Designed self-assemblies based on cooperative noncovalent interactions including anion–π, lone-pair electron–π and hydrogen bonding†
Wei Liu,
Qi-Qiang Wang,
Yuanyuan Wang,
Zhi-Tang Huang and
De-Xian Wang*
Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: dxwang@iccas.ac.cn; Tel: +86-10-6279-6761
First published on 23rd January 2014
Abstract
A dual functional tetraoxacalix[2]arene[2]triazine derivative as a building block was synthesized. Designed self-assemblies were obtained under the guidance of multiple noncovalent interactions including anion–π, lone-pair electron–π and hydrogen bonding.
Inspired by the pioneering theoretical reports by Alkorta, Besnard and Dougherty, in which the favorable interactions between electron-deficient aromatic rings and electron donating small molecules were suggested,1 Mascal, Deyà and Alkorta published independently their theoretical studies on the noncovalent interaction between anions and electron-deficient aromatics such as triazine, hexafluorobenzene and perfluoroaromatic compounds.2 Since then anion–π interactions have attracted increasing interest.3 According to the theoretical calculations, four types anion–π interactions, namely typical noncovalent, weak σ, strong σ and C–H⋯X− hydrogen bonding were proposed. The nature, from the theoretical view, of anion–π interaction was ascribed mainly to electrostatic attraction, anion induced polarization, charge-transfer (CT) and dispersion.2b,3c,4,5a In contrast with the largely developed theoretical results, experimental evidences for anion–π interactions based on charge-neutral electron-deficient aromatics are still rare.5 For example, several groups including ourselves demonstrated the crystal structures of both typical noncovalent and weak σ anion–π interactions.5a,c–g,n,o Recent remarkable reports from Dunbar,5g Saha5k–m and Ballester5p et al. revealed experimentally the charge-transfer nature of anion–π interactions. In spite of the increasing experimental evidences of anion–π interaction, however, study of molecular self-assembly with anion–π interaction as a guiding force is very rare.5b,d,e,g,6
As a typical representative of heteracalixaromatics, tetraoxacalix[2]arene[2]triazines have been shown to be unique anion receptors based on anion–π interactions.5d–f,6,7 We have previously demonstrated that tetraoxacalix[2]arene[2]triazine interacted with halides and water in solid state, forming a ternary complex based on anion–π and lone pair electron–π interactions.5d,6 The included halide anion and water molecule interacted to each other through hydrogen bonding. Moreover, two ternary complexes self assembled into cage structures through a hydrogen bond network between two halides and two water molecules. Noteworthy, such cage self-assemblies are discrete due to the binding saturation of water molecule. Our interests on anion–π interaction and anion–π guided supramolecular self-assembly led us to carry out the current study. We envisioned then to introduce hydroxyl groups on the larger rim of benzene rings of tetraoxacalix[2]arene[2]triazine. Such host molecule is dual functional with electron-deficient triazines as π receptors and hydroxyl groups as lone pair electrons and hydrogen bond donors. In the presence of anions, the hydroxyl groups are expected to form ternary complex with anion and the V-shaped cavity of the host molecule instead of water molecule, which would led to the formation of infinite self-assemblies. Reported herein is the synthesis of dihydroxyl-substituted tetraoxacalix[2]arene[2]triazine and the sequential anion guided self-assemblies in solid state.
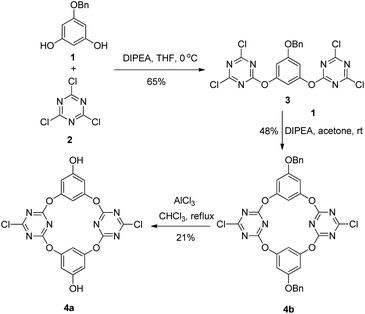
We initiated our synthesis taking benzyl protected phloroglucin 1 and cynuric chloride 2 as the starting materials. In the presence of diisopropylamine (DIPEA), the reaction of 1 and 2 in THF at 0 °C afforded the trimer 3. The macrocyclization reaction of trimer 3 with monomer 1 proceeded smoothly at ambient temperature in acetone to give 4b in 48% yield. The AlCl3-mediated debenzylation of 4b was then performed with CHCl3 as the solvent, affording the larger-rim dihydroxylated tetraoxacalix[2]arene[2]triazine 4a in 21% yield (Scheme 1).
The structures of the synthesized compounds were established on the basis of spectroscopic data and microanalyses (see Fig. S14–19†). From the X-ray molecular structures of 4a, which was shown in Fig. 1, the larger-rim dihydroxylated macrocyclic molecule adopts a 1,3-alternate conformation, similar with other tetraoxacalix[2]arene[2]triazine derivatives.8 Two triazine rings form an electron-deficient V-shaped cavity with the larger-rim distance of the cavity being 9.218 Å (dC1⋯C11). The two benzene rings, meanwhile, construct an electron-rich cavity with the larger-rim distance of the cavity being 5.373 Å (dC6⋯C15). The introduced hydroxyl groups act as hydrogen bond donor and form hydrogen bonds with oxygen of water molecule or nitrogen of triazine rings, leading to linear self-assembly (Fig. S1†).
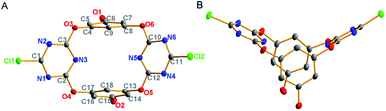 | ||
| Fig. 1 Crystal structure of 4a (A) top view and (B) side view. The ellipsoid probability is 25 percentage. Hydrogens are omitted for clarity. | ||
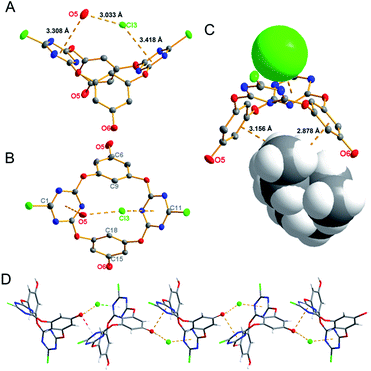
To investigate the anion–π interactions between 4a and anions at molecular level and anion guided self-assemblies, single crystals of the complexes were cultivated through diffusion of ethyl ether into the acetone solution of the host–guest mixtures at room temperature. The crystallography data of the complexes between host 4a and Et4NX (X = Cl−, Br− and NO3−) are listed in Table S1.†
As depicted in Fig. 2 and Fig. S2–S4,† the host molecule 4a forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with Cl−, Br− and NO3−. The anions are included in the V-shaped electron-deficient cavity forming typical anion–π interactions with one of the triazine rings. This was evidenced by the observation of shorter distances between anions and plane of the triazine ring being 3.418 (dCl-plane), 3.486 (dBr-plane) and 2.880 Å (dO7-plane), respectively, than the sums of their van der Waals radius.10 Different interaction modes between anions and 4a in comparison with that between anions and the parent host molecule tetraoxacalix[2]arene[2]triazine were noticeable. In the case of 4a–halide complexes, for instance, one hydroxyl group of another host molecule is included in the cavity, with oxygen atom locating above the other triazine ring and forming short contacts with the plane of triazine ring. Consequently, the deliberately introduced hydroxyl group serves as lone pair electrons and hydrogen bond donor instead of water molecule and form 4a–halide–hydroxyl ternary complexes, which is in contrast to the host–halide–water ternary complex of the parent host molecule.5d In case of Et4N+(4a·NO3−) complex, which is shown in Fig. S4,† nitrate straights up in the cavity with the plane of nitrate orthogonal with one of the triazine rings. Such direction of nitrate in the cavity prevents it from forming typical and weak σ anion–π interactions with both triazine rings, the interaction mode in the complex of nitrate and the parent molecule,5f but renders the formation 4a–NO3−–hydroxyl ternary complex. The location of tetraethylammonium ion in the aforementioned host–guest complexes is interesting. In sharp contrast with the parent host–anion complexes, in which tetraethylammonium ion showed no contact with the macrocyclic receptor, the cation in 4a–anion complexes occupies the electron-rich cavity constructed with the larger rims of benzene rings. The distances of alkyl C–H and planes of benzene rings for the three complexes are in the range of 2.871 to 3.298 Å, suggesting possible cation–π interactions. Noteworthy, in order to accommodate the tetraethylammonium ion, 4a self tuned the cavity formed with larger rims of the benzene rings from 5.373 Å in host 4a to 7.024 in (4a·NO3)−, 7.428 in (4a·Br)− and 7.433 Å in (4a·Cl)− complexes, respectively. Hence the functionalized tetraoxacalix[2]arene[2]triazine 4a serves as a ditopic receptor to simultaneously complex anion with its V-shaped electron-deficient cavity and cation with its V-shaped electron-rich cavity, respectively, leading to ion-pair interaction based on anion–π and cation–π interactions. The aforementioned ion-pair interactions are probably due to the electronic donating effect of the attached hydroxyl groups, which increased the electronic density of the benzene rings and facilitated the cation–π interaction.
1 complexes with Cl−, Br− and NO3−. The anions are included in the V-shaped electron-deficient cavity forming typical anion–π interactions with one of the triazine rings. This was evidenced by the observation of shorter distances between anions and plane of the triazine ring being 3.418 (dCl-plane), 3.486 (dBr-plane) and 2.880 Å (dO7-plane), respectively, than the sums of their van der Waals radius.10 Different interaction modes between anions and 4a in comparison with that between anions and the parent host molecule tetraoxacalix[2]arene[2]triazine were noticeable. In the case of 4a–halide complexes, for instance, one hydroxyl group of another host molecule is included in the cavity, with oxygen atom locating above the other triazine ring and forming short contacts with the plane of triazine ring. Consequently, the deliberately introduced hydroxyl group serves as lone pair electrons and hydrogen bond donor instead of water molecule and form 4a–halide–hydroxyl ternary complexes, which is in contrast to the host–halide–water ternary complex of the parent host molecule.5d In case of Et4N+(4a·NO3−) complex, which is shown in Fig. S4,† nitrate straights up in the cavity with the plane of nitrate orthogonal with one of the triazine rings. Such direction of nitrate in the cavity prevents it from forming typical and weak σ anion–π interactions with both triazine rings, the interaction mode in the complex of nitrate and the parent molecule,5f but renders the formation 4a–NO3−–hydroxyl ternary complex. The location of tetraethylammonium ion in the aforementioned host–guest complexes is interesting. In sharp contrast with the parent host–anion complexes, in which tetraethylammonium ion showed no contact with the macrocyclic receptor, the cation in 4a–anion complexes occupies the electron-rich cavity constructed with the larger rims of benzene rings. The distances of alkyl C–H and planes of benzene rings for the three complexes are in the range of 2.871 to 3.298 Å, suggesting possible cation–π interactions. Noteworthy, in order to accommodate the tetraethylammonium ion, 4a self tuned the cavity formed with larger rims of the benzene rings from 5.373 Å in host 4a to 7.024 in (4a·NO3)−, 7.428 in (4a·Br)− and 7.433 Å in (4a·Cl)− complexes, respectively. Hence the functionalized tetraoxacalix[2]arene[2]triazine 4a serves as a ditopic receptor to simultaneously complex anion with its V-shaped electron-deficient cavity and cation with its V-shaped electron-rich cavity, respectively, leading to ion-pair interaction based on anion–π and cation–π interactions. The aforementioned ion-pair interactions are probably due to the electronic donating effect of the attached hydroxyl groups, which increased the electronic density of the benzene rings and facilitated the cation–π interaction.
Last but not the least, the anion directed self-assemblies were worth addressing. In the presence of anions, as shown in Fig. 2D and Fig. S2–S4,† the hydrogen bonding linked self-assembly of the host molecule 4a was disrupted (Fig. S1†). Alternatively, under the guiding of cooperative anion–π interaction, lone-pair electron–π and intermolecular hydrogen bonding interactions, an infinite linear self-assembly or supramolecular wire was formed (Fig. 2D and Fig. S3E and S4E†). In addition, cavity-included anions also interact with a second hydroxyl group of another host molecule from outside the cavity (Fig. S2A, S3D and S4D†), which links the wires together and leads to a mesh assembly (Fig. S2B, S3F and S4F†).
By means of 1H NMR titrations, the interactions of 4a and anions in solution were investigated. Job's plot experiments demonstrated that the anions formed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with 4a in d6-acetone solution (Fig. S5–S7†). The addition of anions to the solution of 4a led to a downfield shift of the hydroxy-H (H3), indicating the formation of O–H⋯anion hydrogen bonding. The formation of hydrogen bonding between the hydroxyl group and anions also caused the slight downfield movement of aryl-H (H2). The NMR titration data were fitted with a HyperNMR program giving the association constants for anions being 127 ± 10 M−1 (4a·Cl)−, 43 ± 0.3 M−1 (4a·Br)− and 42 ± 0.2 M−1 (4a·NO3)−, respectively. Moreover, ESI-MS experiments also gave 1
1 complex with 4a in d6-acetone solution (Fig. S5–S7†). The addition of anions to the solution of 4a led to a downfield shift of the hydroxy-H (H3), indicating the formation of O–H⋯anion hydrogen bonding. The formation of hydrogen bonding between the hydroxyl group and anions also caused the slight downfield movement of aryl-H (H2). The NMR titration data were fitted with a HyperNMR program giving the association constants for anions being 127 ± 10 M−1 (4a·Cl)−, 43 ± 0.3 M−1 (4a·Br)− and 42 ± 0.2 M−1 (4a·NO3)−, respectively. Moreover, ESI-MS experiments also gave 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between 4a with anions in gaseous state, which is in agreement with the results in solution (Fig. S11–S13†).
1 complexes between 4a with anions in gaseous state, which is in agreement with the results in solution (Fig. S11–S13†).
In addition to the host–guest binding affinity determination, diffusion-ordered NMR spectroscopy (DOSY) experiments were applied to find the possible self-assemblies in solution, taking the interaction between 4a and chloride as a representative example. As depicted in Fig. S8A and B,† in d6-acetone and at 213 K, the host 4a and guest Bu4NCl in the free states give diffusion coefficients of 6.47 × 10−10 cm2 s−1 (Dhost) and 10.34 × 10−10 cm2 s−1 (Dguest), respectively. The larger diffusion coefficient of guest than that of host is reasonable due to its smaller molecular weight. However, in the mixture of 4a and Bu4NCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), only one diffusion band in addition to the band of solvent was observed. The apparent diffusion coefficients decreased from 6.47 × 10−10 cm2 s−1 (Dfree) to 5.12 × 10−10 cm2 s−1 (Dbound) for the host and from 10.34 × 10−10 cm2 s−1 (Dfree) to 5.12 × 10−10 cm2 s−1 (Dbound) for the guest, respectively. The almost identical apparent diffusion coefficients of the bound guest (5.82 × 10−10 cm2 s−1) and the bound host (5.12 × 10−10 cm2 s−1) in the mixture indicate the formation of strong complex in solution.9 In the premise of assuming fast exchange on the NMR timescale, the apparent diffusion coefficient (Dapp) of the host should be a weighted average of the free and bound diffusion coefficients (Dfree and Dbound, respectively).9 According to the bound fraction of the host 4a, which was calculated from the association constant (Ka = 127 M−1) between 4a and Cl−, the calculated bonded diffusion coefficient of 4a was 4.39 × 10−10 cm2 s−1. On the assumption that the molecules are spherical, the molecular weight of the complex calculated from the equation Mcomplex/Mfree = (Dfree/Dbound)3 is 1506, which is double of the sum of host (Mw = 475.2) and guest (Mw = 278.0), suggesting the complex has an approximate structure of [Bu4N+(4a·Cl)−]2. Noticeably, such outcomes also support the apparent 1
2), only one diffusion band in addition to the band of solvent was observed. The apparent diffusion coefficients decreased from 6.47 × 10−10 cm2 s−1 (Dfree) to 5.12 × 10−10 cm2 s−1 (Dbound) for the host and from 10.34 × 10−10 cm2 s−1 (Dfree) to 5.12 × 10−10 cm2 s−1 (Dbound) for the guest, respectively. The almost identical apparent diffusion coefficients of the bound guest (5.82 × 10−10 cm2 s−1) and the bound host (5.12 × 10−10 cm2 s−1) in the mixture indicate the formation of strong complex in solution.9 In the premise of assuming fast exchange on the NMR timescale, the apparent diffusion coefficient (Dapp) of the host should be a weighted average of the free and bound diffusion coefficients (Dfree and Dbound, respectively).9 According to the bound fraction of the host 4a, which was calculated from the association constant (Ka = 127 M−1) between 4a and Cl−, the calculated bonded diffusion coefficient of 4a was 4.39 × 10−10 cm2 s−1. On the assumption that the molecules are spherical, the molecular weight of the complex calculated from the equation Mcomplex/Mfree = (Dfree/Dbound)3 is 1506, which is double of the sum of host (Mw = 475.2) and guest (Mw = 278.0), suggesting the complex has an approximate structure of [Bu4N+(4a·Cl)−]2. Noticeably, such outcomes also support the apparent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry obtained by Job's plot. The NMR titration results indicate that hydrogen bonding is one of the guiding forces of the host–guest interaction in solution. In addition, DOSY experiments showed the formation of low-level preorganzied self-assemblies in dilute solution, suggesting the probable existence of cooperative interactions. We further applied 1-D Selective NOESY experiments (performed at 213 K in order to inhibit the exchange of active hydroxyl-H) to get more information of the interactions in solution. In the absence of chloride (Fig. S9A†), selective excitation of proton H3 on the hydroxyl group does not produce inverted signals of H1, excluding the intermolecular short contact between the two protons. In the presence of chloride, however, an inverted signal of H1 was observed (Fig. S9B†). This result, in combination of the slightly upfield shift of H1 during the titration of 4a with chloride, and the aforementioned formation of O–H⋯chloride hydrogen bonding, indicate probably the existence of cooperative anion–π interaction and intermolecular hydrogen bonding in solution. In addition, 1H NMR spectra showed a upfield shift of the protons of TBA cation in the presence of host (Fig. S10†), and selective excitation of the protons of the butyl groups of TBA caused an inverted signal of H2 and a relatively weak inverted signal of H3 (Fig. S9C†), suggesting the probable inclusion of TBA cation in between the V-shaped benzene rings in solution.
1 stoichiometry obtained by Job's plot. The NMR titration results indicate that hydrogen bonding is one of the guiding forces of the host–guest interaction in solution. In addition, DOSY experiments showed the formation of low-level preorganzied self-assemblies in dilute solution, suggesting the probable existence of cooperative interactions. We further applied 1-D Selective NOESY experiments (performed at 213 K in order to inhibit the exchange of active hydroxyl-H) to get more information of the interactions in solution. In the absence of chloride (Fig. S9A†), selective excitation of proton H3 on the hydroxyl group does not produce inverted signals of H1, excluding the intermolecular short contact between the two protons. In the presence of chloride, however, an inverted signal of H1 was observed (Fig. S9B†). This result, in combination of the slightly upfield shift of H1 during the titration of 4a with chloride, and the aforementioned formation of O–H⋯chloride hydrogen bonding, indicate probably the existence of cooperative anion–π interaction and intermolecular hydrogen bonding in solution. In addition, 1H NMR spectra showed a upfield shift of the protons of TBA cation in the presence of host (Fig. S10†), and selective excitation of the protons of the butyl groups of TBA caused an inverted signal of H2 and a relatively weak inverted signal of H3 (Fig. S9C†), suggesting the probable inclusion of TBA cation in between the V-shaped benzene rings in solution.
In summary, we have synthesized a hydroxyl functionalized tetraoxacalix[2]arene[2]triazine host molecule through a fragment coupling followed by AlCl3 mediated deprotection protocol. The introduction of hydroxyl groups on the larger rim rendered the macrocyclic molecule as an unique donor–acceptor functional building block. Infinite self-assemblies were then obtained under the directing of cooperative anion–π, lone-pair electron–π interactions and intermolecular hydrogen bonding. Our study indicated that anion–π interaction directed self-assembly could be designed through the rational fabrication of building blocks.
Acknowledgements
We thank National Natural Science Foundation of China (91127008, 21072197, 21272239), Ministry of Sciences and Technology (2011CB932501, 2013CB834504) for financial support.Notes and references
- (a) I. Alkorta, I. Rozas and J. Elguero, J. Org. Chem., 1997, 62, 4687 CrossRef CAS; (b) Y. Danten, T. Tassaing and M. Besnard, J. Phys. Chem. A, 1999, 103, 3530 CrossRef CAS; (c) J. P. Gallivan and D. A. Dougherty, Org. Lett., 1999, 1, 103 CrossRef CAS.
- (a) M. Mascal, A. Armstrong and M. D. Bartberger, J. Am. Chem. Soc., 2002, 124, 6274 CrossRef CAS PubMed; (b) D. Quiñonero, C. Garau, C. Rotger, A. Frontera, P. Ballester, A. Costa and P. M. Deyà, Angew. Chem., Int. Ed., 2002, 41, 3389 CrossRef; (c) I. Alkorta, I. Rozas and J. Elguero, J. Am. Chem. Soc., 2002, 124, 8593 CrossRef CAS PubMed.
- (a) P. Gamez, T. J. Mooibroek, S. J. Teat and J. Reedijk, Acc. Chem. Res., 2007, 40, 435 CrossRef CAS PubMed; (b) H. T. Chifotides and K. R. Dunbar, Acc. Chem. Res., 2013, 46, 894 CrossRef CAS PubMed; (c) B. P. Hay and V. S. Bryantsev, Chem. Commun., 2008, 2417 RSC; (d) O. B. Berryman and D. W. Johnson, Chem. Commun., 2009, 3143 RSC; (e) A. Frontera, P. Gamez, M. Mascal, T. J. Mooibroek and J. Reedijk, Angew. Chem., Int. Ed., 2011, 50, 9564 CrossRef CAS PubMed; (f) D.-X. Wang and M.-X. Wang, Chimia, 2011, 65, 939 CrossRef CAS PubMed; (g) P. Ballester, Acc. Chem. Res., 2013, 46, 874 CrossRef CAS PubMed; (h) A. V. Jentzsch, A. Hennig, J. Mareda and S. Matile, Acc. Chem. Res., 2013, 46, 2791 CrossRef PubMed.
- D. Kim, P. Tarakeshwar and K. S. Kim, J. Phys. Chem. A, 2004, 108, 1250 CrossRef CAS.
- (a) O. B. Berryman, V. S. Bryantsev, D. P. Stay, D. W. Johnson and B. P. Hay, J. Am. Chem. Soc., 2007, 129, 48 CrossRef CAS PubMed; (b) B. Han, J. Lu and J. K. Kochi, Cryst. Growth Des., 2008, 8, 1327 CrossRef CAS; (c) Y. S. Rosokha, S. V. Lindeman, S. V. Rosokha and J. K. Kochi, Angew. Chem., Int. Ed., 2004, 43, 4650 CrossRef CAS PubMed; (d) D.-X. Wang, Q.-Y. Zheng, Q.-Q. Wang and M.-X. Wang, Angew. Chem., Int. Ed., 2008, 47, 7485 CrossRef CAS PubMed; (e) D.-X. Wang, Q.-Q. Wang, Y. Han, Y. Wang, Z.-T. Huang and M.-X. Wang, Chem. – Eur. J., 2010, 16, 13053 CrossRef CAS PubMed; (f) D.-X. Wang and M.-X. Wang, J. Am. Chem. Soc., 2013, 135, 892–897 CrossRef CAS PubMed; (g) H. T. Chifotides, B. L. Schottel and K. R. Dunbar, Angew. Chem., Int. Ed., 2010, 49, 7202 CrossRef CAS PubMed; (h) O. B. Berryman, F. Hof, M. J. Hynes and D. W. Johnson, Chem. Commun., 2006, 506 RSC; (i) O. B. Berryman, A. C. Sather, B. P. Hay, J. S. Meisner and D. W. Johnson, J. Am. Chem. Soc., 2008, 130, 10895 CrossRef CAS PubMed; (j) G. Gil-Ramírez, E. C. Escudero-Adán, J. Benet-Buchholz and P. Ballester, Angew. Chem., Int. Ed., 2008, 47, 4114 CrossRef PubMed; (k) S. Guha and S. Saha, J. Am. Chem. Soc., 2010, 132, 17674 CrossRef CAS PubMed; (l) S. Guha, F. S. Goodson, S. Roy, L. J. Corson, C. A. Gravenmier and S. Saha, J. Am. Chem. Soc., 2011, 133, 15256 CrossRef CAS PubMed; (m) S. Guha, F. S. Goodson, L. J. Corson and S. Saha, J. Am. Chem. Soc., 2012, 134, 13679 CrossRef CAS PubMed; (n) M. Giese, M. Albrecht, T. Krappitz, M. Peters, V. Gossen, G. Raabe, A. Valkonen and K. Rissanen, Chem. Commun., 2012, 48, 9983 RSC; (o) P. Arranz-Mascarós, C. Bazzicalupi, A. Bianchi, C. Giorgi, M.-L. Godino-Salido, M.-D. Gutiérrez-Valero, R. Lopez-Garzón and M. Savastano, J. Am. Chem. Soc., 2013, 135, 102 CrossRef PubMed; (p) G. Aragay, A. Frontera, V. Lloveras, J. Vidal-Gancedo and P. Ballester, J. Am. Chem. Soc., 2013, 135, 2620 CrossRef CAS PubMed; (q) A. V. Jentzsch, D. Emery, J. Mareda, P. Metrangolo, G. Resnati and S. Matile, Angew. Chem., Int. Ed., 2011, 50, 11675 CrossRef PubMed.
- D.-X. Wang, S.-X. Fa, Y. Liu, B.-Y. Hou and M.-X. Wang, Chem. Commun., 2012, 48, 11458 RSC.
- (a) S. Li, S.-X. Fa, Q.-Q. Wang, D.-X. Wang and M.-X. Wang, J. Org. Chem., 2012, 77, 1860 CrossRef CAS PubMed; (b) Y. Chen, D.-X. Wang, Z.-T. Huang and M.-X. Wang, Chem. Commun., 2011, 47, 8112 RSC; (c) S. Li, D.-X. Wang and M.-X. Wang, Tetrahedron Lett., 2012, 53, 6226 CrossRef CAS PubMed.
- (a) Q.-Q. Wang, D.-X. Wang, H.-B. Yang, Z.-T. Huang and M.-X. Wang, Chem. – Eur. J., 2010, 16, 7265 CrossRef CAS PubMed; (b) M.-X. Wang and H.-B. Yang, J. Am. Chem. Soc., 2004, 126, 15412 CrossRef CAS PubMed.
- C. Schalley, Analytical methods in supramolecular chemistry, Wiley-VCH Verlag GmbH & KGaA, Weinheim, 2007 Search PubMed.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystal structures, 1H and 13C NMR spectra of products, spectroscopic titration data. CCDC 968607–968610 for Et4N+(4a·Cl)−, Et4N+(4a·Br)−, Et4N+(4a·NO3)− and 4a. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra47748g |
| This journal is © The Royal Society of Chemistry 2014 |