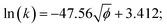Base-catalyzed decomposition of hexachlorobenzene: effect on dechlorination efficiency of different hydrogen donors, alkalis and catalysts
Ye Xiaoa and
Jianguo Jiang*abc
aSchool of Environment, Tsinghua University, Beijing, China. E-mail: jianguoj@tsinghua.edu.cn; Fax: +86 10 62783548; Tel: +86 10 62783548
bKey Laboratory for Solid Waste Management and Environment Safety, Tsinghua University, China
cCollaborative Innovation Center for Regional Environmental Quality, Tsinghua University, China
First published on 21st March 2014
Abstract
The effect of different hydrogen donors, alkalis, and catalysts on the dechlorination efficiency of hexachlorobenzene (HCB) by base-catalyzed decomposition (BCD) technology was investigated. According to an orthogonal experiment, all three factors have notable influence on the dechlorination of HCB, and four different combinations of reagents were suitable for the treatment of HCB at high concentrations: polyethylene glycol (PEG) + hydroxide, octadecane + KOH, glycerin + hydroxide, and glycerin + carbonate. Further research regarding the effects of catalysts determined that ∼100% dechlorination of HCB can be achieved in 3 h at 250 °C in the presence of PEG and hydroxide without any catalyst, and iron rather than nickel was recommended as catalyst when octadecane and KOH were used for HCB dechlorination. In addition, an investigation of the HCB dechlorination kinetics in the presence of PEG and various alkalis determined that the HCB dechlorination rate constant increased linearly with the decreasing of the ionic potential of the metal cation in the hydroxide.
Introduction
Persistent organic pollutants (POPs) are chemical substances, which possess the properties of persistence, bioaccumulation, and toxicity. They can be transported and deposited far from their sources, where they accumulate in ecosystems for long periods, which endangers human beings and the environment.1 Although the production, use, import, and export of POPs have been banned by the Stockholm Convention, large quantities of waste containing POPs exist in obsolete stockpiles and contaminated sites globally. For example, the estimated volumes of POP pesticides stockpiled in the European Union and China are >5370 t and 4059–6093 t, respectively.2,3 The illegal use and leakage of containers of these stockpiles could represent locally and regionally important on-going primary source inputs of POPs to the environment.4 Therefore, appropriate technologies are needed for the environmentally sustainable disposal of wastes consisting of, containing or contaminated with POPs.Recently, many emerging non-incineration technologies have been proposed for the treatment of chlorinated organic compounds. Many focused on the heterogeneous catalytic dechlorination of organic chlorinated compounds in the aqueous phase with a Pd-based catalyst,5–8 nanoscale iron particles9,10 and bimetallic catalysts.7,11–13 However, noble-metal-based catalysts can be self-inhibited and fouled by halide anions generated during the reaction,14 and the heavy metal can be leached into the treated media.11 In addition, regeneration of catalysts at the required efficiency would increase the cost of the degradation process. Thus, appropriate dechlorination technologies without noble metal catalysts are required for the treatment of POP waste in large volumes and at high concentrations.
Base catalyzed decomposition (BCD) technology is a relatively low temperature chemical dehalogenation process that involves the treatment of POP wastes in the presence of hydrogen donor, alkali and a carbon-based, rather than a noble metal, catalyst. It has been identified as an appropriate technology for the disposal of POPs and has been used in the United States, Australia, Japan, and the Czech Republic.15 According to the difference in the hydrogen donor or solvent, two different BCD processes, including the alkali polyethylene glycol (APEG) and the paraffin oil system, had been proposed and investigated.16–18 Different alkalis have been transferred into the BCD process to realize the decomposition of POPs.19 And a strong alkali can increase the degradation efficiency significantly; for instance, by using potassium tert-butoxide, the concentration of PCBs reduced from 120 ppm to 0.02 ppm in a reaction time of only 6 minutes.20 Meanwhile, it has been reported that some substances, including Fe, Ni, and graphite, can increase the degradation efficiency through the catalytic hydrodechlorination pathway.19,21–23 However, most of these researches focused on the removal of target pollutants rather than the dechlorination efficiency. In addition, differences in dechlorination efficiency among the BCD processes with utilization of different reagents has rarely been investigated or discussed.
