An NIR dye encapsulated by a supramolecular assembly for imaging mitochondria in living cells with ultrastable photostability†
Xinwei Songa,
Fengyu Liuab,
Shiguo Sun*a,
Jingyun Wangc,
Jingnan Cuia and
Xiaojun Peng*a
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, P.R. China. E-mail: shiguo@dlut.edu.cn; pengxj@dlut.edu.cn; Fax: +86-411-84986304; Tel: +86-411-84986304
bState Key Laboratory of Fine Chemicals, School of Chemistry, Dalian University of Technology, Dalian, P.R. China
cSchool of Life Science & Biotechnology, Dalian University of Technology, 2 Linggong Road, Dalian, P.R. China
First published on 12th December 2013
Abstract
A near infrared dye was synthesized to form an inclusion complex with cucurbit[8]uril (CB[8]) in aqueous solution which disrupted the aggregation of the dye, leading to a 30-fold fluorescence increase. This inclusion complex exhibited ultrastable photostability with good mitochondria-staining capability.
Detailed visualization and monitoring of subcellular dynamic processes in living cells is one of the main goals in biomedical sciences.1–3 For instance, tracking and assessing the morphological changes of mitochondria in real-time would provide important information for pathology research and clinical diagnosis, for conditions such as Alzheimer's disease, diabetes, cancer and so on.4 However, this monitoring process often requires continuously recording the fluorescence images over a long period of time, for example, several days.5–8 To this end, mitochondria stains are expected to have a high fluorescence quantum yield, selectivity, low cytotoxicity, and potent resistance against photobleaching. Unfortunately, the structural damage from prolonged exposure to irradiation is an undesirable property for most mitochondria stains, especially for dyes with long absorption and emission wavelengths.9–11 Efforts have been made to enhance the photostability of dyes, and structural modification is a popular strategy.4,12–15 Yet, this method can be somewhat complicated or less efficient.
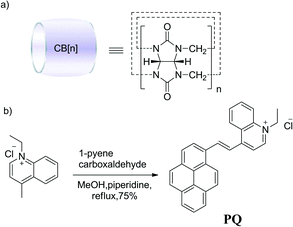
Host-guest supramolecular systems have proven profitable as an ideal strategy to alter the fluorescence characteristics of organic dyes.16–20 Among macrocyclic host molecules, cucurbit[n]uril (CB[n], n = 5–8 Scheme 1a) has a large hydrophobic cavity with polar carbonyl groups surrounding the two portals. Such a structure has enabled positively charged dyes to be encapsulated in the cavity of CB[n] and, consequently, resulted in profound changes in the absorption/emission wavelengths and quantum yields, as well as enhanced photostabilities.5,21–25 Furthermore, CB[n]s have been reported to have no intrinsic cytotoxicity and good uptake by many human cancer cell lines.26–29 These valuable features make CB[n] a promising candidate in the use of life science research.
Amongst fluorescence probes, pyrene and its derivatives have attracted much attention due to their high quantum yields of fluorescence, long excited-state lifetimes and high photostabilities.30–32 However, some disadvantages such as dye aggregation, poor solubility in aqueous solution and relatively short excitation/emission wavelength limit their application to some extent.22,33,34 Herein, to solve these problems mentioned above, we designed a near infrared (NIR) mitochondrial dye, 4-1-pyrenyl-vinyl-N-ethylquinolinium chloride (PQ, Scheme 1b), by introducing a hydrophilic cation moiety N-ethylquinolinium and established a supramolecular approach to enhance the solubility, photostability and fluorescence quantum yield in staining mitochondria by forming the host-guest inclusion complex PQ@CB[8] with the water soluble macrocyclic host CB[8]. The result demonstrated that the CB[8] host forms a stable complex with PQ and prevents PQ aggregation in aqueous solution, resulting in a dramatic increase in fluorescence intensity, and the photostability of this complex was greatly improved compared with that of the PQ dye alone. The MCF-7 cellular staining experiment revealed that the PQ@CB[8] complex has a good cell penetrating ability, and good specificity for mitochondria with a high re-stain rate. Under long term exposure of laser radiation from laser scanning confocal fluorescence microscopy (LSCFM), the PQ@CB[8] complex kept a strong fluorescence intensity without any significant photobleaching to the living cells.
In aqueous solution, PQ exhibits absorption maxima at 334 nm and 460 nm which are the aggregation peaks of PQ. By a deaggregation experiment, the monomeric dye predominated in solution and exhibited a red shifted absorption maximum at 501 nm at an extremely low PQ concentration of 5 μM, and a CB[8] concentration of 80 μM (as shown in Fig. S1†). Upon addition of CB[8], the absorption intensity at 334 nm was increased and red shifted to 343 nm, and the band at 460 nm was red shifted to 500 nm (as shown in Fig. 1). These absorption spectra are consistent with the spectral signature of monomeric dye PQ.
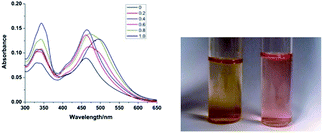 | ||
| Fig. 1 Absorption spectra of PQ (20 μM in water) in the presence of increasing concentration of CB[8] (0 to 1 equiv.), and colour comparison of 20 μM PQ (left) and 20 μM PQ@CB[8] (right) in water. | ||
The changes in spectral signature indicate the deaggregation and monomerization of PQ upon addition of CB[8] due to the formation of a dye inclusion complex (as shown in Scheme 2).
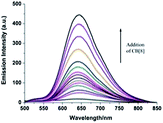
Fluorescence measurements can provide some further evidence. In aqueous solution, PQ exhibits an emission maximum at 645 nm. The addition of CB[8] resulted in a remarkable enhancement in the fluorescence intensity of PQ by 30-fold (as shown in Fig. 2). As the free dyes tend to aggregate in aqueous solution by cation–π interaction,35 PQ stacks exhibit very weak fluorescence.
Control experiments were carried out with a smaller cavity host CB[7], which cannot accommodate the N-ethylquinolinium moiety of PQ. The results indicate that CB[7] does not cause significant changes in absorption band shift or fluorescence intensity (as shown in Fig. S2†). The differences in the UV/vis and fluorescence spectra response between CB[8] and CB[7] confirmed the fact that CB[8] is able to accommodate PQ with a quinolinium moiety. In addition, a continuous variation Job's plot showed that the fluorescence spectrum data fit well to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host-guest inclusion model, whilst the binding constant was determined to be 1.3 × 104 M−1 (as shown in Fig. S3†).
1 host-guest inclusion model, whilst the binding constant was determined to be 1.3 × 104 M−1 (as shown in Fig. S3†).
The thickness of aggregated dye PQ and its CB[8] complex PQ@CB[8] was measured using Atomic Force Microscopy (AFM). As illustrated in Fig. 3, the height profiles obtained for PQ aggregation shows the thickness to be about 15.786 nm, as compared to 4.121 nm for the PQ@CB[8] complex. This result implies that the dye PQ tends to aggregate and stack on the substrate while CB[8] prevents the PQ dye from self-aggregation and forms a thin layer on the substrate.
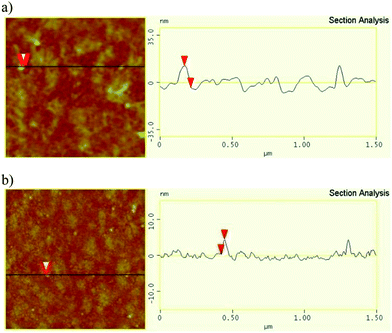 | ||
| Fig. 3 AFM images (left) and height profiles (right) of PQ aggregation (a) and the complex PQ@CB[8] (b). | ||
Furthermore, the emission lifetime of PQ increased from 2.48 ns to 2.91 ns upon addition of CB[8], which is a characteristic feature for the complexation of fluorescent dyes by host molecules, particularly CBs (as shown in Fig. S4†).17
A molecular modelling study was also carried out, and the result is shown in Fig. S5.†36,37 The adequate cavity size of CB[8] is essential so as to fit guest molecule PQ within, and to form a CB[8] inclusion complex with PQ. Which possesses a N-ethylquinolinium moiety. Whereas CB[7] with a smaller cavity is inefficient at preventing the aggregation of PQ.
As shown in Fig. 4, the photostability of the PQ@CB[8] complex was determined relative to the free dye PQ and a NIR mitochondrial probe Mito-Tracker Deep Red FM. After irradiation with a 500 W iodine–tungsten lamp for 540 min at room temperature, the fluorescence intensity of PQ decreased to less than 20% of the original value, implying 80% of the PQ molecules underwent photodecomposition. In the case of Mito-Tracker Deep Red FM, which has a similar emission wavelength to PQ, the fluorescence intensity almost vanished over this long period of irradiation. However, under the same irradiation condition, only 21% of the PQ@CB[8] complex decomposed and the solution still kept a strong fluorescence intensity. As a consequence, the PQ@CB[8] complex exhibits an ultrastable photostability property and falls into the range of the most photostable fluorescent dyes.22,23
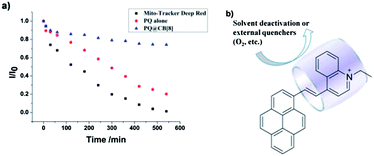 | ||
| Fig. 4 (a) Photodecompositions of PQ (10 μM) in the presence (▲) or absence (●) of 1 equiv. CB[8] as compared to Mito-Tracker Deep Red (10 μM) (■) in aerated water with increasing time of irradiation. (b) The blocking of the photophysical decay pathway of the dye PQ upon CB[8] inclusion.17 | ||
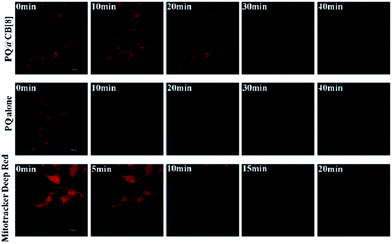
The intracellular localization of the PQ@CB[8] complex and dye PQ alone was investigated by LSCFM with argon laser excitation at 488 nm. Fig. S6† shows confocal fluorescence microscopy images of MCF-7 cells after incubation with PQ alone (2 μM) or PQ (2 μM) in the presence of CB[8] (20 μM) at 37 °C for 10 min. The PQ and PQ@CB[8] complex show an intracellular distribution in the mitochondria. The presence of CB[8] significantly enhanced the red fluorescence of mitochondria. This result is consistent with the fluorescence titration experiment mentioned above. The presence of CB[8] provides the possibility to employ a low concentration of PQ and low power laser excitation, which is essential for the imaging of active mitochondria – known to be damaged by laser exposure – in living cells.4
To estimate the staining efficiency of PQ@CB[8], a colocalization experiment was performed in MCF-7 cell lines. A commercially available, mitochondria localizing dye (Mito-Tracker Green FM) was employed for the colocalization study. The merged image presented in Fig. S7† strongly supports the mitochondrial specificity of PQ@CB[8], and the Pearson correlation coefficient ranges at about 98% (as shown in Fig. S8†).
The remarkable photostability of the PQ@CB[8] complex permits fluorescence imaging for long-term monitoring that is impossible for other NIR mitochondrial dyes, such as Mito-tracker Deep Red. Time series fluorescence images of PQ@CB[8] complex, PQ alone and Mito-tracker Deep Red in MCF-7 cells upon 40 min constant laser exposure were captured (as shown in Fig. 5). No obvious photobleaching can be observed over a 40 minute exposure time for PQ@CB[8]. This result is in contrast with PQ and Mito-tracker Deep Red FM, which illustrate significant fading after 30 min and 15 min exposure times, respectively, and the intracellular monitoring process is consistent with the photodecomposition experiment demonstrated in Fig. 4. These microscopy images highlight the extreme photostability of the PQ@CB[8] complex and also its low phototoxicity.
Conclusions
In summary, we report a mitochondrial specific NIR fluorescent probe PQ, which can form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex with CB[8]. The PQ@CB[8] complex exhibits intensified fluorescence, excellent photostability, good cell penetrating ability and mitochondria staining capability. This study enriches the field of supramolecular complexes in the application of fluorescence imaging, including timelapse microscopy imaging.
1 inclusion complex with CB[8]. The PQ@CB[8] complex exhibits intensified fluorescence, excellent photostability, good cell penetrating ability and mitochondria staining capability. This study enriches the field of supramolecular complexes in the application of fluorescence imaging, including timelapse microscopy imaging.
Acknowledgements
This work was financially supported by State Key Laboratory of Fine Chemicals, Dalian University of Technology, the Fundamental Research Funds for the Central Universities (no. DUT11LK21), the Open Research Fund of State Key Laboratory of Bioelectronics, Southeast University, China Postdoctoral Science Foundation (no. 20100471434), the Open Project Program of Key Laboratory of ECO-Textiles (Jiangnan University), Ministry of Education (no. KLET1102), National Basic Research Program of China (2009CB724700, 2013CB733702) and the National Natural Science Foundation of China (no. 21272030, 21136002, 21072024, 20923006).Notes and references
- S. Versari, A. M. Villa, A. Villa, S. M. Doglia, G. A. Pagani and S. Bradamante, J. Biomed. Opt., 2006, 11, 034014 CrossRef PubMed.
- L. F. Yousif, K. M. Stewart and S. O. Kelley, ChemBioChem, 2009, 10, 1939–1950 CrossRef CAS PubMed.
- A. T. Hoye, J. E. Davoren, P. Wipf, M. P. Fink and V. E. Kagan, Acc. Chem. Res., 2008, 41, 87–97 CrossRef CAS PubMed.
- S. Zhang, T. Wu, J. Fan, Z. Li, N. Jiang, J. Wang, B. Dou, S. Sun, F. Song and X. Peng, Org. Biomol. Chem., 2013, 11, 555–558 CAS.
- W. Lei, Q. Zhou, G. Jiang, Y. Hou, B. Zhang, X. Cheng and X. Wang, ChemPhysChem, 2011, 12, 2933–2940 CrossRef CAS PubMed.
- K. Chu, N. Teele, M. W. Dewey, N. Albright and W. C. Dewey, Radiat. Res., 2004, 162, 270–286 CrossRef CAS.
- H. B. Forrester, N. Albright, C. C. Ling and W. C. Dewey, Radiat. Res., 2009, 154, 625–639 CrossRef.
- E. Meijering, O. Dzyubachyk, I. Smal and W. A. van Cappellen, Seminars in Cell & Developmental Biology, 2009, 20, 894–902 Search PubMed.
- E. Macoas, G. Marcelo, S. Pinto, T. Caneque, A. M. Cuadro, J. J. Vaquero and J. M. G. Martinho, Chem. Commun., 2011, 47, 7374–7376 RSC.
- M.-L. Zheng, K. Fujita, W.-Q. Chen, N. I. Smith, X.-M. Duan and S. Kawata, ChemBioChem, 2011, 12, 52–55 CrossRef PubMed.
- W. J. Rhee and G. Bao, Nucleic Acids Res., 2010, 38, 109 CrossRef PubMed.
- B. Kopainsky, P. Qiu, W. Kaiser, B. Sens and K. Drexhage, Appl. Phys. B, 1982, 29, 15–18 CrossRef.
- B. R. Renikuntla, H. C. Rose, J. Eldo, A. S. Waggoner and B. A. Armitage, Org. Lett., 2004, 6, 909–912 CrossRef CAS PubMed.
- P. Chen, S. Sun, Y. Hu, Z. Qian and D. Zheng, Dyes Pigm., 1999, 41, 227–231 CrossRef CAS.
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2009, 110, 2620–2640 CrossRef PubMed.
- S. Gadde, E. K. Batchelor, J. P. Weiss, Y. Ling and A. E. Kaifer, J. Am. Chem. Soc., 2008, 130, 17114–17119 CrossRef CAS PubMed.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- B. P. Jiang, D. S. Guo and Y. Liu, J. Org. Chem., 2010, 75, 7258–7264 CrossRef CAS PubMed.
- A. Singh, W.-T. Yip and R. L. Halterman, Org. Lett., 2012, 14, 4046–4049 CrossRef CAS PubMed.
- O. Buyukcakir, F. T. Yasar, O. A. Bozdemir, B. Icli and E. U. Akkaya, Org. Lett., 2013, 15, 1012–1015 CrossRef CAS PubMed.
- H. Zhang, L. Liu, C. Gao, R. Sun and Q. Wang, Dyes Pigm., 2012, 94, 266–270 Search PubMed.
- F. Biedermann, E. Elmalem, I. Ghosh, W. M. Nau and O. A. Scherman, Angew. Chem., Int. Ed., 2012, 51, 7739–7743 CrossRef CAS PubMed.
- J. Mohanty and W. M. Nau, Angew. Chem., Int. Ed., 2005, 44, 3750–3754 CrossRef CAS PubMed.
- Z. Li, S. Sun, F. Liu, Y. Pang, J. Fan, F. Song and X. Peng, Dyes Pigm., 2012, 93, 1401–1407 CrossRef CAS PubMed.
- T. Y. Zhang, S. G. Sun, F. Y. Liu, Y. Pang, J. L. Fan and X. J. Peng, Phys. Chem. Chem. Phys., 2011, 13, 9789–9795 RSC.
- G. Hettiarachchi, D. Nguyen, J. Wu, D. Lucas, D. Ma, L. Isaacs and V. Briken, PLoS One, 2010, 5, e10514 Search PubMed.
- V. D. Uzunova, C. Cullinane, K. Brix, W. M. Nau and A. I. Day, Org. Biomol. Chem., 2010, 8, 2037–2042 CAS.
- Y. J. Jeon, S.-Y. Kim, Y. H. Ko, S. Sakamoto, K. Yamaguchi and K. Kim, Org. Biomol. Chem., 2005, 3, 2122–2125 CAS.
- S. Walker, R. Oun, F. J. McInnes and N. J. Wheate, Isr. J. Chem., 2011, 51, 616–624 CrossRef CAS.
- V. De Halleux, W. Mamdouh, S. De Feyter, F. De Schryver, J. Levin and Y. H. Geerts, J. Photochem. Photobiol., A, 2006, 178, 251–257 CrossRef CAS PubMed.
- J. Balapanuru, J. X. Yang, S. Xiao, Q. L. Bao, M. Jahan, L. Polavarapu, J. Wei, Q. H. Xu and K. P. Loh, Angew. Chem., Int. Ed., 2010, 49, 6549–6553 CrossRef CAS PubMed.
- Z. Kostereli and K. Severin, Chem. Commun., 2012, 48, 5841–5843 RSC.
- K. Maie, M. Nakamura, T. Takada and K. Yamana, Bioorg. Med. Chem., 2009, 17, 4996–5000 CrossRef CAS PubMed.
- J. Xiao, C. D. Malliakas, Y. Liu, F. Zhou, G. Li, H. Su, M. G. Kanatzidis, F. Wudl and Q. Zhang, Chem. – Asian J., 2012, 7, 672–675 CrossRef CAS PubMed.
- I. Richter, J. Minari, P. Axe, J. P. Lowe, T. D. James, K. Sakurai, S. D. Bull and J. S. Fossey, Chem. Commun., 2008, 1082–1084 RSC.
- A. Thangavel, C. Sotiriou-Leventis, R. Dawes and N. Leventis, J. Org. Chem., 2012, 77, 2263–2271 CrossRef CAS PubMed.
- Y. Xu, M. J. Panzner, X. Li, W. J. Youngs and Y. Pang, Chem. Commun., 2010, 46, 4073–4075 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization of PQ, measurement equipment and materials, cell culture and staining, UV-vis absorption and fluorescence spectra of PQ and other control experiments. See DOI: 10.1039/c3ra46444j |
| This journal is © The Royal Society of Chemistry 2014 |