Role of basic sites of substituted ferrocenes in interaction with the trinuclear 3,5-bis(trifluoromethyl)pyrazolates: thermodynamics and structure of complexes†
Alexey A. Titova,
Oleg A. Filippova,
Ekaterina A. Gusevaa,
Alexander F. Smol'yakova,
Fedor M. Dolgushina,
Lina M. Epsteina,
Vitaly K. Belskyb and
Elena S. Shubina*a
aA. N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Vavilov str. 28, 119991 Moscow, Russia
bL. Ya. Karpov Institute of Physical Chemistry, Vorontsovo Pole St. 10, 103064, Moscow, Russia
First published on 14th January 2014
Abstract
Formation of complexes of the macrocycles (ML)3, where L = 3,5-(CF3)2Pz = 3,5-bis(trifluoromethyl)pyrazolate, M = Cu and Ag, and the acylferrocenes FcC(O)CH2R (Fc = (C5H5)Fe(C5H4); R = H (1), Ph (2)) was studied by means of variable temperature IR, UV-vis, NMR spectroscopy. The sole site of coordination in solution is the oxygen atom of the CO group. The complex composition (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and thermodynamic parameters in hexane solution were determined, the formation constants and the enthalpies decreasing from 1 to 2 and from Ag to the Cu macrocycle. The same coordination site featuring triple coordination of oxygen to all metal atoms of a macrocycle was found in the solid state by single crystal X-ray diffraction. There are no shortened contacts of the metal in the macrocycles with π-electron system of the ferrocene's cyclopentadienyl ligands in all complexes. The complexes of (ML)3 with 1 have 1
1) and thermodynamic parameters in hexane solution were determined, the formation constants and the enthalpies decreasing from 1 to 2 and from Ag to the Cu macrocycle. The same coordination site featuring triple coordination of oxygen to all metal atoms of a macrocycle was found in the solid state by single crystal X-ray diffraction. There are no shortened contacts of the metal in the macrocycles with π-electron system of the ferrocene's cyclopentadienyl ligands in all complexes. The complexes of (ML)3 with 1 have 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 composition and bipyramidal structure whereas 2 forms the 1
2 composition and bipyramidal structure whereas 2 forms the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with (AgL)3. The latter is packed in the infinite stacks involving additional contacts with Ph groups.
1 complex with (AgL)3. The latter is packed in the infinite stacks involving additional contacts with Ph groups.
Introduction
The fluoro-substituted pyrazolates or imidazoles (ML)3, M = Cu, Ag, Au, were actively studied as compounds possessing significant luminescent and π-acidic properties.1–3 The ability to form complexes with organic arenes as bases was shown.3–5 These adducts form structures of the general formula {[(ML)3]m·Arn}∞ (m, n = 1, 2).4,5 In our previous work we have shown that in the complexes of (ML)3 (M = Cu, Ag) with iron compounds such as (COT)Fe(CO)3 (COT = cyclooctatetraene), Cp2Fe, Fe(CO)5 in solid state and in solution only π-electron system (COT or Cp ligand) is involved in the complex formation.6 There is no coordination of the macrocycles with (Fe)–CO group of (COT)Fe(CO)3 as well as no complexation was found with Fe(CO)5. At the same time in the solid phase ferrocene behaves similar to mesitylene4 and forms infinite stacks {[(ML)3]·[Cp2Fe]}∞ with macrocycles.6 Our recent study of (ML)3 interaction with butanone-2 as the example of aliphatic ketones and benzophenone as a representative of aromatic ketones evidenced that the oxygen atom of CO group is a sole site of coordination for all complexes in solution.7 X-Ray data demonstrated various types of composition and structure in the solid state for the complexes of (ML)3 with Ph2CO among which there are sandwiches with additional contacts to phenyl ring,6 but the main site of coordination is again the oxygen atom.The aim of this work was to reveal the site competition in the substituted ferrocenes (acetylferrocene (1) and (phenylacetyl)ferrocene (2)) interacting with copper(I) and silver(I) trinuclear pyrazolates in solution and in the solid state.
Experimental
General
The macrocycles ([3,5-(CF3)2Pz]Ag)3 and ([3,5-(CF3)2Pz]Cu)3 were synthesized according to the published procedure.8 IR spectra of the compounds in CH2Cl2 and hexane solutions were measured on Nicolet 6700 FTIR spectrometer in CaF2 cells (d = 0.04–0.4 cm), using a home modified cryostat (Carl Zeiss Jena) for variable temperature measurements. The accuracy of the temperature adjustment was ±1 K. IR studies in the ν(CO) region (1690–1610 cm−1) were carried out at various concentrations (10−3 to 10−2 M) and ratios of the reagents. The composition of the complexes in solution was determined by the saturation and the continuous variation (Job) methods. Room and low temperature NMR measurements were carried out on Bruker Avance 600 spectrometer operating at 600.22 MHz (1H) and 150.93 MHz (13C{1H}). The temperature was controlled using Bruker BVT-3000 accessory; the accuracy of the temperature adjustment and stability was ±1 K. The spectra were calibrated with the residual solvent resonance relative to TMS (1H, 13C). UV-vis spectra were measured on Cary50 spectrometer using a Carl Zeiss Jena cryostat for variable temperature measurements.Isolation of crystals
The crystals obtained this way were suitable for X-ray analysis.
X-ray studies
Single-crystal X-ray diffraction study was carried out with a Bruker SMART APEX II diffractometer (graphite monochromated Mo-Kα radiation, λ = 0.71073 Å, ω-scan technique, T = 100 K). The APEX II software9 was used for collecting frames of data, indexing reflections, determination of lattice constants, integration of intensities of reflections, scaling and absorption correction, and SHELXTL10 was used for space group and structure determination, refinements, graphics, and structure reporting. The structures were solved by direct methods and refined by the full-matrix least-squares technique against F2 with the anisotropic thermal parameters for all non-hydrogen atoms. The H(C) atoms were placed geometrically and included in the structure factors calculation in the riding motion approximation. In the crystal of {[([3,5-(CF3)2Pz]Cu)3]·2[1]}, one of six CF3 groups is disordered over two positions with 0.65/0.35 occupancies. The principal experimental and crystallographic parameters are listed in Table 1.| Compound | {[([3,5-(CF3)2Pz]Cu)3]·2[1]} | {[([3,5-(CF3)2Pz]Ag)3]·2[1]} | {[([3,5-(CF3)2Pz]Ag)3]·[2]} |
|---|---|---|---|
| a R1 = ∑∣∣Fo∣ − ∣Fc∣∣/∑∣Fo∣.b wR2 = {∑[w(Fo2 − Fc2)2]/∑w(Fo2)2}1/2. | |||
| Molecular formula | C39H27F18N6O2Fe2Cu3 | C39H27F18N6O2Fe2Ag3 | C33H19F18N6OFeAg3 |
| Formula weight | 1255.99 | 1388.98 | 1237.00 |
| Dimension, mm | 0.24 × 0.21 × 0.09 | 0.22 × 0.06 × 0.05 | 0.17 × 0.14 × 0.08 |
| Crystal system | Orthorhombic | Monoclinic | Orthorhombic |
| Space group | Pbca | C2/c | Pna21 |
| a, Å | 11.5920(11) | 19.8409(8) | 14.9390(5) |
| b, Å | 16.9249(16) | 13.5269(5) | 24.6385(8) |
| c, Å | 44.395(4) | 17.6393(7) | 10.4127(3) |
| α, deg. | 90.00 | 90.00 | 90.00 |
| β, deg. | 90.00 | 108.393(1) | 90.00 |
| γ, deg. | 90.00 | 90.00 | 90.00 |
| V, Å3 | 8710.0(14) | 4492.3(3) | 3832.6(2) |
| Z | 8 | 4 | 4 |
| ρcalc, g cm−3 | 1.916 | 2.054 | 2.144 |
| Linear absorp. (μ), cm−1 | 22.13 | 20.33 | 20.08 |
| Tmin/Tmax | 0.642/0.826 | 0.713/0.905 | 0.727/0.856 |
| 2θmax, deg. | 54 | 60 | 60 |
| No. unique refl. (Rint) | 9481 (0.0892) | 6544 (0.0420) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112(0.0587) 112(0.0587) |
| No. obs. refl. (I > 2σ(I)) | 6653 | 5349 | 9542 |
| No. parameters | 642 | 318 | 559 |
| R1 (on F for obs. refl.)a | 0.0493 | 0.0291 | 0.0318 |
| wR2 (on F2 for all refl.)b | 0.1103 | 0.0672 | 0.0567 |
| GOOF | 1.052 | 1.021 | 1.003 |
Physical measurements
Steady-state luminescence and excitation spectra were recorded on Fluorolog FL 3-22 Horiba-Jobin-Yvon photon counting emission spectrometer equipped with a 450 W xenon source and double monochromators for excitation and emission. The luminescence spectra observed were corrected for the nonlinear response of the instrument using predetermined factors. The crystals for these measurements were packed in quartz capillaries. Phosphorescence lifetimes (τ) were averaged for at least three independent measurements, monitoring the decay at the maxima of the emission spectra. The single decays were analyzed with Origin 7.0 software.Results and discussion
(a) Complex formation in solution
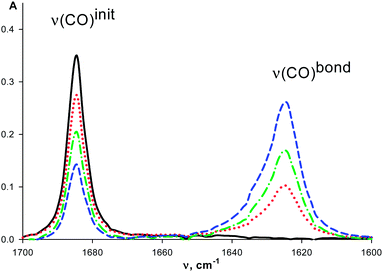
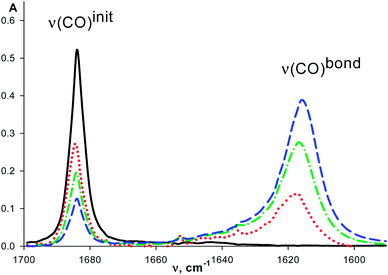
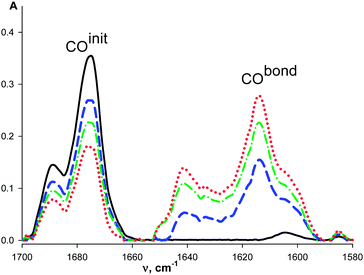
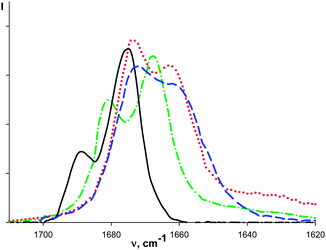
The intensity of ν(CO)init bands decreases and the intensity of ν(CO)bond bands increases with the macrocycle concentration (Fig. 1) similarly to that observed for organic ketones upon complexation with these macrocycles.7 The redistribution of ν(CO)init–ν(CO)bond intensities is observed with the temperature change (see for example, Fig. 2). It is caused by the shift of the complex formation equilibrium to the right upon cooling. Moreover, these changes are reversible.
Variable temperature 1H and 13C NMR spectra of FcC(O)CH3–(AgL)3 mixture in CD2Cl2 confirm formation of the complex with the CO group as the site of coordination. 13C NMR resonance of the carbon atom in CO group (δ 201.41 ppm) undergoes the low field shift (Δδ = 0.91 ppm) upon addition of the equimolar amount of macrocycle at room temperature, the value of the shift (Δδ) increases upon cooling to 1.63 ppm at 213 K. The larger shifts at low temperatures are due to the increase of the formation constants relative to those at 297 K. The 13C signals of other carbon atoms are less sensitive (Δδ ≤ 0.45 ppm) even at low temperatures. The 1H resonances of all the protons of 1 undergo small high-field shift (Δδ ≤ 0.12 ppm) in the presence of (AgL)3 at 213 K. At room temperature no changes in the 1H NMR spectra were observed.
 | ||
| Fig. 3 IR spectra FcC(O)CH2Ph in different solvents: hexane (solid line) THF (dash-dotted line), CH2Cl2 (dash line), CH3CN (dotted line), at room temperature. | ||
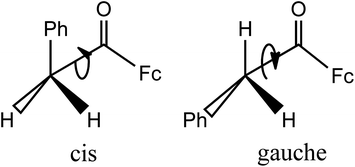
The relative intensities of the ν(CO) bands depend on the temperature: the lower frequency band becomes more intense upon cooling. These features appear to be similar to those of the substituted acetophenones: Ph–C(O)–CH2–X (X = Ph, Hal and CN), evidencing coexistence of the cis- and gauche-conformations in solution due to the hindered rotation around the C(O)–CH2X bond (Scheme 1).14
The authors14 have attributed the high frequency band to the cis-conformer, confirming assignment of Bellamy et al. (made at the examples of α-halogenated cyclohexanones15) where the cis-form was considered as thermodynamically more stable. So, the character of conformational equilibrium is the same in phenylacetophenone14 and (phenylacetyl)ferrocene.
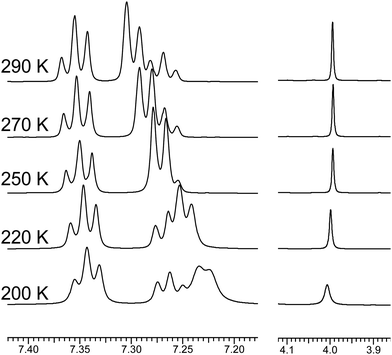
The dynamic behavior of the 1H and 13C NMR resonances of the interacting groups of 2 (in CD2Cl2) also confirms the idea about hindered rotation. Thus, the doublet of the phenyl o-protons in 1H NMR shifts from 7.30 ppm to 7.23 ppm upon cooling. In contrast the resonances of p-, m-protons and of CH2 group exhibit the negligible shift (Fig. 4). The 13C NMR signal of CO group of 2 depends slightly on temperature being 201.80 ppm at 290 K and 201.92 ppm at 200 K (Δδ = 0.12 ppm) whereas the corresponding acetylferrocene signal is substantially more sensitive to cooling (Δδ = 1.51 ppm). The competition between the effects of hindered rotation inducing significant up-field shift and of temperature change leading to down-field shift could explain abnormally small temperature dependence of the 13C(O) resonance of 2.
Significant up-field shift upon the temperature change from 290 to 200 K is observed for the 13C NMR resonance of CH2 group carbon and C-1 atom of the phenyl ring (from 47.10 to 46.15 ppm, Δδ = 0.95 ppm; and from 136.02 to 134.95 ppm, Δδ = 1.07 ppm, respectively). Smaller but again up-field shifts of the other Ph ring carbon atoms resonances were observed (Δδ = 0.65–0.46 ppm). We believe all these changes evidence hindered rotation around CH2–Ph bond slowed at the NMR time scale by the temperature decrease.
Low temperature 13C NMR spectra in CD2Cl2 support the formation of complex: the signal of CO group (δ = 201.91 ppm at 213 K) shifts significantly to low field upon addition of the equimolar amount of (AgL)3 (Δδ = 1.98 ppm), while other 13C signals exhibit the small high-field shifts (Δδ ≤ 0.4 ppm). Under these conditions the 1H spectra reveal very small low-field shifts (Δδ ≤ 0.1 ppm).
Thus, all the IR and NMR data confirm interaction between CO group of (phenylacetyl)ferrocene 2 and the macrocycle as in the case of acetylferrocene 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, i.e. one molecule of the ketone per one molecule of (ML)3, are formed at molar ratios from 7
1, i.e. one molecule of the ketone per one molecule of (ML)3, are formed at molar ratios from 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 for all four ketone–macrocycle combinations (see for example, Fig. 6).
7 for all four ketone–macrocycle combinations (see for example, Fig. 6).
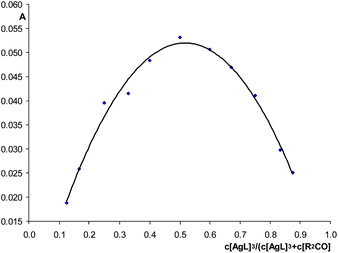 | ||
| Fig. 6 The Job's plot: dependence of the ν(CO)bond band intensity of {[(AgL)3]·[1]} (1624 cm−1) on the composition of the isomolar solution of 1 and (AgL)3, hexane. | ||
The complex formation constants at different temperatures were determined from the ν(CO)free bands intensity changes; the enthalpy and entropy values of the complexes {[(ML)3]·[1]} in hexane were calculated by the Van't Hoff method.
Very poor solubility of 2 in hexane at low temperatures and small intensity ν(CO)free of (phenylacetyl)ferrocene comparing to the acetylferrocene prevented the use of the IR spectra for the determination of the thermodynamic parameters for {[(ML)3]·[2]} complexes formation. So, we used UV-vis spectroscopy, that allowed to measure the spectra at smaller concentrations (c0 = 0.002 M) without precipitation at low temperatures (<270 K).
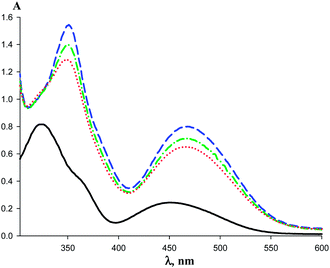
UV-vis spectra of (phenylacetyl)ferrocene in hexane feature two bands: at 450 nm (ε = 305 l mol−1 cm−1) and at 322 nm (ε = 1020 l (mol−1 cm−1)). The broad band with maximum at 450 nm is assigned to bathochromically shifted (by 9 nm) transition of the ferrocene which intensity is increased (cf. εferrocene = 87 l (mol−1 cm−1)) due to the mixing of charge transfer and the ligand field transition as in acylferrocenes.16–18 The band at 322 nm belongs to MLCT transition with a charge transfer from Cp to CO-substituent which stabilizes the resulting exited states.16 The inflection at 384 nm caused by conjugation between the orbitals of the cyclopentadienyl ring and the adjacent carbonyl.17 The UV bands of acetylferrocene have the same structure (447 nm; ε = 302 l (mol−1 cm−1) and 319 nm; ε = 1144 l (mol−1 cm−1)).
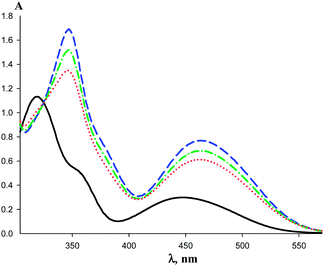
Addition of the macrocycle (AgL)3 to the solution of 2 in hexane induces the noticeable bathochromic shift and the intensity increase of the initial ferrocenyl ketone bands. The bands intensities grow further upon cooling (Fig. 7). The similar spectral changes are observed for 1 in the presence of (AgL)3 (Fig. 8). The band maximum of the ferrocene transition in the complex was chosen as the analytical point for determination of the formation constants (463 nm for {[(AgL)3]·[1]} and 465 nm for {[(AgL)3]·[2]}). Unfortunately, the shifted band of {[(AgL)3]·[R2CO]} complex is overlapping with the initial band of ferrocenyl ketone 1 or 2 (Fig. 7) and this necessitated the evaluation of the need to take into account an impact of the non-bonded ferrocenyl ketone absorbance in the intensity of the band of the complex. To solve this problem we studied the UV-vis spectra of 1 complexation with (AgL)3, for which the thermodynamic data were determined by IR.
Employment of formation constants of {[(AgL)3]·[1]} derived from IR measurements at 290 K allowed us to calculate the equilibrium concentrations for both forms of the ketone (bonded and non-bonded) and the extinction coefficients for their UV-vis bands (see ESI† for procedure details). This way the formation constants at different temperatures were determined taking into account the impact of the non-bonded ketone absorption and using the maximum of the band as the analytical point. The comparison of the linear dependences of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kform on 1/T derived from the UV-vis and IR data (Fig. 9) shows that the Van't Hoff plots have close slopes. Consequently the difference in thermodynamic characteristics of {[(AgL)3]·[1]} formation is very small (ΔH° = −10.4 ± 0.4 kcal mol−1 and ΔS° = −22 ± 3 e.u. from UV-vis data and ΔH° = −10.9 ± 0.3 kcal mol−1 and ΔS° = −24 ± 3 e.u. determined by IR), being in the limits of the experimental error.
Kform on 1/T derived from the UV-vis and IR data (Fig. 9) shows that the Van't Hoff plots have close slopes. Consequently the difference in thermodynamic characteristics of {[(AgL)3]·[1]} formation is very small (ΔH° = −10.4 ± 0.4 kcal mol−1 and ΔS° = −22 ± 3 e.u. from UV-vis data and ΔH° = −10.9 ± 0.3 kcal mol−1 and ΔS° = −24 ± 3 e.u. determined by IR), being in the limits of the experimental error.
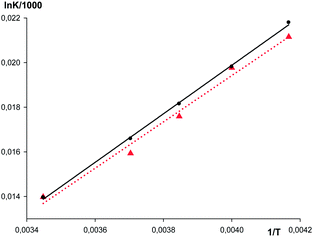 | ||
Fig. 9 Van't-Hoff plots (dependence R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K/1000 on 1/T) for {[(AgL)3]·[1]} from IR (solid) and from UV-vis (dotted) experiments in hexane. K/1000 on 1/T) for {[(AgL)3]·[1]} from IR (solid) and from UV-vis (dotted) experiments in hexane. | ||
Thus we calculated the thermodynamic parameters of {[(AgL)3]·[2]} using this approach: Kform (290 K) was determined from the IR spectra and those at lower temperatures – from UV-vis spectra.
All determined thermodynamic parameters of complexes are gathered in Table 2.
| Kform (290 K), l/mol × 10−3 | Kform (270 K), l/mol × 10−3 | Kform (250 K), l/mol × 10−3 | −ΔH°, kcal mol−1 | −ΔS°, cal (mol−1 K−1) | |
|---|---|---|---|---|---|
| {[(CuL)3]·[1]} | 0.2 | 0.6 | 1.5 | 7.8 ± 0.3 | 16 ± 2 |
| {[(AgL)3]·[1]} | 1.1 | 4.2 | 21.4 | 10.9 ± 0.3 | 24 ± 3 |
| {[(AgL)3]·[2]} | 0.7 | 1.3 | 5.4 | 7.3 ± 0.1 | 12.5 ± 0.5 |
Formation constants of complexes with acetylferrocene are 1.5–4 times higher than those of (phenylacetyl)ferrocene, this difference is larger at low temperatures which leads to the relatively large entropy effect in the case of [(ML)3]·[1] complexes. The complex of 2 with (AgL)3 is significantly weaker (has smaller ΔH°) in comparison with {[(AgL)3]·[1]} that is in line with the electron withdrawing effect of CH2Ph group in comparison with the CH3 group.
Formation constants of copper complexes are an order of magnitude lower than those of silver complexes for 1 at all temperatures as well as room temperature constants for 2 (Kform (290 K) = 0.1 × 103 l mol−1 for {[(CuL)3]·[2]} and 0.7 × 103 l mol−1 for {[(AgL)3]·[2]}). Thus, complexes with Ag-macrocycles are significantly more stable than those with (CuL)3. This trend is qualitatively the same as for the complexes with organic ketones7 but opposite to the complexes of these macrocycles with π-electron ligands.6
(b) Complexes in the solid state
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, that is two molecules of 1 per one molecule of macrocycle differing from that in solution (1
1, that is two molecules of 1 per one molecule of macrocycle differing from that in solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and feature bipyramidal structure. Notable the 2
1) and feature bipyramidal structure. Notable the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes are obtained from solutions of both 1
1 complexes are obtained from solutions of both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
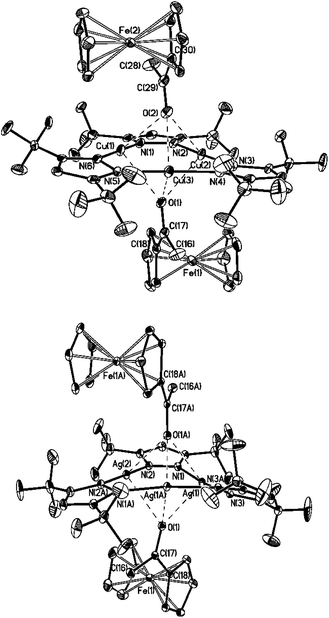
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the reactants. The structure is characterized by triple coordination of oxygen atom to each metal atom of the macrocycle (Fig. 10). No observable contacts between Fc-fragments and the macrocyclic molecule were found in the complexes. All significant interatomic distances are presented in Table 3.
1 molar ratio of the reactants. The structure is characterized by triple coordination of oxygen atom to each metal atom of the macrocycle (Fig. 10). No observable contacts between Fc-fragments and the macrocyclic molecule were found in the complexes. All significant interatomic distances are presented in Table 3.
| {[([3,5-(CF3)2Pz]Cu)3]·2[1]} | |||
|---|---|---|---|
| a The atoms labeled with A were generated with symmetry transformation of −x + 1, y, −z + 1.5. | |||
| Cu(1)–O(1) | 2.643(3) | Cu(1)–O(2) | 2.599(3) |
| Cu(2)–O(1) | 2.567(3) | Cu(2)–O(2) | 2.665(3) |
| Cu(3)–O(1) | 2.587(3) | Cu(3)–O(2) | 2.624(3) |
| Cu(1)–N(1) | 1.885(4) | O(1)–C(17) | 1.238(5) |
| Cu(1)–N(6) | 1.891(3) | C(16)–C(17) | 1.499(6) |
| Cu(2)–N(2) | 1.883(3) | C(17)–C(18) | 1.456(6) |
| Cu(2)–N(3) | 1.879(3) | O(2)–C(29) | 1.241(5) |
| Cu(3)–N(4) | 1.891(3) | C(28)–C(29) | 1.488(6) |
| Cu(3)–N(5) | 1.880(3) | C(29)–C(30) | 1.457(7) |
| N(1)–Cu(1)–N(6) | 176.8(2) | C(18)–C(17)–C(16) | 118.2(4) |
| N(3)–Cu(2)–N(2) | 177.5(2) | O(2)–C(29)–C(30) | 120.9(4) |
| N(5)–Cu(3)–N(4) | 176.8(2) | O(2)–C(29)–C(28) | 120.8(5) |
| O(1)–C(17)–C(18) | 121.0(4) | C(30)–C(29)–C(28) | 118.3(4) |
| O(1)–C(17)–C(16) | 120.7(4) | ||
| {[([3,5-(CF3)2Pz]Ag)3]·2[1]}a | |||
|---|---|---|---|
| Ag(1)–O(1) | 2.615(2) | Ag(2)–O(1) | 2.675(2) |
| Ag(1A)–O(1) | 2.763(2) | ||
| Ag(1)–N(1) | 2.136(2) | O(1)–C(17) | 1.233(3) |
| Ag(1)–N(3) | 2.147(2) | C(16)–C(17) | 1.500(3) |
| Ag(2)–N(2) | 2.115(2) | C(17)–C(18) | 1.461(3) |
| N(1)–Ag(1)–N(3) | 174.25(7) | O(1)–C(17)–C(18) | 120.6(2) |
| N(2)–Ag(2)–N(2A) | 179.07(11) | O(1)–C(17)–C(16) | 120.6(2) |
| C(18)–C(17)–C(16) | 118.8(2) | ||
There is an appreciable difference in the structure of the Cu and Ag containing complexes in spite their general similarity. The two crystallographically independent molecules of acetylferrocene in the complex {[(CuL)3]·2[1]} are disposed symmetrically relative to the macrocycle plane and are related by the pseudocenter of symmetry. The macrocycle has near to planar structure (maximum deviation from the median plane equals to 0.08 Å for the N(5) atom). All six independent distances Cu⋯O are in the narrow range 2.567(3)–2.665(3) Å (mean value 2.61 Å), that is less than the formal sum of the van der Waals radii for Cu⋯O (2.92 Å).19 These distances are appreciably shorter than those observed previously in the crystal of the complex of (CuL)3 with benzophenone (Ph2CO).7 The latter is composed of two molecules of (CuL)3 per one molecule of ketone. Its wedge-shaped sandwich structure is formed by coordination of the oxygen atom of the CO group to only one copper atom in both macrocycles [Cu⋯O 2.879(4) Å]. The lengths of the Cu⋯O contacts in the complex {[(CuL)3]·2[1]} correspond to the weak coordination found between the two coordinated copper(I) atoms and oxygen containing anions (perchlorate, sulfate, nitrate, triflate and others −2.50 to 2.75 Å).20–23
The complex {[(AgL)3]·2[1]} occupies a special position in the crystal on the twofold axis passing through the Ag(2) and the middle point of N(3)–N(3A) bond, see Fig. 10. Despite the presence of a crystallographic symmetry this complex has less symmetric structure than it's “copper” analogue. The macrocycle is bent (maximal deviation from the medium plane is 0.24 Å for the N(3) atom). The lengths of Ag⋯O interactions (2.615(2)–2.763(2) Å, mean value 2.68 Å) are, just as expected, larger than those in the copper analogue, but significantly shorter than the sum of the van der Waals radii for Ag⋯O (3.42 Å).19 Note that such multicenter coordination was observed for the complex {[(AgL)3]·[Ph2CO]}, however the Ag⋯O distances were longer (2.768(3)–2.952(4) Å).7
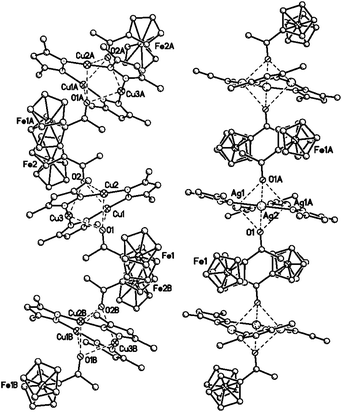 | ||
| Fig. 11 Fragments of polymer chains in the crystals of {[([3,5-(CF3)2Pz]Cu)3]·2[1]} (left) and {[([3,5-(CF3)2Pz]Ag)3]·2[1]} (right). The H and F atoms are omitted for clarity. | ||
Besides the mentioned difference in molecular structure the association of complexes {[(CuL)3]·2[1]} and {[(AgL)3]·2[1]} in crystals is also significantly different. It is possible to identify a common motif of their crystal structures corresponding to 1D polymeric stack in which the complexes are integrated by stacking interactions between the acetylferrocene molecules (Fig. 11). However in the case of “copper” complex the stacking interaction is implemented through the ten carbon atoms of the two substituted Cp rings of the crystallographically independent FcC(O)CH3 molecules of the neighboring complexes (the distances C⋯C0.5−x, 0.5+y, z = 3.388(6)–3.602(6) Å, the distance between the planes of the adjacent Cp rings is 3.45 Å, the distance between their centroids is 3.47 Å, the dihedral angle is 0.0°). In “silver” complex the stacking interaction is implemented through the carbon atom of the acetyl group and the substituted Cp fragment of the adjacent molecule of 1 (the distance C(17)⋯C(22)1−x, −y, 1−z is 3.241(3) Å, the distance between the planes of the adjacent Cp rings is 3.05 Å, the distance between their centroids −4.75 Å, the dihedral angle is equal to 0.0°). In both cases the pairs of approaching each other acetylferrocene molecules are sandwiched between the two macrocyclic molecules, which planes are parallel to each other in the crystal of the “silver” complex and inclined with dihedral angle of 41.4° in the crystal of the “copper” complex.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition in the solid state and the pyramidal structure (Fig. 12), as in the case of the complex of (AgL)3 with Ph2CO.7 The complex is formed by cooperative interaction of oxygen atom with three metal atoms of the macrocycle. All Ag⋯O distances (2.676(2)–2.908(2) Å) (Table 4) are appreciably shorter than the formal sum of the van der Walls radii. These values are close to those obtained for the pyramidal 1
1 composition in the solid state and the pyramidal structure (Fig. 12), as in the case of the complex of (AgL)3 with Ph2CO.7 The complex is formed by cooperative interaction of oxygen atom with three metal atoms of the macrocycle. All Ag⋯O distances (2.676(2)–2.908(2) Å) (Table 4) are appreciably shorter than the formal sum of the van der Walls radii. These values are close to those obtained for the pyramidal 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Ph2CO, but longer than the Ag⋯O distances in the bipyramidal complex with acetylferrocene. As in the case of complex {[(AgL)3]·2[1]} the macrocycle is noticeably bent in {[(AgL)3]·[2]} (maximal deviation from the plane passing through silver and nitrogen atoms is 0.27 Å for the N(5) atom).
1 complex with Ph2CO, but longer than the Ag⋯O distances in the bipyramidal complex with acetylferrocene. As in the case of complex {[(AgL)3]·2[1]} the macrocycle is noticeably bent in {[(AgL)3]·[2]} (maximal deviation from the plane passing through silver and nitrogen atoms is 0.27 Å for the N(5) atom).
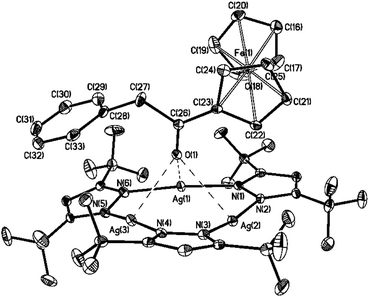 | ||
| Fig. 12 Molecular structure of complex {[([3,5-(CF3)2Pz]Ag)3]·[2]} in the crystal. The hydrogen atoms are omitted for clarity; thermal ellipsoids are drawn at the 30% probability level. | ||
| a The atoms labeled with A were generated with symmetry transformation of −0.5 + x, 1.5 − y, z. | |||
|---|---|---|---|
| Ag(1)–O(1) | 2.803(2) | C(29)–Ag(3A)a | 3.367(4) |
| Ag(2)–O(1) | 2.908(2) | C(31)–Ag(2A)a | 3.417(4) |
| Ag(3)–O(1) | 2.676(2) | C(32)–Ag(2A)a | 3.056(4) |
| C(33)–Ag(2A)a | 3.187(4) | ||
| Ag(1)–N(1) | 2.091(3) | Ag(1)–N(6) | 2.087(3) |
| Ag(2)–N(2) | 2.133(3) | O(1)–C(26) | 1.227(4) |
| Ag(2)–N(3) | 2.129(3) | C(23)–C(26) | 1.452(5) |
| Ag(3)–N(4) | 2.111(3) | C(26)–C(27) | 1.521(5) |
| Ag(3)–N(5) | 2.117(3) | ||
| N(6)–Ag(1)–N(1) | 174.4(1) | O(1)–C(26)–C(23) | 122.7(3) |
| N(3)–Ag(2)–N(2) | 169.8(1) | O(1)–C(26)–C(27) | 121.1(3) |
| N(4)–Ag(3)–N(5) | 175.9(1) | C(23)–C(26)–C(27) | 116.2(3) |
The presence of the additional coordination site in (phenylacetyl)ferrocene (the phenyl substituent) in comparison to acetylferrocene leads to a significant difference in the crystal structure of their complexes. The infinite stacks of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition are formed by {[(AgL)3]·[2]} via alternating the macrocycle and (phenylacetyl)ferrocene molecules (Fig. 13). Each (phenylacetyl)ferrocene molecule is bonded with the two neighboring macrocycles in the stack by different ways: in addition to the multicentered coordination of the oxygen atom of CO-group to three Ag atoms of one macrocycle there is the Ag⋯π interaction of the phenyl group with the second macrocycle (Ag⋯C distances are 3.056(4)–3.417(4) Å; the phenyl plane is parallel to the plane of this macrocycle with the angle between them 1.7°). Cp ligands do not participate in the intermolecular interactions with macrocycles. The planes of two macrocycles form the angle 23.0°.
1 composition are formed by {[(AgL)3]·[2]} via alternating the macrocycle and (phenylacetyl)ferrocene molecules (Fig. 13). Each (phenylacetyl)ferrocene molecule is bonded with the two neighboring macrocycles in the stack by different ways: in addition to the multicentered coordination of the oxygen atom of CO-group to three Ag atoms of one macrocycle there is the Ag⋯π interaction of the phenyl group with the second macrocycle (Ag⋯C distances are 3.056(4)–3.417(4) Å; the phenyl plane is parallel to the plane of this macrocycle with the angle between them 1.7°). Cp ligands do not participate in the intermolecular interactions with macrocycles. The planes of two macrocycles form the angle 23.0°.
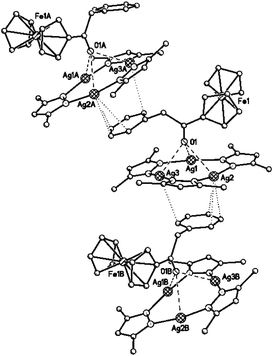 | ||
| Fig. 13 Fragment of infinite chain in the crystal of {[([3,5-(CF3)2Pz]Ag)3]·[2]}. The H and F atoms are omitted for clarity. | ||
Note, the ferrocenyl fragments do not participate in intermolecular interactions with macrocycles in all three structures with 1 and 2. In addition, no metallophilic interactions are observed which are typical for the crystal structures of the macrocycles containing d10 metals and observed in all structures of the complexes of (CuL)3 and (AgL)3 with Ph2CO.7
In the case of {[(AgL)3]·[2]} complex it is difficult to discriminate which of the two types of interactions between 2 and (AgL)3 (cooperative interaction of the carbonyl group oxygen atom with Ag atoms or Ag⋯π interaction with phenyl substituent) should be considered as the key interaction (stronger and structure forming). On the one hand, the Ag⋯O distances are significantly shorter than Ag⋯C(Ph) contacts and the spectral data pointed to involvement of the carbonyl group in the interaction in solution. On the other hand, maximal distortion of the linear coordination of the metal atoms is observed for Ag(2) atom (N(3)–Ag(2)–N(2) angle is 169.8(1)°) whose participation in the interaction with π-system of the Ph substituent is dominant.
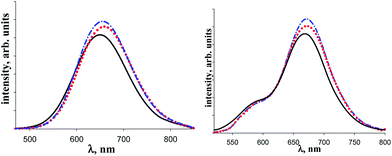
The obtained photophysical data for the initial macrocycle (CuL)3 and complexes {[(CuL)3]·[1]} and {[(CuL)3]·[2]} are provided in Table 5 and photoluminescence spectra are presented in Fig. 14. Notice, that λemmax positions and the life time (τ) values obtained for (CuL)3 are in line with the literature data.1 The complexation of (CuL)3 with 1 and 2 leads to rather small bathochromic λemmax shifts (3–12 cm−1) and slight increase of the τ values. The determined lifetime values are in the range of 45–77 μsec that corresponds to the phosphorescence. Thermochromism is evident from the observed lifetime increase upon cooling (Table 5). All characteristics increase in the same sequence as the strength of the complexes: {[(CuL)3]·[2]} < {[(CuL)3]·[1]}.
Thus, the luminescent properties are strongly dependent on the metal, being quenched in the case of complexes of 1 and 2 with Ag containing macrocycle, and appearing as bright red phosphorescence with Cu analogue. This fact calls for the future study with DFT calculations support and a larger series of complexes.
Conclusions
The results of the (AgL)3 and (CuL)3 complexation with carbonyl substituted ferrocenes and comparison with the previously obtained data on the complexes of these macrocycles with organic ketones allow to draw the first conclusions about the general features of the structure, the sites of coordination and the strength of the complexes. The oxygen atom of the CO group is the site of the ketone coordination in all these compounds both in solution and in the solid state. This is the first case when the functional group competes successfully with π-system of organic and organometallic compounds. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition was established for all complexes in solution. The strength (formation constants and enthalpies) of the complexes decreases in the sequence: FcC(O)CH3 > FcC(O)CH2Ph > MeC(O)Et ≥ Ph2CO. The silver complexes are stronger Lewis acids than the copper ones in the interaction with the same base. Importantly, the luminescent properties of ([3,5-(CF3)2Pz]M)3 (M = Ag, Cu) bonded with acetylylferrocene and (phenylacetyl)ferrocene are strongly dependent on the metal and show the bright red phosphorescence in the case of Cu.
1 composition was established for all complexes in solution. The strength (formation constants and enthalpies) of the complexes decreases in the sequence: FcC(O)CH3 > FcC(O)CH2Ph > MeC(O)Et ≥ Ph2CO. The silver complexes are stronger Lewis acids than the copper ones in the interaction with the same base. Importantly, the luminescent properties of ([3,5-(CF3)2Pz]M)3 (M = Ag, Cu) bonded with acetylylferrocene and (phenylacetyl)ferrocene are strongly dependent on the metal and show the bright red phosphorescence in the case of Cu.
The composition and the structure vary in the solid state, however the main feature of ketone-macrocycle complexes – CO group as a primary coordination site – is preserved. In all cases the interactions with the π-density of the ferrocene Cp rings are absent. The structural diversity of the complexes is based on the macrocycles aptitude to coordinate the external guest molecules at both sides of the macrocycle. Obviously when the guest molecule does not possess several coordination sites (e.g. (COT)Fe(CO)3,6 FcC(O)CH3), the saturation of (ML)3 sites is achieved in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes (two guest molecules per one (ML)3), while formally 1
2 complexes (two guest molecules per one (ML)3), while formally 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes turned to have the composition 2
1 complexes turned to have the composition 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and are packed as dimers due to argentophilic interactions (e.g. [(AgL)3]·[Ph2CO]).7 When the guest molecule provides the possibility to coordinate two macrocycles yielding infinite stacks of 1
2 and are packed as dimers due to argentophilic interactions (e.g. [(AgL)3]·[Ph2CO]).7 When the guest molecule provides the possibility to coordinate two macrocycles yielding infinite stacks of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 composition as in the case of complexes with 2, ferrocene6 or mesitylene.4
1 composition as in the case of complexes with 2, ferrocene6 or mesitylene.4
Acknowledgements
Authors thank Dr L. Puntus and Prof. N. Belkova for helpful discussions. This work was supported by the Russian Foundation for Basic Research (projects 12-03-00872 and 13-03-01176), the Division of Chemistry and Material Sciences of RAS.Notes and references
- M. A. Omary, M. A. Rawashdeh-Omary, M. W. A. Gonser, O. Elbjeirami, T. Grimes and T. R. Cundari, Inorg. Chem., 2005, 44, 8200 CrossRef CAS PubMed.
- M. A. Omary, A. A. Mohamed, M. A. Rawashdeh-Omary and J. P. Fackler, Coord. Chem. Rev., 2005, 249, 1372 CrossRef CAS PubMed.
- M. Kleinwächter, L. Vendier, C. Dinoi and M. Etienne, Dalton Trans., 2013, 42, 10102 RSC.
- H. V. R. Dias and C. S. P. Gamage, Angew. Chem., Int. Ed., 2007, 46, 2192 CrossRef CAS PubMed.
- M. A. Omary, O. Elbjeirami and H. V. R. Dias, Inorg. Chem., 2009, 48, 1784 CrossRef CAS PubMed.
- V. N. Tsupreva, A. A. Titov, O. A. Filippov, A. N. Bilyachenko, A. F. Smol'yakov, F. M. Dolgushin, I. A. Godovikov, D. V. Agapkin, L. M. Epstein and E. S. Shubina, Inorg. Chem., 2011, 50, 3325 CrossRef CAS PubMed.
- A. A. Titov, O. A. Filippov, A. N. Bilyachenko, A. F. Smol'yakov, F. M. Dolgushin, V. K. Belsky, I. A. Godovikov, L. M. Epstein and E. S. Shubina, Eur. J. Inorg. Chem., 2012, 5554 CrossRef CAS.
- H. V. R. Dias, S. A. Polach and Z. J. Wang, Fluorine Chem., 2000, 103, 163 CrossRef CAS.
- APEX II software package, Bruker AXS Inc., 5465, East Cheryl Parkway, Madison, WI 5317, 2005 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- N. Sugiyama, H. Suzuki, Y. Shiora and T. Teitei, Bull. Chem. Soc. Jpn., 1962, 35, 767 CrossRef CAS.
- J. J. Li, W. K. Su, J. D. Lin, M. Chen and J. Li, Synth. Commun., 2005, 35, 1929 CrossRef CAS PubMed.
- R. Wang, X. Hong and Z. Shan, Tetrahedron Lett., 2008, 49, 636 CrossRef CAS PubMed.
- R. N. Jones and E. Spinner, Can. J. Chem., 1958, 36, 1020 CrossRef CAS.
- L. J. Bellamy, L. C. Thomas and R. L. Williams, J. Chem. Soc., 1966, 3704 Search PubMed.
- G. B. Zaslavskaya, B. M. Yavorsky, N. S. Kochetkova and H. P. Gambaryanm, DAN, 1968, 179, 589 CAS.
- Y. Yamagushi and Ch. Kutal, Macromolecules, 2000, 33, 1152 CrossRef.
- Y. Yamagushi and Ch. Kutal, Inorg. Chem., 1999, 38, 4861 CrossRef PubMed.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- L. M. Engelhardt, C. Pakawatchai, A. H. White and P. C. Healy, J. Chem. Soc., Dalton Trans., 1985, 117 RSC.
- M. Munakata, H. Haiyang, T. Kuroda-Sowa, M. Maekawa and Y. Suenaga, J. Chem. Soc., Dalton Trans., 1998, 1499 RSC.
- A. Boni, G. Pampaloni, R. Peloso, D. Belletti, C. Graiff and A. Tiripicchio, J. Organomet. Chem., 2006, 691, 5602 CrossRef CAS PubMed.
- M. Munakata, M. Maekawa, S. Kitagawa, M. Adachi and H. Masuda, Inorg. Chim. Acta, 1990, 167, 181 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The IR and NMR spectra of 1, 2 and in presence of macrocycles in hexane, crystallographic information files (CIF) for the complexes {[(CuL)3]·2[1]}, {[(AgL)3]·2[1]} and {[(AgL)3]·[2]}. CCDC 948156–948158. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra47040g |
| This journal is © The Royal Society of Chemistry 2014 |