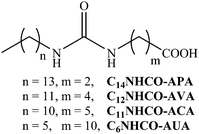
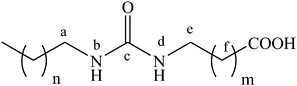
Effect of the position of the urea group in the hydrocarbon tail of fatty acid amphiphiles on the physical gelation of organic liquids
Amrita Pal and
Joykrishna Dey*
Department of Chemistry, Indian Institute of Technology, Kharagpur, Kharagpur-721 302, India. E-mail: joydey@chem.iitkgp.ernet.in; Fax: +91-3222-255303; Tel: +91-3222-283308
First published on 24th March 2014
Abstract
To investigate the effect of the position of the urea group in the hydrocarbon tail of an amphiphilic gelator, three positional isomers of 3-N-(n-tetradecylcarbamoyl)aminopropanoic acid (C14NHCO-APA) were designed and synthesized. The gelation behavior of these three amphiphiles in different organic solvents was investigated and compared. The amphiphiles were found to gelate organic solvents employed, but only in presence of H2O. The gelation in all the solvents employed was observed to be thermoreversible, having gel-to-sol transition temperatures (Tgs) above the physiological temperature (37 °C). It was observed that the critical gelation concentration (CGC) of the amphiphiles decreased; that is, gelation ability increased with the increase of spacer length between the –COOH and –NHCONH groups. The results suggested that H-bonding and van der Waals interactions have a major contribution in the gelation process. The morphology of the organogels was studied by XRD spectroscopy and electron microscopy. The amphiphiles were observed to form ribbon-like aggregates of high aspect ratio in organic solvents. The mechanical strength of the gels was studied by rheology. Their gelation abilities, Tgs values and values of yield stress (σy) of the organogels follow a similar trend, indicating that the gel becomes stronger when the urea linkage is in the middle of the hydrocarbon tail of the amphiphile.
Introduction
Gelators are small organic molecules which turn a liquid into gel above a critical concentration.1 Researchers have been working on urea- or bisurea-based hydrogelators2,3 and organogelators4–11 for decades because of their biodegradability and use in different fields, such as water purifier2 and biocatalysis.4 The urea (–NHCONH–) linkage is similar to amide (–NHCO–) linkage, but unlike the amide group it has two H-bond donor sites and one H-bond acceptor site and therefore can be used as a very useful H-bond building block for supramolecular organization.12,13 Urea derivatives form “bifurcated H-bonding”14,15 in which H-bonding network formation occurs between a carbonyl group of one urea unit and two hydrogen atoms of a neighboring molecule. Long-chain urea derivatives have attracted attention in the recent past16,17 because of their characteristic structure-forming abilities derived from this bifurcated H-bonding. The urea amphiphiles studied earlier have urea linkage directly attached to the α- or β-carbon of the amino acid head-group.18 Results have indicated that the β-alanine derivative, 3-N-(n-tetradecylcarbamoyl)aminopropanoic acid (C14-NHCO-APA)18 exhibits gelation behavior closely similar to that of N-(n-tetradecylcarbamoyl)-L-alanine (C14-NHCO-Ala).18 The major driving forces for the gelation were the van der Waals interactions between long hydrocarbon tails and the H-bonding interaction of the urea linkages between two amphiphiles. The –COOH group of the amphiphiles is also involved in intermolecular H-bonding between the amphiphile molecules. In this study, the effect of position of the urea linkage in the hydrocarbon chain on the gelation behavior is investigated. Three positional isomers 5-N-(n-dodecylcarbamoyl)-aminovaleric acid (C12-NHCO-AVA), 6-N-(n-undecylcarbamoyl)aminocaproic acid (C11-NHCO-ACA), and 11-N-(n-hexylcarbamoyl)aminoundecanoic acid (C6-NHCO-AUA) (see Chart 1 for structures) of C14-NHCO-APA were synthesized and the gelation behavior of these three amphiphiles in different organic solvents was investigated and compared with