In this study, the influence of hydrogen donors, alkalis and catalysts on HCB dechlorination efficiency was investigated by using hexachlorobenzene as a model compound. The main aims of this study were (1) to confirm the major factors that have a significant influence on the dechlorination efficiency of HCB; (2) to determine the possibility of increasing HCB dechlorination efficiency using inexpensive catalysts and (3) to determine the relationship between the basicity of the alkali and the rate of HCB dechlorination.
Experimental
Materials
Hexachlorobenzene (HCB, purity 99.0%) was purchased from Beijing HengYe ZhongYuan Chemical Co., Ltd (Beijing, China). Polyethylene glycol (PEG200, molecular weight 190–210), glycerin (≥99.0%) and octadecane (≥97.0%) were obtained from Tianjin Guanfu Fine Chemical Research Institute (Tianjin, China). Sodium hydroxide (≥96.0%), potassium hydroxide (≥82.0%), sodium carbonate (≥99.8%), iron powder, nickel powder and graphite powder were purchased from Beijing Modern Oriental Fine Chemistry Co., Ltd (Beijing, China). Iron, nickel and graphite powders with diameters <75 μm were used.Dechlorination experiment procedure
All reactions were performed in a 10 mL hydrothermal synthesis reactor with an inner cup (inner diameter 2.0 cm, height 3.3 cm) made of polytetrafluoroethylene (PTFE). Typically, about 0.1 g HCB, 0.3 g alkali, 0.6 g hydrogen donor and 0.1 g catalyst were transferred into the reactor, with an initial HCB concentration of 9.09 wt%. The reactor was then sealed with a screw thread and heated to 250 °C within 30 min in a muffle furnace. The temperature was held at 250 °C for 3 h. The reactor was then removed from the furnace and cooled to room temperature with running water.The reagents remaining after the reaction were completely transferred from the reactor into a 100 mL volumetric flask and diluted with deionized water. The chloride ion concentration was quantified by silver nitrate titration in standard method.24 The HCB dechlorination efficiency (HDE) was calculated by eqn (1) based on the amount of chloride ion remaining after the reaction:
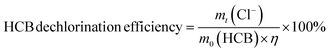 | (1) |
Orthogonal experiment on the influence of different hydrogen donors, alkalis, and catalysts
According to the previous studies as well as the consideration of using inexpensive reagents, three hydrogen donors or solvents (PEG200, glycerin, octadecane), three alkalis (NaOH, KOH, Na2CO3) and four catalysts (Fe, Ni, Ni/Fe, graphite) were selected to investigate the effects of different reagents on HDE. The physical and chemical properties of hydrogen donors selected are shown in Table 1. The boiling points of the hydrogen donors selected were relatively high for security. An orthogonal experiment (shown in Table 2) was designed to identify the major factors affecting HCB dechlorination, in which the dosage of all the reagents and other reaction conditions was the same as the typical experiment. All the experiments in this section were run in duplicate.| Test | Hydrogen donor | Catalyst | Alkali | HDE, % | Deviation, % |
|---|---|---|---|---|---|
| a Reaction conditions: HCB 0.1 g, hydrogen donor 0.6 g, catalyst 0.1 g, alkali 0.3 g; reaction temperature 250 °C; reaction time 3 h.b The yield exceeds 100% because of the experimental error in the chlorine ion assay.c The Ni/Fe catalyst was prepared by mixing the equivalent nickel powder with iron powder. | |||||
| 1# | PEG200 | Ni | NaOH | 98.11 | 1.36 |
| 2# | PEG200 | Fe | KOH | 102.44b | 0.64 |
| 3# | Glycerin | Fe | NaOH | 89.22 | 0.44 |
| 4# | Octadecane | Ni | NaOH | 7.15 | 2.50 |
| 5# | Octadecane | Fe | Na2CO3 | 2.04 | 0.63 |
| 6# | Glycerin | Ni/Fec | NaOH | 87.16 | 3.10 |
| 7# | Glycerin | Graphite | KOH | 92.65 | 2.74 |
| 8# | Octadecane | Graphite | NaOH | 4.26 | 1.25 |
| 9# | Glycerin | Ni | Na2CO3 | 66.73 | 1.17 |
| 10# | Glycerin | Ni | KOH | 82.40 | 6.73 |
| 11# | Glycerin | Graphite | Na2CO3 | 29.58 | 5.79 |
| 12# | PEG200 | Graphite | NaOH | 101.41b | 1.99 |
| 13# | Octadecane | Ni/Fec | KOH | 94.81 | 2.02 |
| 14# | PEG200 | Ni/Fec | Na2CO3 | 7.70 | 5.88 |
Experiments on the influence of catalysts
The alkali polyethylene glycol system and paraffin oil with hydroxide system were the two BCD systems that have been mainly studied in previous researches. So we focused on the investigation of the effect of different catalysts (including Fe, Ni, and graphite) on the HCB dechlorination efficiency in these two BCD systems. According to the experiment design, several experiments have been included in Table 2, which are test 1#, 2#, 12#, and 13#. And remaining tests of other catalysts or without catalyst have been supplemented in this section (shown in Table 3). In all these experiments, about 0.1 g different catalysts or none catalyst and typical amount of other reagents were transferred into the reactor and calcined at 250 °C for 3 h.| Test | Hydrogen donor/solvent | Catalyst | Alkali | Yield, % |
|---|---|---|---|---|
| a No catalyst was added into these tests.b The yield exceeds 100% because of the experimental error in the chlorine ion assay. | ||||
| 15# | Octadecane | Ni | KOH | 92.41 |
| 16# | Fe | KOH | 98.34 | |
| 17# | Graphite | KOH | 88.60 | |
| 18# | Nonea | KOH | 98.17 | |
| 19# | PEG200# | Nonea | KOH | 102.78b |
| 20# | Nonea | NaOH | 104.63b | |
Experiments on the influence of alkalis
Four different type of hydroxide, including NaOH, KOH, Ca(OH)2, and Ba(OH)2, have been used to examined the effect of alkalis on HCB dechlorination efficiency in the alkali polyethylene glycol system. The HCB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alkali ratio was 1
alkali ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 (mol mol−1) in all experiments. And the dosage of HCB and PEG200 was 0.1 g and 0.6 g respectively. All the experiments in this section were conducted at 150 °C. The yield of chlorine ion was evaluated after 0.5, 1.0, 2.0, and 3.0 h.
21 (mol mol−1) in all experiments. And the dosage of HCB and PEG200 was 0.1 g and 0.6 g respectively. All the experiments in this section were conducted at 150 °C. The yield of chlorine ion was evaluated after 0.5, 1.0, 2.0, and 3.0 h.
Results and discussion
Results of the orthogonal experiment
Table 2 shows the results of the orthogonal experiment, in terms of the HDE with various hydrogen donors, alkalis and catalysts.As shown in the Table 2, there were significant differences among the various alkalis. In addition, as shown in Fig. 1, the average dechlorination efficiencies were 26.51%, 64.55%, and 93.08% using Na2CO3, NaOH, and KOH, respectively. The chlorine ion yields in tests 4#, 5#, 8#, and 13# were 7.15 ± 2.50%, 2.04 ± 0.63%, 4.26 ± 1.25%, and 94.81 ± 2.02%, respectively. In these tests with the presence of octadecane as the hydrogen donor, little dechlorination of HCB into inorganic chlorine ions occurred with NaOH and Na2CO3 after a 3 h reaction at 250 °C. In contrast, the HDE was high in the presence of KOH in test 13# under the same reaction conditions. This result is higher than that after a 4 h reaction in the presence of Fe at 360 °C, which was identified in our previous study.21 The difference between the tests with NaOH and KOH is remarkable. This illustrates that the alkali used is a key factor in the base catalyzed dechlorination of HCB.
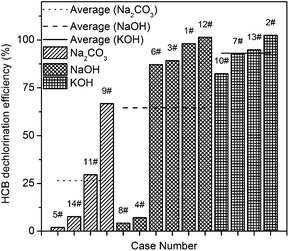 | ||
| Fig. 1 Effect of various alkalis on HCB dechlorination efficiency. The average HDE of a given alkali was calculated based on all the tests using this alkali in the orthogonal experiment. | ||
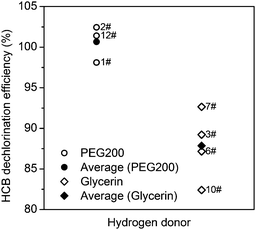
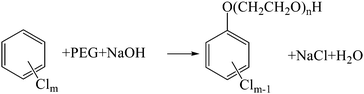
The hydrogen donor or solvent used also has a significant influence on the dechlorination of HCB. In the tests using NaOH as the alkali, HDE decreased in the order: PEG200 > glycerin ≫ octadecane. Octadecane is a type of alkane, the C–H bond in which is difficult to crack under the reaction conditions (250 °C, in the presence of NaOH). However, PEG200 and glycerin are alcohols which can react with NaOH to form sodium alkoxide. According to the investigation of Brunelle and Singleton,16 the reaction of chlorobenzene with PEG and hydroxide can be explained by eqn (2). Considering the similarity of PEG200 and glycerine, the dechlorination mechanism with glycerine may be similar with PEG200, which can be interpreted by eqn (3). However, the average dechlorination efficiency in tests with glycerin and hydroxide was 87.86%, which was lower than the tests with PEG200 and hydroxide (100.65%), as shown in Fig. 2. The chlorine in HCB can be completely transformed into chloride ion in the tests with PEG200# and hydroxide. However, partial chlorine atoms in HCB were converted into inorganic chlorine in the tests with glycerin. Steric hindrance of the substituent groups, with hydroxyl rather than hydrogen on the β-carbon, may account for the decreased dechlorination efficiency in the tests with glycerine compared to those with PEG200.
 | (2) |
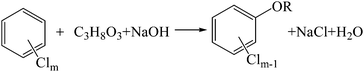 | (3) |
Comparing cases 9# and 11#, there is a significant difference in the HDE with different catalysts in the presence of both glycerin and Na2CO3. The HDE with Ni is 2.26 times higher than with graphite. In Shen et al.'s study, reduction of CO2 and NaHCO3 into formate using glycerin as a reducing agent has been reported, and a hydrogen-transfer reduction pathway was proposed.25 In our research, the hydride ion produced in the carbonate reduction may be involved in the reduction of HCB with the assistance of Ni, which can catalyze the hydrodechlorination reaction above 200 °C.26,27
As described above, the alkali, hydrogen donor and catalyst used all have important influence on the HDE in some situations. However, according to the analysis of variance (ANOVA) of the orthogonal experiment (shown in Table 4), the alkali used is the most significant factor affecting the HDE, followed by the hydrogen donor and catalyst, under these experimental conditions (250 °C, 3 h, mass ratio of HCB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydrogen donor
hydrogen donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alkali
alkali![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 1
catalyst = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
| Degree of freedom | Sum of squares | Mean square | F | p | |
|---|---|---|---|---|---|
| Hydrogen donor | 2 | 7727.65 | 3863.82 | 5.183 | 0.036 |
| Catalyst | 3 | 467.35 | 155.79 | 0.209 | 0.887 |
| Alkali | 2 | 9316.32 | 4658.16 | 6.248 | 0.023 |
| Error | 8 | 5964.20 | 745.52 | ||
| Total | 15 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475.51 475.51 |
The results of the orthogonal experiment also indicate that the following four combinations of reagents can be used for the successful treatment of chlorinated organic compounds at high concentrations: PEG + hydroxide, octadecane + potassium hydroxide, glycerin + hydroxide and glycerin + carbonate + Ni; however, their application requires further investigation.
The effect of catalyst on HCB dechlorination efficiency
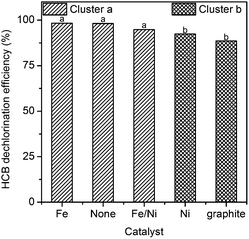
The effect of catalyst on the HDE is shown in Table 3. The presence of a catalyst made little difference to the dechlorination efficiency with PEG200# and hydroxide after a 3 h reaction at 250 °C, which indicated that catalyst is not necessary for the complete dechlorination of HCB in the PEG/hydroxide system under these reaction conditions.However, there were differences among the tests with octadecane and KOH under the same reaction conditions. K-Means clustering analysis was carried out to classify the tests performed in the presence of octadecane and KOH into two clusters (Fig. 3). Through variance analysis, we found that the difference between cluster a (tests 13#, 16#, 18#) and cluster b (tests 15#, 17#) was significant (p = 0.049 < α = 0.05). The HDE in the presence of Fe or without a catalyst was higher than that with Ni and graphite.
In the tests of Ni and Fe as catalysts, we found that the HDE was lower in the presence of Ni (tests 13# and 15#) than in the presence of Fe (test 16#). This is in agreement with the report by Wu et al.,28 which demonstrated that the extent of dechlorination decreases in the order Fe > Ni > Zn > Cu. As proposed by Kawahara and Michalakos and our previous study,19,21 the cracking of the paraffin oil is the hydrogen source in the hydrodechlorination process. However, the catalytic hydrogenation ability of Ni is higher than that of Fe.29 Further hydrogenation of biphenyl to cyclohexyl-benzene was reported in the catalytic dechlorination of polychlorinated biphenyls (PCBs) with a Ni/Fe catalyst.30 Therefore, the presence of Ni may lead to the competition between the dechlorination of HCB and the hydrogenation of dechlorination product, benzene.
In a comparison of tests 16# and 17#, the presence of iron powder slightly improved the HDE. The reduction of intermediates in the HCB dechlorination process in the presence of zero-valent iron has also been proven in our former study.21 The catalytic dechlorination ability of Fe at temperatures above 250 °C has also been reported by Sun et al.31,32 And the small quantity of chlorobenzenes which maybe remained in the residue can be degraded by Fenton-like system with the presence of remaining iron.33 In addition, zero-valent iron is inexpensive and environmentally sustainable which can be readily obtained as commercial products or wastes in factory. Therefore, the utilization of Fe as a dechlorination catalyst is worth considering.
The effect of alkali on HCB dechlorination efficiency
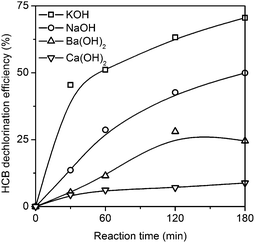
The dechlorination efficiencies of HCB with the various alkalis are presented in Fig. 4. In the presence of KOH, NaOH, Ba(OH)2, and Ca(OH)2 with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 (HCB/alkali, mol mol−1) at 150 °C, the final HCB dechlorination efficiencies were 70.57%, 49.96%, 24.55%, and 8.85% after 3 h, respectively. This illustrates that the basicity of the alkali has an important effect on the HDE. A pseudo-first-order reaction model was proposed to describe the dechlorination of HCB according the research of Filippis et al.34
21 (HCB/alkali, mol mol−1) at 150 °C, the final HCB dechlorination efficiencies were 70.57%, 49.96%, 24.55%, and 8.85% after 3 h, respectively. This illustrates that the basicity of the alkali has an important effect on the HDE. A pseudo-first-order reaction model was proposed to describe the dechlorination of HCB according the research of Filippis et al.34
The first order rate constants of HCB dechlorination were 0.470 h−1, 0.253 h−1, 0.116 h−1 and 0.036 h−1 when KOH, NaOH, Ba(OH)2, and Ca(OH)2 were used as the alkali, respectively. The pseudo-first-order rate constant of HCB dechlorination in the presence of KOH is about twice that in the presence of NaOH at 150 °C with PEG200#. The rate of HCB dechlorination decreases in the order K > Na > Ba > Ca, which is similar to results presented by Berkessel et al.,35 where the hydrogenation rate of benzophenone decreased in the order Cs > Rb ≈ K ≫ Na ≫ Li. Chan and Radom demonstrated considerable dependence of the hydrogenation reaction rate on the nature of the metal cation, the alkoxide base and the substrate.36 In our research, the alkoxide base was produced by the reaction between the PEG200# and hydroxide, and the substrates in all the experiments were HCB. Therefore, the nature of the metal cation in the hydroxide has the most significant influence on the HCB dechlorination reaction rate.
In this study, the ionic potential, as described in eqn (4), is proposed to represent the nature of a metal cation, which can affect the basicity of the hydroxide and the enthalpy change of reactions.37,38
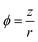 | (4) |
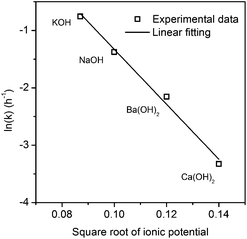
The relationship between the square root of ionic potential and the natural logarithm of the rate constant is shown in Fig. 5. A linear relationship exists between the square root of ionic potential and the natural logarithm of the HCB dechlorination rate constant. The relationship can be described as the flowing model, as shown in eqn (5), which is similar to the Arrhenius formula, represented by eqn (6):
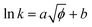 | (5) |
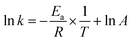 | (6) |
The square root of ionic potential 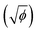 has a linear relation with ionization potential within the alkali and alkaline earth families, which indicated that
has a linear relation with ionization potential within the alkali and alkaline earth families, which indicated that 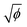 has a linear relation with the energy required to remove an electron from the atom.37 Similarly,
has a linear relation with the energy required to remove an electron from the atom.37 Similarly, 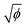 of the cation may have an influence on the energy required to dissociate the hydroxyl ion (OH−) from the hydroxide, as shown in eqn (7). In addition, ln
of the cation may have an influence on the energy required to dissociate the hydroxyl ion (OH−) from the hydroxide, as shown in eqn (7). In addition, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k is linearly proportional to the activation energy of a chemical reaction at a certain temperature according to the Arrhenius equation.
k is linearly proportional to the activation energy of a chemical reaction at a certain temperature according to the Arrhenius equation.
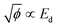 | (7) |
According to the eqn (5)–(7), we can easily conclude that there was a linear relation between the activation energy of the dechlorination of HCB and the dissociation energy of the hydroxide in the reaction media. This relationship indicated that the dissociation of the hydroxide was the most crucial step in HCB dechlorination in presence of PEG200 and hydroxide.
With an increasing ionic radius, the hydroxide can readily dissociate into a metal cation (M+) and the hydroxyl radical (OH−), which was considered one of the most important factors in the activation of PCBs in previous research.19 The metal cation with a high ionic potential is small with a high charge density. The energy barrier to formation of the transition state will increase due to the considerably higher solvent stabilization than exists for low-ionic-potential ions such as K+.36 The decrease in dissociation energy and activation energy with low ionic potential may account for the increasing HCB dechlorination reaction rate.
Conclusions
The main findings of this study can be summarized as follows:(1) The selected hydrogen donors, alkalis, and catalysts all had an influence on the base-catalyzed decomposition of HCB. The most significant factor was the alkali used followed by the hydrogen donor and the catalyst.
(2) Four combinations could be successfully used for the treatment of POPs on a large scale and at high concentrations: PEG + hydroxide, octadecane + KOH, glycerine + hydroxide and glycerine + carbonate. The most efficient combination was PEG200 and NaOH/KOH, which yielded approximately 100% HCB dechlorination without any catalyst in 3 h at 250 °C.
(3) To increase the dechlorination efficiency of HCB in the presence of octadecane and KOH, addition of Fe rather than Ni is recommended.
(4) There was a linear relationship between the square root of the ionic potential of the metal cation and the natural logarithm of the HCB dechlorination rate constant. This suggests that the HCB dechlorination rate increased with an increase in alkali basicity. The pseudo-first-order rate constant of HCB dechlorination in the presence of KOH was about twice that in the presence of NaOH at 150 °C with PEG200.
Acknowledgements
The authors thank the National Hi-Tech R&D Project (863) Key Program (no. 2009AA064001) of the PR China for providing the financial support.Notes and references
- M. H. Wong, A. Leung, J. Chan and M. Choi, Chemosphere, 2005, 60, 740–752 CrossRef CAS PubMed.
- Commission of the European Communities, Community Implementation plan for the Stockholm Convention on Persistent Organic Pollutants, 2007, Available from: http://chm.pops.int/Implementation/NIPs/NIPSubmissions/tabid/253/Default.aspx, January 14, 2014.
- The People's Republic of China, National Implementation Plan for the Stockholm Convention on Persistent Organic Pollutants, 2007, Available from: http://chm.pops.int/Implementation/NIPs/NIPSubmissions/tabid/253/Default.aspx, January 14, 2014.
- K. Breivik, R. Alcock, Y. F. Li, R. E. Bailey, H. Fiedler and J. M. Pacyna, Environ. Pollut., 2004, 128, 3–16 CrossRef CAS PubMed.
- G. V. Lowry and M. Reinhard, Environ. Sci. Technol., 2000, 34, 3217–3223 CrossRef CAS.
- M. M. Zheng, J. G. Bao, P. Liao, K. Wang, S. H. Yuan, M. Tong and H. Y. Long, Chemosphere, 2012, 87, 1097–1104 CrossRef CAS PubMed.
- B. Yang, Y. Zhang, S. Deng, G. Yu, Y. Lu, J. Wu, J. Xiao, G. Chen, X. Cheng and L. Shi, Chem. Eng. J., 2013, 234, 346–353 CrossRef CAS.
- H. Kominami, T. Nishi, K. Fuku and K. Hashimoto, RSC Adv., 2013, 3, 6058–6064 RSC.
- C. Wang and W. Zhang, Environ. Sci. Technol., 1997, 31, 2154–2156 CrossRef CAS.
- J. Feng, B. W. Zhu and T. T. Lim, Chemosphere, 2008, 73, 1817–1823 CrossRef CAS PubMed.
- L. F. Wu and S. Ritchie, Chemosphere, 2006, 63, 285–292 CrossRef CAS PubMed.
- Y. Zhuang, S. Ahn, A. L. Seyfferth, Y. Masue-Slowey, S. Fendorf and R. G. Luthy, Environ. Sci. Technol., 2011, 45, 4896–4903 CrossRef CAS PubMed.
- X. Q. Nie, J. G. Liu, D. B. Yue, X. W. Zeng and Y. F. Nie, Chemosphere, 2013, 90, 2403–2407 CrossRef CAS PubMed.
- B. P. Chaplin, M. Reinhard, W. F. Schneider, C. Schuth, J. R. Shapley, T. J. Strathmann and C. J. Werth, Environ. Sci. Technol., 2012, 46, 3655–3670 CrossRef CAS PubMed.
- Basel Convention, Updated general technical guidelines for the environmentally sound management of wastes consisting of, containing or contaminated with persistent organic pollutants (POPs), 2012, Available from: http://www.basel.int/Implementation/TechnicalMatters/DevelopmentofTechnicalGuidelines/AdoptedTechnicalGuidelines/tabid/2376/Default.aspx, January 14, 2014.
- D. J. Brunelle, A. K. Mendiratta and D. A. Singleton, Environ. Sci. Technol., 1985, 19, 740–746 CrossRef CAS PubMed.
- A. S. C. Chen, A. R. Gavaskar, B. C. Alleman, A. Massa, D. Timberlake and E. H. Drescher, J. Hazard. Mater., 1997, 56, 287–306 CrossRef CAS.
- B. Z. Wu, H. Y. Chen, S. Wang, C. M. Wai, W. S. Liao and K. H. Chiu, Chemosphere, 2012, 88, 757–768 CrossRef CAS PubMed.
- F. K. Kawahara and P. M. Michalakos, Ind. Eng. Chem. Res., 1997, 36, 1580–1585 CrossRef CAS.
- M. Ohno and H. Kaneda, Organohalogen Compd., 1997, 31, 415–419 CAS.
- Y. Xiao, J. G. Jiang, Y. Yang and G. L. Gao, Chem. Eng. J., 2011, 173, 415–421 CrossRef CAS.
- B. Kamarehie, A. J. Jafari and H. A. Mahabadi, J. Mater. Cycles Waste Manage., 2013, 1–10, DOI:10.1007/s10163-013-0185-y.
- E. V. de Pava and E. Battistel, Chemosphere, 2005, 59, 1333–1342 CrossRef PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 19th edn, 1995 Search PubMed.
- Z. Shen, Y. L. Zhang and F. M. Jin, RSC Adv., 2012, 2, 797–801 RSC.
- B. Veriansyah, H. M. Choi, Y. W. Lee, J. W. Kang, J. D. Kim and J. Kim, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2009, 44, 1538–1544 CrossRef CAS PubMed.
- R. Baran, I. I. Kaminska, A. Srebowata and S. Dzwigaj, Microporous Mesoporous Mater., 2013, 169, 120–127 CrossRef CAS.
- Q. X. Wu, A. Majid and W. D. Marshall, Green Chem., 2000, 2, 127–132 RSC.
- E. Byambajav and Y. Ohtsuka, Appl. Catal., A, 2003, 252, 193–204 CrossRef CAS.
- N. M. Zhu, Y. Li and F. S. Zhang, Chem. Eng. J., 2011, 171, 919–925 CrossRef CAS.
- Y. F. Sun, M. Takaoka, N. Takeda, T. Matsumoto and K. Oshita, Chemosphere, 2006, 65, 183–189 CrossRef CAS PubMed.
- Y. F. Sun, M. Takaoka, N. Takeda, W. Wang, X. L. Zeng and T. L. Zhu, Chemosphere, 2012, 88, 895–902 CrossRef CAS PubMed.
- M. H. Cao, L. L. Wang, L. Wang, J. Chen and X. H. Lu, Chemosphere, 2013, 90, 2303–2308 CrossRef CAS PubMed.
- P. De Filippis, M. Scarsella and F. Pochetti, Ind. Eng. Chem. Res., 1999, 38, 380–384 CrossRef CAS.
- A. Berkessel, T. Schubert and T. N. Muller, J. Am. Chem. Soc., 2002, 124, 8693–8698 CrossRef CAS PubMed.
- B. Chan and L. Radom, J. Am. Chem. Soc., 2005, 127, 2443–2454 CrossRef CAS PubMed.
- G. H. Cartledge, J. Am. Chem. Soc., 1928, 50, 2863–2872 CrossRef CAS.
- L. L. Wu, A. Navrotsky, Y. Lee and Y. Lee, Microporous Mesoporous Mater., 2013, 167, 221–227 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |