A novel high-temperature naphthyl-based phthalonitrile polymer: synthesis and properties
Fenghua Zhaoa,
Ruojin Liua,
Chao Kanga,
Xiaoyan Yu*a,
Kimiyoshi Naitob,
Xiongwei Qua and
Qingxin Zhang*a
aInstitute of Polymer Science and Engineering, School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China
bHybrid Materials Center, National Institute for Materials Science, Tsukuba, 305-0047, Japan
First published on 14th January 2014
Abstract
A novel naphthyl-based phthalonitrile monomer, 1,6-bis(3,4-dicyanophenoxy) naphthalene (1,6-BDCN), was prepared, and the phthalonitrile resin was cured with 4,4′-diaminodiphenyl ether (ODA) via two steps, namely, preparation of prepolymer and postcuring prepolymer at elevated temperatures. The 1,6-BDCN polymer might form triazine and phthalocyanine rings as demonstrated by FTIR spectra. The prepolymer shows fine solubility in organic solvents. The 1,6-BDCN polymer exhibits excellent structural integrity and superior thermal stability as indicated by thermogravimetric analysis (TGA). Dynamic mechanical analysis (DMA) revealed that the phthalonitrile resin has a high storage modulus and glass transition temperature (Tg). The water uptake is about 3% by weight after submersion in boiling water for 50 hours. The influence of curing processes on thermal stability and flame retardancy was also explored.
1. Introduction
Because of good mechanical properties and high thermal stability, high-temperature polymers and their composites are increasingly utilized for aerospace and microelectronic applications.1,2 Currently, polyimide (PI),3 polyphenylene sulfide (PPS),4 and polyether-ether-ketone (PEEK),5,6 are typical high-temperature polymers with a long-term use temperature as high as 200 °C. In preparation of polyimide (PI), water is inevitably produced and difficult to remove, which might lead to micro voids in resulted structures. For polyphenylene sulfide (PPS), the impurities which are generated in the production process, are difficult to eliminate. Compared with poly(ether-ether-ketone) (PEEK), the Tg of phthalonitrile resin is much higher. Thus, the phthalonitrile resin is a better high-temperature material.Phthalonitrile polymers, as a member of the high-temperature polymer families, possess outstanding thermal stability at elevated temperatures, superior fire resistance relative to other conventional polymers, and low water uptake.7 They are firstly developed by Naval Laboratory (NRL, the Naval Research Laboratory).8 Keller and his coworkers have prepared various phthalonitrile polymers9–16 and advanced composites17,20,21, including 4,4-bis(3,4-dicyanophenoxy)biphenyl (BPh),18 2,2-bis[4-(3,4-dicyano-phenoxy)phenyl] propane (BAPh),19 and 1,3-bis(3,4-dicyano phenoxy)benzene (resorcinol-based phthalonitrile).7 Phthalonitrile-glass fiber composites21 and phthalonitrile–carbon fiber composites20 were researched, and the results reveal that the phthalonitrile composites postcured at 375 °C for 2, 4 and 8 h respectively do not exhibit any glass transition when the temperature is up to 450 °C and around 90% of their initial modulus retains (phthalonitrile–carbon fiber composites).
The performance of the polymer affects the processing condition and processing equipment. Compared with other high-temperature polymers (PI, PPS, PEEK), phthalonitrile polymers utilized addition polymerization reaction are not need solvent to help processing. In addition, the phthalonitrile polymer has large processing window (the temperature difference between the melting point of the monomer and the exothermic curing temperature) which is significantly important for processing. Compared with other phthalonitrile polymers,18 1,6-BDCN polymer presented in this report has lower melting point and larger processing window so it is more advantageous to the processing and worthy of studying.
This report presents the synthesis and properties of a new phthalonitrile resin based on 1,6-dihydroxynaphthalene. The novel 1,6-BDCN polymer displays good processing properties, extremely high Tg, excellent thermal and thermo-oxidative properties, limited water uptake rate, and outstanding flame retardancy. Thus it is an appropriate candidate for applications such as structural components for advanced aerospace and microelectronics applications.
2. Experimental
2.1 Materials
1,6-Dihydroxynaphthalene (1,6-DHN) (+99.0%) and 4-nitrophthalonitrile (NPN) (98.0%) were purchased from Lideshi (Beijing) chemical technology Co., Ltd. and Wuhan Chifei Chemical Corporation, China, respectively. 4,4′-Diaminodiphenyl ether (ODA) (+98.0%) was obtained from Beijing Hengye Zhongyuan Chemical Corporation. Potassium carbonate (K2CO3) (+99.0%) was purchased from Tianjin Fengchuan Chemical Corporation. The above chemicals were dried overnight at 85 °C under vacuum before use. N,N-Dimethylformamide (DMF) (+99.5%) (with molecular sieves to remove water) and concentrated hydrochloric acid (36–38%) were supplied by Tianjin Fuchen and Beijing Chemical Corporation, and they were used without further purification.2.2 Preparation of 1,6-BDCN monomer
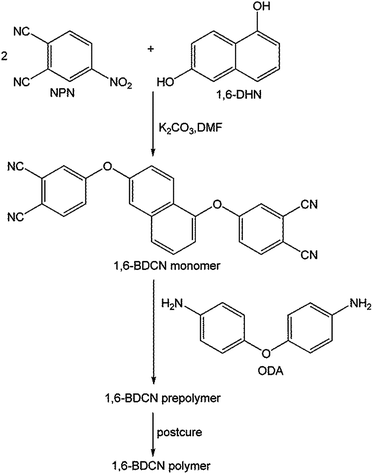
1,6-DHN (8.0085 g, 0.05 mol), K2CO3 (17.25 g, 0.125 mol), NPN (17.313 g, 0.10 mol) and dry DMF (80 mL) were added to a 250 mL, three-necked flask equipped with a reflux condenser. The resulting mixture was kept at 85–90 °C for 6 h under ambient conditions. The product was mixed with diluted hydrochloric acid (300 mL, 1 M). The reddish brown solid 1,6-BDCN monomer (19.22 g, 93% yield) was collected by suction filtration and washed with plenty of distilled water until the filtrate was neutral.2.3 Preparation of 1,6-BDCN prepolymer and polymer
Preparation of polymer was typically via two steps (Fig. 1). The first step was to add ODA (0.097 g, 0.485 mmol) to the monomer (10.0 g, 0.024 mol) melt at 200 °C with continuous stirring for about 1 min and then the phthalonitrile prepolymer was obtained through quenching the molten mixture to room temperature for 10–15 min. The prepolymer was pulverized and placed in a vacuum oven (−0.1 MPa) at 170 °C for 2 h, and then postcured at elevated temperatures with different postcuring processes (Table 1) in a mold with cavity dimensions of 30 mm × 10 mm × 2 mm for DMA experiments.| Sample | The postcuring processes of prepolymer |
|---|---|
| a | 270 °C for 5 h, 300 °C for 5 h |
| b | 270 °C for 5 h, 300 °C for 5 h, 340 °C for 5 h |
| c | 270 °C for 5 h, 300 °C for 5 h, 340 °C for 5 h, 370 °C for 5 h |
| d | 270 °C for 5 h, 300 °C for 5 h, 340 °C for 5 h, 370 °C for 5 h, 400 °C for 5 h |
2.4 Water-uptake test
A sample of polymer produced by the curing of monomer with 2 mol% ODA to 400 °C was immersed in boiling distilled water at 100 °C for 50 hours. The sample was removed from the water, dried, and weighed periodically to determine the amount of water absorbed.2.5 Characterization
Differential scanning calorimetric (DSC) experiment was conducted with the mixture of monomer containing curing additive (2 mol%) in a PE Diamond DSC. Samples were heated from 50 to 400 °C at the rate of 10 °C min−1 with a nitrogen flow of 100 cm3 min−1. Thermogravimetric analysis was carried out from room temperature to 1000 °C using a Q600 thermogravimetric analyzer (TGA) at a heating rate of 10 °C min−1 under nitrogen and air atmosphere with a flow rate of 100 cm3 min−1. IR: A Bruker Vector 22 FTIR spectrophotometer was used to record FTIR spectra (KBr pellet). In a typical experiment, an average of 32 scans per sample was made in the range of 400–4000 cm−1. Dynamic mechanical measurements (DMA) were performed on a DMA Q800 (TA Instruments) in single cantilever mode to measure the dynamic storage modulus (E′) of rectangular 1,6-BDCN polymer samples (dimensions 30 mm × 10 mm × 2 mm) in nitrogen atmosphere from 30 to 465 °C with a heating rate of 5 °C min−1, a frequency of 1 Hz and an amplitude of 10 μm. 1H NMR was performed on a Bruker 400 MHz spectrometer with DMSO-d6 as a solvent and tetramethylsilane as an internal reference. The morphology of the polymer and the residues after TGA tests were determined by a LEO 1530 VP field emission SEM. The specimen was coated with Au prior to observation.3. Results and discussion
3.1 Characterization of monomer
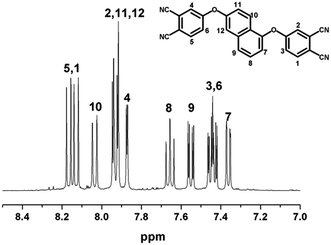
The novel naphthyl-based phthalonitrile monomer, 1,6-BDCN, was synthesized by a nucleophilic displacement of a labile nitro-substituent from 4-nitrophthalonitrile (NPN) as illustrated in Fig. 1. The reaction took place in DMF solution in the presence of alkaline substance K2CO3 as catalyst. Finally, the product was mixed with dilute hydrochloric acid in order to remove the excess K2CO3 and then washed with plenty of distilled water. IR (KBr pellet) [cm−1].(Fig. 9): ν 3422 (O–H), 3076 (Ca C–H), 1594, 1564, 1484, 1423 (aromatic), 1365 (CH3), 1248, 1227, 1211, 1168, 1138 (C–O), 968 (C–OH), 835, 797, 524 (aromatic). 1H NMR (400 MHz, DMSO-d6) [ppm] (Fig. 3): δ 8.165–8.104 (d, H1, d, H5), 8.008–8.031 (d, H10), 7.935–7.904 (d, H2, dd, H11, t, H12), 7.863–7.857 (d, H4), 7.661–7.622 (t, H8), 7.551–7.523 (dd, H9), 7.452–7.402 (dd, H3, dd, H6), 7.357–7.340 (dd, H7).
3.2 Thermal and dynamic mechanical properties
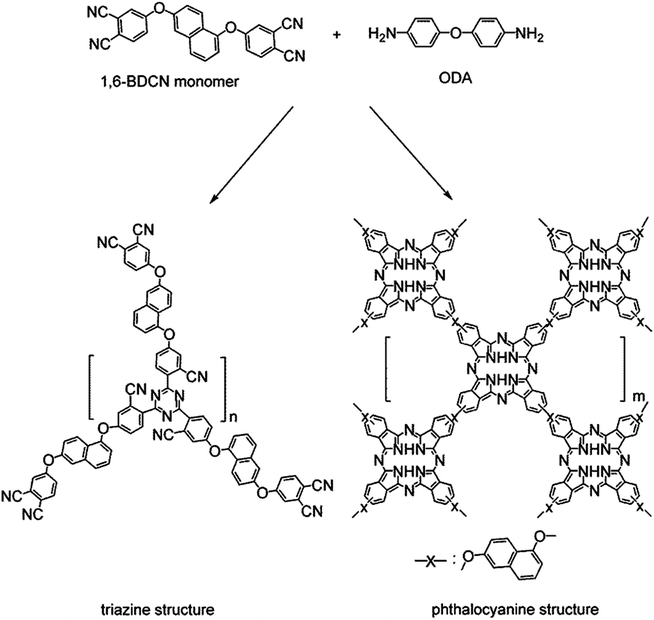
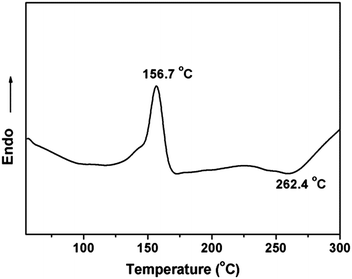
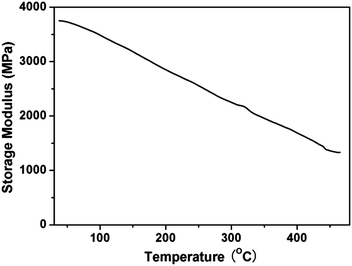
Polymerization reaction of phthalonitrile monomer with ODA (2 mol%) was studied by DSC from 50 to 400 °C. An endothermic transition centered on 156.7 °C of the thermogram appears, which corresponds to the melting point of the monomer. And at about 262.4 °C of the exothermic peak is shown in Fig. 4. This exothermic peak stands for the reaction of nitrile groups on the monomer with the aromatic diamine. The polymerization reaction of phthalonitrile monomer can be catalyzed by phenolic,22 strong organic acids,23 organic amine,24 metal salt and metal,25 organic acids/amine salt.26 Here, organic amine (4,4′-diaminodiphenyl ether) was used to make the nitrile groups react with other nitrile groups forming crosslinking structure (triazine rings and phthalocyanine rings) which was shown in Fig. 2. The large processing window (105.7 °C) between the melting point (156.7 °C) and the broad, gentle curing exothermic peak (262.4 °C) is observed in the DSC curve which proves the polymerization of phthalonitrile-based monomer. Thus, a curing temperature program is formulated based on the DSC.The dynamic mechanical properties of the cured 1,6-BDCN polymer was evaluated to ascertain the modulus. The storage modulus (E′) versus temperature curve which ranges from about 30 to 465 °C are presented in Fig. 5. As seen, the 1,6-BDCN polymer exhibits a high storage modulus of 3.75 GPa at 38 °C, which decreases gradually along with increasing temperature. The high modulus should be ascribed to the aromatic and crosslinked microstructure. When the temperature reaches 450 °C, the storage modulus of the phthalonitrile polymer is decreased to approximately 35% of the value at room temperature, but it is still about 1.5 GPa. The decrease of modulus with increasing temperature is due to stress relaxation of the polymer network. In addition, storage modulus does not decrease dramatically from 30 °C to 465 °C, which indicates that glass transition temperature does not appear. Thus, Tg of the phthalonitrile polymer is higher than 465 °C and it exhibits excellent thermal-mechanical properties.
In our group, the 2,7-bis(3,4-dicyanophenoxy) naphthalene (2,7-BDCN) has been successfully synthesized through the reaction between 2,7-dihydroxynaphthalene (2,7-DHN) and 4-nitrophthalonitrile (NPN).27 Compared with 1.6-BDCN polymer, though the 2,7-BDCN polymer is a high performance and high-temperature polymer, the 1,6-BDCN polymer shows higher Tg, lower melting temperature, wide processing window and higher storage modulus. Therefore, the 1,6-BDCN polymer is a superior high-temperature polymer with excellent mechanical properties and fine processability.
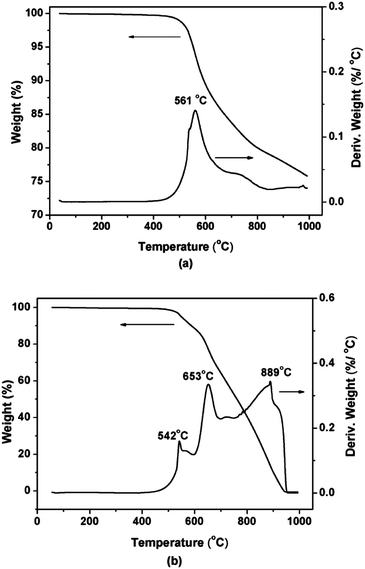
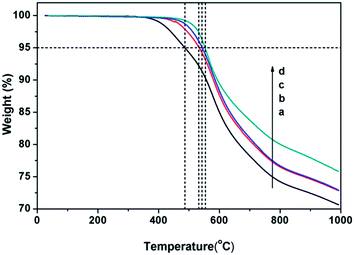
The thermal stability of 1,6-BDCN polymer was evaluated by TGA (Fig. 6) in air and nitrogen atmosphere, and the specific parameters were collected in Table 2. When cured with 2 mol% ODA at the highest temperature up to 400 °C, the sample exhibits excellent thermal property with 75% weight retention approximately at 1000 °C in nitrogen atmosphere, and the 1,6-BDCN polymer shows the weight residual of 99% (Tid), 95% (T5%) and 90% (T10%) at 498 °C, 553 °C and 594 °C respectively. In air atmosphere, the 1,6-BDCN polymer also demonstrates outstanding stability as shown in the TGA curve and Table 2, and shows the Tid, T5% and T10% at 488 °C, 549 °C and 588 °C, respectively. And the maximum decomposition temperature (Tdmax) appears at 561 °C in nitrogen while at 542, 653, 889 °C in air and then suddenly drops. It is obvious that the rapid weight loss at high temperatures should be attributed to the oxidation reaction.
| Atmosphere | Tida (°C) | T5%a (°C) | T10%a (°C) | Tdmaxb (°C) | Char yieldc (%) | LOId |
|---|---|---|---|---|---|---|
| a Temperature at which 1%, 5% and 10% weight loss respectively were recorded by TGA at heating rate of 10 °C min−1 in air or nitrogen.b Temperature at which maximum loss rate was recorded by TGA at heating rate of 10 °C min−1 in air or nitrogen.c Residual weight retention at 1000 °C in nitrogen.d Limiting oxygen index. | ||||||
| Air | 488 | 549 | 588 | 542, 653, 889 | — | — |
| N2 | 498 | 553 | 594 | 561 | 75.80 | 47.82 |
Generally, thermal oxidation stability of a polymer depends on the density of active H atoms and dissociation energy of chemical bonds.27 For the 1,6-BDCN polymer, all of the referred bonds are C–O (332.2 kJ mol−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (736.2 kJ mol−1) in triazine and phthalocyanine rings, the conjugated C
N (736.2 kJ mol−1) in triazine and phthalocyanine rings, the conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (478.6 kJ mol−1), C–H (334.3–462.8 kJ mol−1) in phenyl structure, and C–C (553.5 kJ mol−1) between CN and phenyl structure.28,29 As a consequence, it can be found that the thermal stability of polytriazine and phthalocyanine rings is high. So the three decomposition peaks temperature might be ascribed to the decomposition of ether linkages, phenyl structures, and phthalocyanine and polytriazine rings in order. Thus, the cross-linked naphthyl-based phthalonitrile polymer with low level of active H atoms shows superior thermal performance compared to most of heat-resistant polymers which may be also ascribed to the naphthalene backbone structure.
C (478.6 kJ mol−1), C–H (334.3–462.8 kJ mol−1) in phenyl structure, and C–C (553.5 kJ mol−1) between CN and phenyl structure.28,29 As a consequence, it can be found that the thermal stability of polytriazine and phthalocyanine rings is high. So the three decomposition peaks temperature might be ascribed to the decomposition of ether linkages, phenyl structures, and phthalocyanine and polytriazine rings in order. Thus, the cross-linked naphthyl-based phthalonitrile polymer with low level of active H atoms shows superior thermal performance compared to most of heat-resistant polymers which may be also ascribed to the naphthalene backbone structure.
Compared with 2,7-BDCN polymer,27 T5%, T10%, Tdmax and char yield at 1000 °C in N2 of 1,6-BDCN polymer are all higher. The more weight residue retains the better flame retardancy the material. Two reasons may be able to account for this result. In addition to the different curing temperature and time of curing process of the two polymers, the difference of their polymer structures should be considered as another important reason. Polypyrrole structure, the main structure in the 2,7-BDCN polymer, is a five-member rings structure, while the 1,6-BDCN polymer forms six-member rings of polytriazine rings which are more stable and contribute to its higher stability.
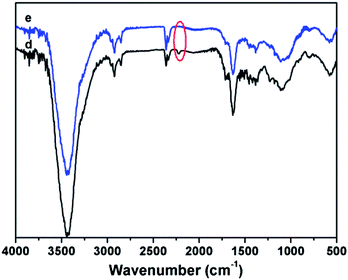
In addition, FTIR and SEM were used to evaluate the results after TGA test in nitrogen atmosphere. Compared with Fig. 7(d), after TGA test in N2 atmosphere, the nitrile group (–CN) at 2232 cm−1 is disappeared in Fig. 7(e), which demonstrates the bonds between carbon and nitrogen of unreacted CN or C–C bonds between CN and phenyl structure may be broken while other bonds are not destroyed. The morphology of the 1,6-BDCN polymer surface and the residues after the TGA test are shown in Fig. 8. No voids can be observed for the 1,6-BDCN polymer under a magnification of 1000 times which proves the void-free structure of 1,6-BDCN polymer and it also guarantees the excellent thermal and mechanical properties. Moreover, the addition polymerization mechanism of the monomer is one of the most important factors for preparing void-free components. Compared with the polymer (Fig. 8(A)), no changes of the residues after TGA test can be observed in the Fig. 8(B) which fully demonstrates the high-temperature properties of 1,6-BDCN polymers.
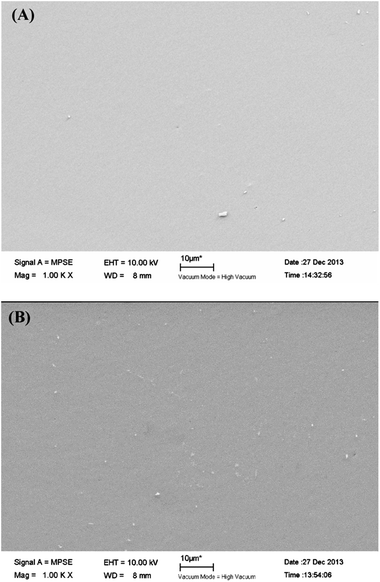 | ||
| Fig. 8 SEM images of (A) 1,6-BDCN polymer surface and (B) the residues after TGA test of 1,6-BDCN polymer. | ||
According to van Krevelen,30 there is a linear relationship between LOI (limiting oxygen index) and CR (char yield) for halogen-free polymers, namely,
| LOI = 17.5 + 0.4[CR] |
LOI is defined as the minimum fraction of oxygen in an oxygen–nitrogen mixture that is just sufficient to sustain combustion of the specimen after ignition31 and CR is the char residue in weight percent in nitrogen. At 1000 °C, the char residue of the cured 1,6-BDCN (cured at 400 °C) is as high as 75.80%, and the LOI value is up to 47.82. LOI value of 26 or higher is rated as a flame-retardant material.31 Therefore, it can be found that the 1,6-BDCN polymer is an excellent flame retardant material.
3.3 Effects of curing procedures
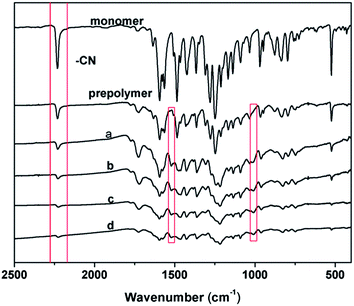
FTIR spectra were used to track the different curing processes as shown in Table 1. As seen, the spectrum of prepolymer is very similar to that of the monomer, apart from the weak peaks around 1520 cm−1 and around 1010 cm−1, which are signified as the characteristic absorptions of triazine and phthalocyanine rings, respectively.During the second stage, the prepolymer was postcured into cross-linked polymer by heating at higher temperatures above 270 °C, which can be observed in Fig. 9(a–d). With the increase of curing degree, the characteristic absorbing peaks of the nitrile group (–CN) at 2232 cm−1 was gradually disappeared. In this reaction, more nitrile groups are polymerized gradually to form triazine and phthalocyanine rings, as seen by the decreasing intensity of the nitrile absorption and the growth of the intensity of peaks at 1520 cm−1 and 1010 cm−1 in the IR spectra.
TGA was also used to study the effect of different curing processes (Fig. 10), and the results are listed in Table 3. With the extension of curing temperature and time, the decomposition temperatures become higher and the char yield rates at 1000 °C are increased. The highly cross-linked 1,6-BDCN polymer (cured at 400 °C) remains the weight of 95% at 553 °C and an overall char yield of 75.80% at 1000 °C. The initial reduced char yield (about 1%) could be a result of surface adsorption of small molecules.
| Sample | Tid (°C) | T5% (°C) | Char yield (%) at 1000 °C | LOI |
|---|---|---|---|---|
| a | 401 | 486 | 70.64 | 45.76 |
| b | 452 | 534 | 72.85 | 46.64 |
| c | 477 | 543 | 72.92 | 46.67 |
| d | 498 | 553 | 75.80 | 47.82 |
According to the char yield, the limit oxygen index (LOI) is calculated, which increases gradually with the increase of curing temperature and time, so that the flame retardancy of the polymer becomes better. The results indicate that curing time and temperature in the polymerization reaction could affect the thermal stability of the 1,6-BDCN polymer which might be ascribed to the difference in curing degrees.
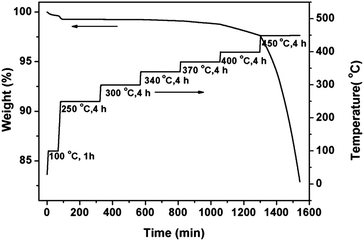
3.4 Thermal oxidative property
Thermo-oxidative aging of the cured polymer postcured at elevated temperatures was carried out to assess the thermo-oxidative stability (Fig. 11). The values of weight residue (at 100 °C for 1 h, 250, 300, 340, 370, 400, and 450 °C for 4 h, respectively) are listed in Table 4. It illustrates the total cumulative weight residue at different segments. After aging at 250 and 300 °C in air for a total of 8 hours, the 1,6-BDCN polymer retained 99.25% of its initial weight. At 340 °C, 370 °C and 400 °C, the polymer showed less than 1%, 2% and 3% weight loss respectively. The majority (17.11%) of the weight loss occurred during the final heat segment at 450 °C. The above results demonstrate the superior thermo-oxidative properties of the naphthyl-based phthalonitrile polymer.| Segment | Temperature (°C) × time (h) | Weight residue (%) |
|---|---|---|
| (1) | 100 × 1 | 99.63 |
| (2) | 250 × 4 | 99.26 |
| (3) | 300 × 4 | 99.25 |
| (4) | 340 × 4 | 99.11 |
| (5) | 370 × 4 | 98.78 |
| (6) | 400 × 4 | 97.64 |
| (7) | 450 × 4 | 82.89 |
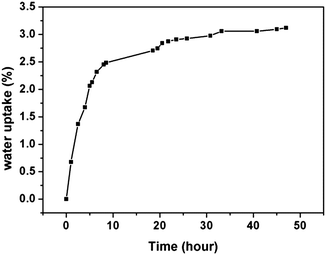
3.5 Water uptake
The water uptake was calculated using the following equation:| Water uptake (%) = (M2 − M1)/M1 × 100 |
Another important feature of the naphthyl-based phthalonitrile polymer is the limited water uptake. Fig. 12 shows a plot of the water uptake versus time in boiling distilled water. The maximum water uptake capacity of 50 hours at 100 °C was approximately 3.0% by weight. The water uptake appears to level off after approximately 15–20 hours depending on the experimental temperature. The low water uptake of the polymer should be ascribed to the high crosslinking structure and the low degree of hydrophilic groups on molecular chains.32 For the use of polymer in a high humidity or aqueous environment, the lower water uptake is a significant advantage over other high temperature polymers.
4. Conclusion
A novel naphthyl-based phthalonitrile monomer, 1,6-bis(3,4-dicyanophenoxy) naphthalene (1,6-BDCN), was prepared by a nucleophilic displacement reaction, and the phthalonitrile resin cured with 4,4′-diaminodiphenyl ether shows high Tg and outstanding thermal stability. The lower melting point (156.7 °C) of the monomer and the higher curing temperature (262.4 °C) provide a large processing window (105.7 °C) for the material. In the polymerization reaction, nitrile groups form triazine rings and phthalocyanine rings as proved by the FTIR spectra. The thermal stability of the phthalonitrile polymer was improved with the increase of postcuring temperature and time. The prepared phthalonitrile polymer exhibits excellent thermal stability together with high Tg and storage modulus. Thus, it is a good candidate for applications such as structural components, high temperature adhesives and packing materials in aerospace and microelectronic industries.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant no. 51073049 and 21001039). The authors also thank the financial support from Natural Science Foundation of Hebei Province (B2011202081) and Technology Foundation for Selected Overseas Chinese Scholar, Department of Human Resources and Social Security of Hebei Province. We express our appreciation to Pange Liu and Rongqin Ji for experimental assistance.Notes and references
- T. M. Keller, J. Polym. Sci., Part A: Polym. Chem., 1988, 26, 3199 CrossRef CAS.
- Y. K. Zou and X. B. Lui, J. Appl. Polym. Sci., 2013, 129, 130 CrossRef CAS.
- D. M. Deloziera, R. A. Orwolla, J. F. Cahoona, J. S. Ladislawa, J. G. Smith Jr and J. W. Connellb, Polymer, 2003, 44, 2231 CrossRef.
- Z. Y. Jiang, L. A. Gyurova, A. K. Schlarb, K. Friedrich and Z. Zhang, Compos. Sci. Technol., 2008, 68, 734 CrossRef CAS PubMed.
- T. K. Mishra, K. Ashish, V. Verma, K. N. Pandey and V. Kumar, Compos. Sci. Technol., 2012, 72, 1627 CrossRef CAS PubMed.
- Q. H. Wang, Q. J. Xue, H. W. Liu, W. C. Shen and J. F. Xu, Wear, 1996, 198, 216 CrossRef CAS.
- T. M. Keller and D. D. Dominguez, Polymer, 2005, 46, 4614 CrossRef CAS PubMed.
- S. B. Sastri and T. M. Keller, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 1885 CrossRef CAS.
- D. D. Dominguez and T. M. Keller, Polymer, 2007, 48, 91 CrossRef CAS PubMed.
- T. M. Keller, Polymer, 1993, 34, 952 CrossRef CAS.
- D. D. Dominguez and T. M. Keller, J. Appl. Polym. Sci., 2008, 110, 2504 CrossRef CAS.
- Z. Brunovska, R. Lyon and H. Ishida, Thermochim. Acta, 2000, 195, 357 Search PubMed.
- D. D. Dominguez and T. M. Keller, High Perform. Polym., 2006, 18, 283 CrossRef CAS PubMed.
- L. Matthew, D. D. Dominguez and T. M. Keller, Polymer, 2006, 47, 3727 CrossRef PubMed.
- T. M. Keller, Chem. Mater., 1994, 6, 302 CrossRef CAS.
- M. Laskoski, D. D. Dominguez and T. M. Keller, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4774 CrossRef CAS.
- X. B. Liu, S. R. Long, D. W. Luo, W. J. Chen and G. P. Cao, Mater. Lett., 2008, 62, 19 CrossRef CAS PubMed.
- S. B. Sastri and T. M. Keller, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 2105 CrossRef CAS.
- M. Laskoski, D. D. Dominguez and T. M. Keller, J. Polym. Sci., Part A: Polym Chem., 2005, 43, 4136 CrossRef CAS.
- S. B. Sastri, J. P. Armistead and T. M. Keller, Polym. Compos., 1996, 17, 816 CrossRef CAS.
- S. B. Sastri, J. P. Armistead and T. M. Keller, Polym. Compos., 1997, 18, 48 CrossRef CAS.
- M. J. Sumner, M. Sankarapandian, J. E. Mcgrath, J. S. Riffle and U. Sorathia, Polymer, 2002, 43, 5069 CrossRef CAS.
- T. M. Keller, Polym. Prepr., 1992, 33, 422 CAS.
- T. M. Keller and T. R. J. Macromol, Sci. Chem., 1982, 18, 931 Search PubMed.
- K. Jia, R. Zhao, J. C. Zhong and X. B. Liu, J. Mater. Sci.: Mater. Electron., 2010, 21, 708 CrossRef CAS.
- P. J. Burchill, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 1 CrossRef CAS.
- X. Y. Yu, K. Naito, C. Kang, X. W. Qu and Q. X. Zhang, Macromol. Chem. Phys., 2013, 214, 361 CrossRef CAS.
- J. B. Pedley, R. D. Naylor and S. P. Kirby, Thermochemical data of organic compounds, Chapman and Hall, New York, 2nd edn, 1986.
- R. G. Wenthold and R. R. Squires, J. Am. Chem. Soc., 1994, 116, 6401 CrossRef.
- D. W. van Krevelen, Polymer, 1975, 16, 6156 CrossRef.
- C. H. Lin and C. S. Wang, Polymer, 2001, 42, 1877 Search PubMed.
- H. T. Sheng, Mater. Chem. Phys., 2013, 142, 740 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |