Solution reduction synthesis of amine terminated carbon quantum dots†
Keith Linehan and
Hugh Doyle*
Tyndall National Institute, University College Cork, Lee Maltings, Cork, Ireland. E-mail: hugh.doyle@tyndall.ie; Tel: +353-21-2346300
First published on 19th February 2014
Abstract
Highly luminescent water soluble carbon quantum dots (CQDs) with narrow size distributions have been prepared via a simple room temperature, solution-phase synthesis. The CQDs, stabilised by covalently bound allylamine ligands to minimise surface oxidation, exhibit an excitation wavelength dependent blue luminescence with a quantum yield of 25%.
Carbon quantum dots (CQDs) have attracted great interest in recent years as a new class of fluorescence nanomaterial with attractive photophysical properties, high quantum yields, low toxicity and biocompatibility.1–3 Compared to conventional organic dyes and semiconductor nanocrystals, CQDs possess several advantages in terms of chemical inertness, easy functionalisation, high resistance to photobleaching, an absence of fluorescence intermittency and the potential for low cost production. As a result, much attention has been paid to their potential application in areas from biological labelling and imaging to fluorescence nanosensors and optoelectronic devices.2,4,5 As CQDs may be prepared using cheap precursor materials, moderate reaction conditions and relatively simple equipment, numerous synthetic approaches have been reported in the literature, which may be broadly divided into two main categories. Physical methods include arc discharge,6 laser ablation/passivation7,8 and plasma treatment.9 Chemical methods include electrochemical synthesis,10–12 combustion and acidic oxidation,13,14 hydrothermal and pyrolysis routes,15 supported synthesis16–18 and microwave/ultrasonic synthesis.19,20 However, all these methods possess some drawbacks including, e.g. extensive post-synthetic purification, lack of control of CQD surface chemistry and sample polydispersity. In addition, many of these methods also generate relatively broad size distributions, necessitating tedious size separation processes to obtain monodisperse CQDs.1
The use of microemulsion based synthetic methods have been the most promising due to their ability to control the size, shape and surface chemistry of the CQDs. Rhee and co-workers reported the solution-phase synthesis of CQDs using reverse micelles as nanoscale reactors.21 The CQDs were formed via condensation polymerisation and subsequent carbonization of glucose within AOT reverse micelles at 160 °C. Control of the water–surfactant ratio within the micelle allowed the CQD diameter to be tuned from 1.8 to 4.1 nm, but with increasing polydispersity at larger diameters. They later reported a similar hydrothermal synthesis of nearly monodisperse (1.4 ± 0.15 nm) CQDs using octanol as the surfactant.22 In both cases, subsequent in situ surface passivation resulted in formation of hexadecylamide-capped CQDs. More recently, Gao et al. reported on the hydrothermal synthesis of CQDs by oxidation of C60 by hydrogen peroxide under alkaline conditions within CTAB reverse micelles at 150 °C.23
Previously, we reported the synthesis of size monodisperse carbon quantum dots using a room temperature microemulsion strategy.24 The surfaces of the CQDs are terminated with a covalently attached alkyl monolayer, rendering the resulting hydrophobic quantum dots dispersible in a wide range of non-polar solvents. However, for carbon quantum dots to be used effectively in many biological applications, it is essential that they are water-soluble and stable against aggregation and precipitation within a biological system, possess a high photoluminescence quantum yield in the visible region and exhibit excellent photostability under typical illumination conditions.
In this communication, we report the room temperature synthesis of highly monodisperse, amine terminated CQDs that form stable aqueous dispersions and exhibit strong visible emission. The CQDs are synthesized in reverse micelles via the reduction of carbon tetrachloride using a hydride reducing agent; see the ESI† for further synthetic details. The hydrogen-terminated CQDs are functionalised using a platinum-catalysed concerted reaction to covalently attach allylamine ligands to the surface, chemically passivating the surface and rendering the CQDs dispersible in polar solvents, see Scheme 1.
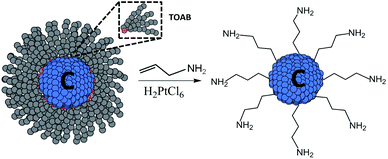 | ||
| Scheme 1 Chemical passivation and functionalisation of the CQDs using a platinum-catalysed concerted reaction. CQDs are synthesized by reduction of CCl4 within TOAB reverse micelles in toluene. | ||
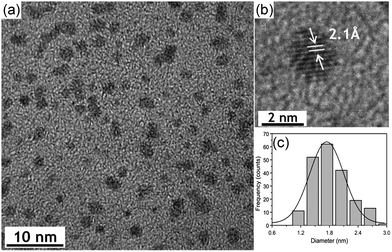
Fig. 1(a) shows a transmission electron microscope (TEM) image of the as-synthesized CQDs. The CQDs are highly size and shape monodisperse, see Fig. 1(a) and S1 and S2 of the ESI.† Fig. 1(b) shows a high resolution TEM image of a single CQD, illustrating that the nanocrystals are highly crystalline and form a single contiguous crystalline phase. The lattice fringes shown in Fig. 1(b) correspond to a d spacing of 2.1 Å, matching the (100) spacing reported for graphitic carbon. Fig. 1(c) shows a histogram of the CQD diameters, determined by analysis of TEM images of ca. 200 CQDs located at different locations on the grid. The mean diameter of the CQDs is 1.8 ± 0.3 nm, based on fitting the histogram to a Gaussian model.
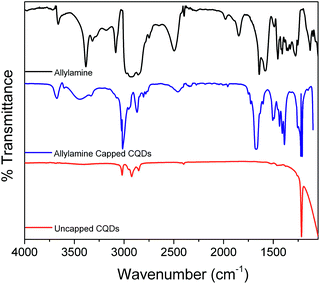
The surface chemistry of the quantum dots was characterized by infrared spectroscopy; Fig. 2 shows the FTIR spectra of the allylamine ligand, together with allylamine-capped and uncapped CQDs. Spectra of allylamine-capped CQDs and the allylamine ligand show peaks from ca. 3700–3500 cm−1, assigned to the N–H stretching of the amine, while the peaks observed between 3000 and 2850 cm−1 are attributed to C–H stretching modes. The large feature centred near 1670 cm−1 for the allylamine-capped CQDs is consistent with amine N–H deformation modes, but also with stretching modes of carboxylate species caused by surface oxidation. The peaks observed between 1500 and 1400 cm−1 are attributed to C–C bending modes. The absence of the characteristic CH ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 peaks at 1640 and 3080 cm−1 for the capped CQDs, which is observed in the spectrum of neat allylamine, is consistent with successful binding of the allylamine ligand to the CQD surface, as previously reported.24 In contrast, the FTIR spectrum of the uncapped CQDs shows no evidence of peaks that may be assigned to amine groups. The presence of peaks between 3000 and 2850 cm−1 are assigned to C–H stretching modes at the CQD surface, while the additional peaks below 1300 cm−1 are due to the presence of oxygenic species, indicating significant surface oxidation.
CH2 peaks at 1640 and 3080 cm−1 for the capped CQDs, which is observed in the spectrum of neat allylamine, is consistent with successful binding of the allylamine ligand to the CQD surface, as previously reported.24 In contrast, the FTIR spectrum of the uncapped CQDs shows no evidence of peaks that may be assigned to amine groups. The presence of peaks between 3000 and 2850 cm−1 are assigned to C–H stretching modes at the CQD surface, while the additional peaks below 1300 cm−1 are due to the presence of oxygenic species, indicating significant surface oxidation.
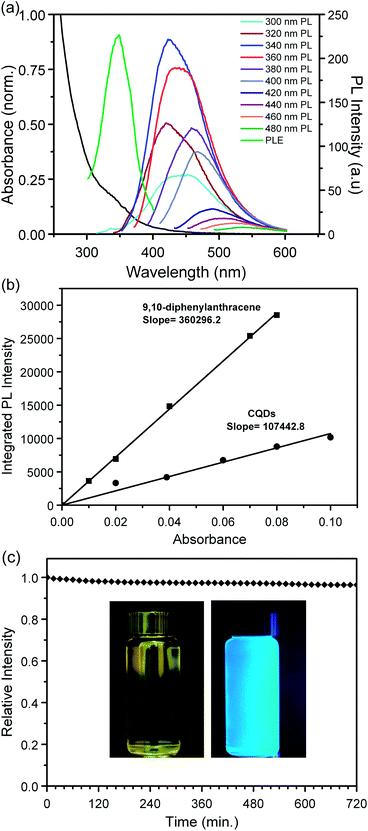
The optical properties of the CQDs were studied using UV-vis absorption, photoluminescence (PL), photoluminescence excitation (PLE) and quantum yield measurements. The allylamine capped CQDs exhibit a strong absorption in the UV region, with a shoulder at ca. 320 nm and a tail extending into the visible range. The PL spectra were recorded using excitation wavelengths ranging from 300 to 400 nm in 20 nm increments. The carbon quantum dots exhibit a primarily blue luminescence, clearly dependent on the excitation wavelength used, with the wavelength position of the PL maximum red-shifting from 441 nm to 465 nm, as the excitation wavelength is increased from 300 nm to 400 nm. This is in good agreement with literature reports for CQDs dispersed in water.7,16
Photoluminescence from CQDs still remains a widely debated topic despite the extensive research carried out in recent years. A number of different interpretations explaining the origin of PL in CQDs have been proposed.1,3 Sun et al. suggested that the PL is due to the presence of energetic trap states near the CQD surface.7 In contrast, Zhao et al. attributed the dependency on excitation wavelength to size differences rather than emissive trap states.10 Although the exact mechanism still remains unresolved, evidence exists that both mechanisms can contribute to both CQD emission colour and intensity. Given the high degree of size monodispersity in the CQDs reported here, the most plausible explanation is that the blue luminescence originates from recombination of photogenerated excitons at shallow surface trap states. PLE spectra recorded at the PL intensity maximum (423 nm, see Fig. 3(a)) show a narrow peak centred at ca. 347 nm, considerably above the band gap energy of the CQDs, suggesting radiationless transfer and recombination at states close to the CQD surface. Interestingly, the relative luminescence intensity of the amine-terminated CQDs at different excitation wavelengths shows a maximum at 340 nm, in contrast to similarly sized CQDs with alkyl-terminated surfaces,24 which exhibit a monotonically decreasing luminescence intensity at increasing excitation wavelengths, see Fig. S3† for a comparison of the excitation wavelength dependence of amine- and alkyl-terminated CQDs. This underlines the importance of the surface in determining the photophysical properties of CQDs, which has been widely observed for CQDs prepared using different preparation methods and surface functionalities.1–3 Carbon quantum dots capped with either ligand show the same red-shift in the position of the PL maximum at increasing excitation wavelengths. The CQDs showed similar emission characteristics when dispersed in different polar solvent media, see Fig. S4.† PL spectra of allylamine-terminated CQDs recorded in different pH environments (see Fig. S5†) showed fluctuations in the PL intensity, but no overall trend was observed.
Photoluminescence quantum yield of the allylamine capped carbon quantum dots in water were determined using the comparative method by Williams et al., see ESI† for further details. Fig. 3(b) shows the integrated PL intensity of the amine terminated CQDs compared to the 9,10-diphenylanthracene emission standard used; the quantum yield (QY) was determined to be ca. 25% at an excitation wavelength of 320 nm. This QY value is comparable to values obtained for CQDs reported in the literature. The CQDs also exhibit excellent long-term PL stability, decreasing by less than 3.5% after continuous illumination for 12 hours; see Fig. 3(c).
In conclusion, a simple, room temperature synthetic route for production of size monodisperse CQDs has been demonstrated, with close control of internal structure and surface chemistry. The surfaces of the CQDs are chemically functionalised with allylamine, producing the hydrophilic CQDs that are readily dispersible in water and polar organic solvents. The CQDs possess an excitation wavelength dependent emission in the visible, and exhibit excellent photostability and high (25%) quantum yields, making them attractive candidates for biological labelling and fluorescence sensing applications.
Acknowledgements
This work was supported by the European Commission under the FP7 Projects SNAPSUN (grant agreement no. 246310) and CommonSense (grant agreement no. 2618309) and the Irish Higher Education Authority under the PRTLI program (Cycle 3 “Nanoscience” and Cycle 4 “INSPIRE”).Notes and references
- H. Li, Z. Kang, Y. Liu and S. T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC
.
- J. C. G. Esteves da Silva and H. M. R. Gonçalves, TrAC, Trends Anal. Chem., 2011, 30, 1327–1336 CrossRef CAS PubMed
.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed
.
- W. Kwon, S. Do, D. C. Won and S.-W. Rhee, ACS Appl. Mater. Interfaces, 2013, 5, 822–827 CAS
.
- F. Wang, Y.-H. Chen, C.-Y. Liu and D.-G. Ma, Chem. Commun., 2011, 47, 3502–3504 RSC
.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed
.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed
.
- H. Goncalves and J. C. G. Esteves da Silva, J. Fluoresc., 2010, 20, 1023–1028 CrossRef PubMed
.
- H. Jiang, F. Chen, M. G. Lagally and F. S. Denes, Langmuir, 2009, 26, 1991–1995 CrossRef PubMed
.
- Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Chem. Commun., 2008, 5116–5118 RSC
.
- L. Zheng, Y. Chi, Y. Dong, J. Lin and B. Wang, J. Am. Chem. Soc., 2009, 131, 4564–4565 CrossRef CAS PubMed
.
- J. Zhou, C. Booker, R. Li, X. Zhou, T. K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744–745 CrossRef CAS PubMed
.
- H. Liu, T. Ye and C. Mao, Angew. Chem., Int. Ed., 2007, 119, 6593–6595 CrossRef
.
- X. Wang, L. Cao, F. Lu, M. J. Meziani, H. Li, G. Qi, B. Zhou, B. A. Harruff, F. Kermarrec and Y. P. Sun, Chem. Commun., 2009, 3774–3776 RSC
.
- D. Pan, J. Zhang, Z. Li, C. Wu, X. Yan and M. Wu, Chem. Commun., 2010, 46, 3681–3683 RSC
.
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 121, 4668–4671 CrossRef
.
- J. Zong, Y. Zhu, X. Yang, J. Shen and C. Li, Chem. Commun., 2011, 47, 764–766 RSC
.
- A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, V. Georgakilas and E. P. Giannelis, Chem. Mater., 2008, 20, 4539–4541 CrossRef CAS
.
- H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118–5120 RSC
.
- X. Wang, K. Qu, B. Xu, J. Ren and X. Qu, J. Mater. Chem., 2011, 21, 2445–2450 RSC
.
- W. Kwon and S. W. Rhee, Chem. Commun., 2012, 48, 5256–5258 RSC
.
- W. Kwon, S. Do and S.-W. Rhee, RSC Adv., 2012, 2, 11223–11226 RSC
.
- M. X. Gao, C. F. Liu, Z. L. Wu, Q. L. Zeng, X. X. Yang, W. B. Wu, Y. F. Li and C. Z. Huang, Chem. Commun., 2013, 49, 8015–8017 RSC
.
- K. Linehan and H. Doyle, RSC Adv., 2014, 4, 18–21 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic method, characterisation and additional figures. See DOI: 10.1039/c3ra47770c |
| This journal is © The Royal Society of Chemistry 2014 |