secondary amines, structurally diverse cyclic and acyclic amines were evaluated and found to undergo the photo-oxidative cyclization very smoothly.
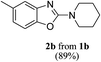
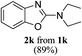
Furthermore, owing to an easy, rapid and high yielding conversion of benzoxazoles into o-phenolic amidines,10 we proceeded for one-pot synthesis of 2-aminobenzoxazoles starting from benzoxazoles and secondary amines (Scheme 2). Thus, we stirred benzoxazole (1 mmol) and piperidine (2 mmol) under neat condition at rt for 10 min. After confirming the complete conversion of benzoxazole to corresponding amidine by TLC, CH3CN (3 mL) and CBr4 (1 mmol) were added and the reaction mixture was irradiated with an 18 W CFL for 1.5 h. To our delight, the one-pot procedure was also effective and the desired product, 2-aminobenzoxazole 2a was obtained in a decent yield (89%).
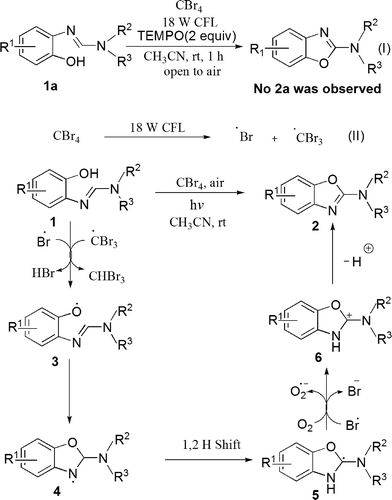
On the basis of the above observations and the literature precedents,12,14,17,18 a plausible mechanism for the present visible-light-initiated photo-oxidative cyclization of o-phenolic amidines 1 to afford 2-aminobenzoxazoles 2 is depicted in Scheme 3. The cyclization of amidines might follow a radical pathway, as the reaction was quenched on addition of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy), which is a traditional radical scavenger (Scheme 3, eqn (I)).
The key steps include: (i) on photo-irradiation, CBr4 is activated to give ˙Br and ˙CBr3 radicals which initiate the reaction (Scheme 3, eqn (II)). (ii) ˙CBr3 or ˙Br radical abstracts phenolic H to give 3 which undergoes intramolecular cyclization to generate an aminyl radical 4. (iii) The aminyl radical 4 is prone to undergo 1,2 H shift to form a more stable carbon radical 5, and (iv) the radical 5 is efficiently oxidized by giving an electron to ˙Br or O2 to generate a highly stabilised carbocation 6, which finally aromatizes through deprotonation to give the product 2.
Conclusion
In summary, we have developed a highly efficient, mild and unique strategy for a one-pot synthesis of 2-aminobenzoxazoles. The protocol involves ring opening of benzoxazoles with secondary amines followed by photo-oxidative intramolecular cyclization of the resulting o-phenolic amidines using visible-light as a mild source of energy and CBr4 as an oxidant. The use of visible-light irradiation without the need for any metal or non-metal photosensitizers or photocatalysts makes our method sustainable, ecofriendly and superior to the other existing methods for the amination of benzoxazoles.Acknowledgements
We sincerely thank SAIF, Punjab University, Chandigarh, for providing microanalyses and spectra. T. K. is grateful to the CSIR, New Delhi, for the award of a junior Research Fellowship (File no. 09/001(0370)/2012-EMR-1). V. P. S. is grateful to the Department of Science and Technology (DST) Govt. of India, for the award of a DST-Inspire Faculty position (Ref. IFA-11CH-08) and financial support.References
- For selected reviews, see: (a) K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785 CrossRef CAS PubMed; (b) P. Melchiorre, Angew. Chem., Int. Ed., 2009, 48, 1360 CrossRef CAS PubMed; (c) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527 CrossRef CAS PubMed; (d) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC; (e) F. Teply, Collect. Czech. Chem. Commun., 2011, 76, 859 CrossRef CAS; (f) J. W. Tucker and C. R. J. Stephenson, J. Org. Chem., 2012, 77, 1617 CrossRef CAS PubMed.
- (a) G. Ciamician, Science, 1912, 36, 385 CAS; (b) X. Sala, I. Romero, M. Rodriguze, E. Lluis and A. Llobet, Angew. Chem., Int. Ed., 2009, 48, 2842 CrossRef CAS PubMed.
- For reviews, see: (a) K. Kalyanasundaram, Coord. Chem. Rev., 1982, 46, 159 CrossRef CAS; (b) A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85 CrossRef CAS; (c) L. Flamigni, A. Barbieri, C. Sabatini, B. Ventura and F. Barigelletti, Top. Curr. Chem., 2007, 281, 143 CrossRef CAS.
- (a) Y. Sato, M. Yamada, S. Yoshida, T. Soneda, M. Ishikawa, T. Nizato, K. Suzuki and F. Konno, J. Med. Chem., 1998, 41, 3015 CrossRef CAS PubMed; (b) M. Cheung, P. Harris, M. Hasegawa, S. Ida, K. Kano and N. Nishigaki, PCT WO 02/44156A2, 2002, Chem. Abstr., 2002, 137, 1679; (c) S. Yoshida, T. Watanabe and Y. Sato, Bioorg. Med. Chem., 2007, 15, 3515 CrossRef CAS PubMed; (d) K. G. Liu, J. R. Lo, T. A. Comery, G. M. Zhang, J. Y. Zhang, D. M. Kowal, D. L. Smith, L. Di, E. H. Kerns, L. E. Schechter and A. J. Robichaud, Bioorg. Med. Chem. Lett., 2009, 19, 1115 CrossRef CAS PubMed; (e) A. Armstrong and J. C. Collins, Angew. Chem., Int. Ed., 2010, 49, 2282 CrossRef CAS PubMed.
- (a) D. Monguchi, T. Fujiwara, H. Furukawa and A. Mori, Org. Lett., 2009, 11, 1607 CrossRef CAS PubMed; (b) Q. Wang and S. L. Schreiber, Org. Lett., 2009, 11, 5178 CrossRef CAS PubMed; (c) T. Kawano, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2010, 132, 6900 CrossRef CAS PubMed; (d) N. Matsuda, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2011, 13, 2860 CrossRef CAS PubMed.
- (a) F. Haviv, J. D. Ratajczyk, R. W. DeNet, F. A. Kerdesky, R. L. Walters, S. P. Schmidt, J. H. Holms, P. R. Young and G. W. Carter, J. Med. Chem., 1988, 31, 1719 CrossRef CAS; (b) R. Lok, R. E. Leone and A. J. Williams, J. Org. Chem., 1996, 61, 3289 CrossRef CAS; (c) M. W. Hooper, M. Utsunomiya and J. F. Hartwig, J. Org. Chem., 2003, 68, 2861 CrossRef CAS PubMed; (d) M. S. R. Murty, K. R. Ram, R. V. Rao, J. S. Yadav, U. S. N. Murty and K. P. Kumar, Med. Chem. Res., 2011, 20, 626 CrossRef CAS.
- (a) T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner and B. Nachtsheim, Org. Lett., 2011, 13, 3754 CrossRef CAS PubMed; (b) S. Wertz, S. Kodama and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 11511 CrossRef CAS PubMed; (c) Y. S. Wagh, D. N. Sawant and B. M. Bhanage, Tetrahedron Lett., 2012, 53, 3482 CrossRef CAS PubMed.
- C. L. Cioffi, J. J. Lansing and H. Yuksel, J. Org. Chem., 2010, 75, 7942 CrossRef CAS PubMed.
- T. Guntreddi, B. K. Allam and K. N. Singh, RSC Adv., 2013, 3, 9875 RSC.
- J. Joseph, J. Y. Kim and S. Chang, Chem.–Eur. J., 2011, 17, 8294 CrossRef CAS PubMed.
- Y. S. Wagh, N. J. Tiwari and B. M. Bhanage, Tetrahedron Lett., 2013, 54, 1290 CrossRef CAS PubMed.
- V. P. Srivastava and L. D. S. Yadov, Synlett, 2013, 24, 2758 CrossRef PubMed.
- (a) R. Patel, V. P. Srivastava and L. D. S. Yadav, Synlett, 2010, 1797 CAS; (b) A. Rai and L. D. S. Yadav, Tetrahedron Lett., 2011, 52, 3933 CrossRef CAS PubMed; (c) A. Rai, V. K. Rai, A. K. Singh and L. D. S. Yadav, Eur. J. Org. Chem., 2011, 4302 CrossRef CAS; (d) A. K. Singh, R. Chawla, A. Rai and L. D. S. Yadav, Chem. Commun., 2012, 48, 3766 RSC; (e) A. Rai and L. D. S. Yadav, Tetrahedron, 2012, 68, 2459 CrossRef CAS PubMed; (f) V. P. Srivastava, A. K. Yadav and L. D. S. Yadav, Synlett, 2013, 24, 0465 CrossRef CAS PubMed.
- Y. Nishina, B. Ohtani and K. Kikushima, Beilstein J. Org. Chem., 2013, 9, 1663 CrossRef PubMed.
- M. Lamani and K. R. Prabhu, J. Org. Chem., 2011, 76, 7938 CrossRef CAS PubMed.
- T. Kawano, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2010, 132, 6900 CrossRef CAS PubMed.
- S. Maity and N. Zheng, Angew. Chem., Int. Ed., 2012, 51, 9562 CrossRef CAS PubMed.
- (a) Y. Su, L. Zhang and N. Ziao, Org. Lett., 2011, 13, 2168 CrossRef CAS PubMed; (b) Y. Q. Zou, L. Q. Lu, L. Fu, N. J. Chang, J. Rong, J. R. Chen and W. J. Xiao, Angew. Chem., Int. Ed., 2011, 50, 7171 CrossRef CAS PubMed; (c) S. Cai, X. Zhao, X. Wang, Q. Liu, Z. Li and Z. D. Wang, Angew. Chem., Int. Ed., 2012, 51, 8050 CrossRef CAS PubMed; (d) H. Sun, C. Yang, F. Gao, Z. Lia and W. Xia, Org. Lett., 2013, 14, 624 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures, 1H, 13C NMR and HRMS spectral data of all the products. See DOI: 10.1039/c3ra46314a |
| This journal is © The Royal Society of Chemistry 2014 |