that of the former. These amphiphiles with the same H-bonding (–NHCONH– and –COOH) functionalities are of the same total length. That is, they have the same number of C, H, N, and O atoms. The position of the –NHCONH– group is only changed along the hydrocarbon tail. All the amphiphiles have an almost linear backbone. The melting point values of the solid gelators suggest that their intermolecular interactions decrease in the order C14-NHCO-APA > C12-NHCO-AVA > C11-NHCO-ACA > C6-NHCO-AUA. If H-bonding is the major driving force, then gelation abilities should also decrease in the same order. Therefore, the gelation abilities of the amphiphiles were investigated in different solvents.Experimental section
Materials
3-Aminopropanoic acid (3-APA), 5-aminovaleric acid (5-AVA), 6-aminocaproic acid (6-ACA) and 11-aminoundecanoic acid (11-AUA), mesitylene (Ph(Me)3), hexyl isocyanate, dodecyl isocyanate, tetradecyl isocyanate, and the deuterated solvents (D2O, NaOD) were purchased from Sigma-Aldrich (Bangalore, India). Hydrochloric acid and triethylamine (TEA) were procured from SRL. These reagents were used directly from the bottle. The organic solvents, such as benzene (PhH), toluene (PhMe), o-xylene (o-Ph(Me)2), m-xylene (m-Ph(Me)2), p-xylene (p-Ph(Me)2), chlorobenzene (PhCl), nitrobenzene (PhNO2), chloroform (CHCl3), and tetrachloromethane (CCl4) were of the highest purity commercially available from Spectrochem (Mumbai, India) and were dried and distilled fresh before use. Ethanol was obtained from Jiangsu Huaxi International Trade Co., Ltd., and was distilled fresh before use. All the surfactants employed in this study were synthesized in the laboratory as described below.Synthesis of N-(n-alkylcarbamoyl)aminoalkanoic acid
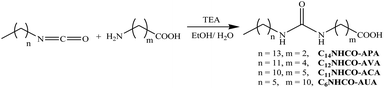
The reactions (Scheme 1) of tetradecyl isocyanate, dodecyl isocyanate, undecyl isocyanate or hexyl isocyanate were carried out with the appropriate amino acids (3APA, 5AVA, 6ACA or 11AUA) as described in previous publication.18 Briefly, amino acids and the corresponding alkyl isocyanate (1 eq.) were reacted in 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) ethanol–water mixture (10 mL) containing TEA (1.5 eq.) in a round bottomed flask at room temperature for 20 h. The compound was precipitated from ethanol–water mixture upon acidification (pH 2) with 1 N HCl as white solid. The compound was purified by recrystallizing from ethanol–water mixture (50
20 (v/v) ethanol–water mixture (10 mL) containing TEA (1.5 eq.) in a round bottomed flask at room temperature for 20 h. The compound was precipitated from ethanol–water mixture upon acidification (pH 2) with 1 N HCl as white solid. The compound was purified by recrystallizing from ethanol–water mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). Chemical structures of the amphiphiles were determined by FT-IR, 1H-NMR spectra, and elemental analysis.
50). Chemical structures of the amphiphiles were determined by FT-IR, 1H-NMR spectra, and elemental analysis.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O); 1H-NMR: δH in ppm (200 MHz, D2O) 0.96 (3H, t, CH3), 1.29 (24H, m, alkyl chain), 3.16 (2H, m, CH2–NH), 2.49 (2H, t, (NH)CH2CH2COOH), 3.43 (2H, t, (NH)CH2CH2COOH); CHN analysis: calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.75%, H: 11.19%, N: 8.92%.
O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O); 1H-NMR: δH in ppm (200 MHz, D2O) 0.96 (3H, t, CH3), 1.29 (24H, m, alkyl chain), 3.16 (2H, m, CH2–NH), 2.49 (2H, t, (NH)CH2CH2COOH), 3.43 (2H, t, (NH)CH2CH2COOH); CHN analysis: calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.75%, H: 11.19%, N: 8.92%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O). 1H-NMR: δH in ppm (400 MHz, D2O): 0.96 (3H, t, CH3), 1.29 (18H, m, alkyl chain), 1.55 (6H, m, CH2CH2NH, NHCH2CH2, CH2CH2COOH), 2.23 (2H, t, CH2CH2COOH), 3.15 (2H, t, CH2CH2NH); CHN analysis: calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.92%, H: 11.10%, N: 8.69%.
O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O). 1H-NMR: δH in ppm (400 MHz, D2O): 0.96 (3H, t, CH3), 1.29 (18H, m, alkyl chain), 1.55 (6H, m, CH2CH2NH, NHCH2CH2, CH2CH2COOH), 2.23 (2H, t, CH2CH2COOH), 3.15 (2H, t, CH2CH2NH); CHN analysis: calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.92%, H: 11.10%, N: 8.69%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O). 1H-NMR: δH in ppm (200 MHz, D2O): 0.96 (3H, t, CH3), 1.29 (18H, m, alkyl chain), 1.55 (6H, m, CH2CH2NH, NHCH2CH2, CH2CH2COOH), 2.23 (2H, t, CH2CH2COOH), 3.15 (2H, t, CH2CH2NH), CHN analysis: calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.55%, H: 11.19%, N: 8.72%.
O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O). 1H-NMR: δH in ppm (200 MHz, D2O): 0.96 (3H, t, CH3), 1.29 (18H, m, alkyl chain), 1.55 (6H, m, CH2CH2NH, NHCH2CH2, CH2CH2COOH), 2.23 (2H, t, CH2CH2COOH), 3.15 (2H, t, CH2CH2NH), CHN analysis: calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.55%, H: 11.19%, N: 8.72%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O); 1H-NMR: δH in ppm (D2O, 200 MHz) 0.96 (3H, t, CH3), 1.29 (18H, m, alkyl chain), 1.55 (6H, m, CH2CH2NH, NHCH2CH2, CH2CH2COOH), 2.23 (2H, t, CH2CH2COOH), 3.15 (2H, t, CH2CH2NH); CHN analysis calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.45%, H: 11.39%, N: 8.22%.
O of acid), 1628 (amide-I), 1563 (amide-II), 1219 (C–O); 1H-NMR: δH in ppm (D2O, 200 MHz) 0.96 (3H, t, CH3), 1.29 (18H, m, alkyl chain), 1.55 (6H, m, CH2CH2NH, NHCH2CH2, CH2CH2COOH), 2.23 (2H, t, CH2CH2COOH), 3.15 (2H, t, CH2CH2NH); CHN analysis calcd for C18H36N2O3 C: 65.81%, H: 11.05%, N: 8.53%; found C: 65.45%, H: 11.39%, N: 8.22%.Methods and instrumentation
Melting point (m.p.) of solid compounds was measured using Instind (Kolkata) melting point apparatus with open capillaries. The FTIR spectra were measured with a Perkin-Elmer (Model Spectrum Rx I) spectrometer. The 1H-NMR spectra were recorded on an AVANCE DAX-400 (Bruker, Sweden) 400 MHz NMR spectrometer in D2O–NaOD solvent with CH3CN as a standard. All measurements were done at 298 K unless otherwise mentioned.For field emission scanning electron microscopy (FESEM) measurement, the hot sample solution was placed on an aluminum foil. It was first air dried at room temperature and then kept in a desiccator for 24 h. A layer of gold was sputtered on top to make a conducting surface and finally the specimen was transferred into the FESEM (Zeiss, Supra-40) operating at 5–10 kV to get a micrograph.
X-ray diffraction (XRD) spectra were taken at room temperature for all air-dried organogel samples prepared on a glass slide. The experiment was performed on a Pan analytical X'Pert pro X-ray diffractometer using Cu target (Cu-Kα) and Ni filter at a scanning rate of 0.001 s−1 between 2 to 12°, operating at a voltage of 40 kV and a current of 30 mA.
Rheology measurements were performed on a Bohlin RS D-100 (Malvern, UK) rheometer using parallel-plate (PP-20) geometry. The gap between the plates was fixed at 100 μm. The organogel was placed on the rheometer and a stress-amplitude sweep experiment was performed at a constant oscillation frequency of 1.0 Hz at 25 °C. From this measurement, a linear viscoelastic region was identified and then a frequency sweep measurement was performed over a wide frequency range 0.01–100 Hz at a particular stress taken from the linear viscoelastic region at 25 °C.
The gelation behavior of the amphiphiles was studied following the same procedure as described for C14-NHCO-APA.18 Because the gelation studies of C14-NHCO-APA gelator were carried out in the presence of H2O at a H2O/gelator mole ratio of 50, the gelation behavior of the other three amphiphiles was also studied in the presence of the same amount of H2O. Gelation abilities of the amphiphiles were determined in terms of critical gelation concentration (CGC), which is defined as the minimum concentration of gelator required to gelate a certain amount of solvent. Gelation was studied by dissolving 5 mg of a solid gelator in a screw capped vial in requisite volume of organic solvent by heating in a hot water bath (∼50–80 °C as per solvent's boiling point) and subsequently allowed to cool at 298 K in a temperature controlled water bath (Julabo, Model F12). The gelation was confirmed by the vial inversion test; it was considered to be a gel when the material did not flow due to gravity upon inversion of the vial.
Results and discussion
Gelation behavior
The amphiphiles were found to gelate all the organic solvents employed, but only in the presence of a small quantity of H2O. The gelation abilities measured in terms of CGC values are summarized in Table 1. It was observed that all four gelators exhibit good gelation abilities; the CGC values ranged between 0.2 and 1.5% (w/v) in the solvents employed. Though the CGC values, within the experimental error limit, of C6NHCO-AUA and C11NHCO-ACA were comparable in some solvents, it was observed that among these gelators, C11NHCO-ACA had the lowest CGC value in most of the solvents. This suggests that the CGC value of the amphiphiles decreased (i.e., gelation ability increased) with an increase in spacer chain length. In order to understand this, the molecular geometry of the amphiphiles was analyzed. The molecular geometry of these amphiphiles was optimized by use of Chemdraw 7.0 software.19 It was observed that for C11NHCO-ACA the spacer chain is linear, as suggested by the large dihedral angles (∼180°) (Table 2), in contrast to the other amphiphiles for which the dihedral angles are smaller, corresponding to bent structure. As a result, C11NHCO-ACA molecules pack more tightly in the self-assembly without any steric hindrance. In the case of C6NHCO-AUA, though the dihedral angle is not as large as in C11NHCO-ACA, it is greater than those of amphiphiles 1 and 2. Thus, it has a slightly less bent structure, which is responsible for the better packing in the self-assembly and hence a higher gelation capacity.| Solvent | CGC (±0.1% w/v) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| PhH | 1.5 | 1.0 | 0.2 | 0.4 |
| PhMe | 1.4 | 0.8 | 0.2 | 0.2 |
| o-Ph(Me)2 | 0.7 | 0.5 | 0.2 | 0.3 |
| m-Ph(Me)2 | 0.8 | 0.6 | 0.2 | 0.2 |
| p-Ph(Me)2 | 0.6 (330) | 0.3 (333) | 0.2 (335) | 0.3 (337) |
| Ph(Me)3 | 0.9 | 0.5 | 0.3 | 0.3 |
| PhCl | 0.8 | 0.4 | 0.2 | 0.3 |
| PhNO2 | 0.7 | 0.3 | 0.2 | 0.2 |
| CHCl3 | 1.0 | 1.0 | 0.7 | 0.8 |
| CCl4 | 1.3 | 1.1 | 0.8 | 0.9 |
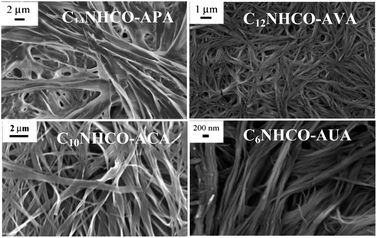
Morphology
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, which suggests an ordered one-dimensional (1-D) lamellar phase. These peaks are due to the length of a repeat unit along the long axis of the molecule. The inter-planar distance of the lamella is less than twice the extended molecular length19 (Table 3). It was observed that the d-values for the gels formed by C14NHCO-APA (4.01 nm) and C12NHCO-AVA (4.03 nm) are higher than that of the other two. In these amphiphiles, the urea linkage is in the –COOH end with a long C14 or C12 alkyl chain. The other two amphiphiles have urea linkage in the middle part causing strong van der Waals interactions from both sides of the urea linkage, along with H-bonding between urea groups and between carboxylic acid groups. So these amphiphiles have greater interdigitation, which is responsible for the shorter bilayer thickness. But all the organogels have similar morphology and are crystalline in nature.
4, which suggests an ordered one-dimensional (1-D) lamellar phase. These peaks are due to the length of a repeat unit along the long axis of the molecule. The inter-planar distance of the lamella is less than twice the extended molecular length19 (Table 3). It was observed that the d-values for the gels formed by C14NHCO-APA (4.01 nm) and C12NHCO-AVA (4.03 nm) are higher than that of the other two. In these amphiphiles, the urea linkage is in the –COOH end with a long C14 or C12 alkyl chain. The other two amphiphiles have urea linkage in the middle part causing strong van der Waals interactions from both sides of the urea linkage, along with H-bonding between urea groups and between carboxylic acid groups. So these amphiphiles have greater interdigitation, which is responsible for the shorter bilayer thickness. But all the organogels have similar morphology and are crystalline in nature.
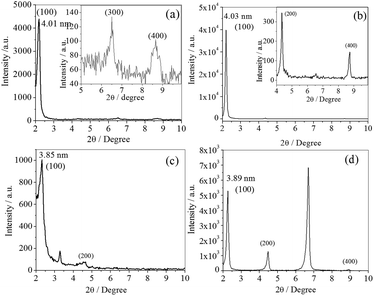 | ||
| Fig. 2 X-ray diffractograms of the air-dried p-Ph(Me)2 organogels of (a) C14NHCO-APA, (b) C12NHCO-AVA, (c) C11NHCO-ACA, and (d) C6NHCO-AUA. | ||
| Gelator | lc (nm) | 2θ (degree) | d (nm) | Plane |
|---|---|---|---|---|
| C14NHCO-APA | 2.25 | 2.19, 6.52, 8.70 | 4.01 | 100, 200, 300 |
| C12NHCO-AVA | 2.25 | 2.19, 4.35, 8.74 | 4.03 | 100, 200, 400 |
| C11NHCO-ACA | 2.25 | 2.31, 3.27, 4.61 | 3.85 | 100, 200 |
| C6NHCO-AUA | 2.25 | 2.26, 4.46, 6.68, 8.92 | 3.89 | 100, 200, 400 |
Thermal stability
The organogels were very stable at room temperature (25 °C) and they were thermo-reversible, i.e., when the temperature was increased above a certain value, the gel melted to produce a solution/dispersion and after cooling to room temperature, the gel was formed again. The thermal stability of the organogels was measured in terms of the gel-to-sol transition temperature (Tgs). The Tgs values of the p-Ph(Me)2 organogels containing 0.05 M C14NHCO-APA, C12NHCO-AVA, C11NHCO-ACA and C6NHCO-AUA are 330 K, 333 K, 335 K and 337 K, respectively. This means that the intermolecular interactions increase as the –NHCONH– group shifts toward the middle of the hydrocarbon chain. This is due to stronger H-bonding interactions between urea linkages of neighboring amphiphiles as a result of linear backbone. The results are consistent with the CGC values of the gelators.Mechanical stability
The mechanical strength of a gel is measured by the storage modulus (G′) and loss modulus (G′′), which are dependent on gelator concentration. Therefore, to compare the viscoelastic properties of the p-Ph(Me)2 organogels, rheological measurements were performed with a fixed (0.15 M) gelator concentration. The mechanical strength of the organogels was estimated by the amplitude sweep measurements. Fig. 3 shows the plots of G′ and G′′ versus applied stress (σ) at a constant frequency of 1 Hz. It can be observed that above a critical stress, called yield stress (σy), G′ and G′′ of the organogels fall abruptly to a very low value, indicating flow of the gel. The σy values of the organogels increase in the order C14NHCO-APA (2205 Pa) < C12NHCO-AVA (2481 Pa) < C11NHCO-ACA (2617 Pa) < C6NHCO-AUA (2853 Pa). Interestingly, this order is same as the order of change of Tgs and CGC values of the organogels discussed above. This further emphasizes the fact that the mechanical strength of the organogels is dependent on the intermolecular interactions involved in the gelation process. Frequency sweep measurement was performed in the linear viscoelastic regime that was obtained from the stress sweep measurement. The frequency sweep (Fig. 4) measurements indicate that G′ and G′′ values of the organogels are independent of frequency (f) in a wide range. Also, the higher value of G′ compared to G′′ suggests that the organogels are highly viscoelastic.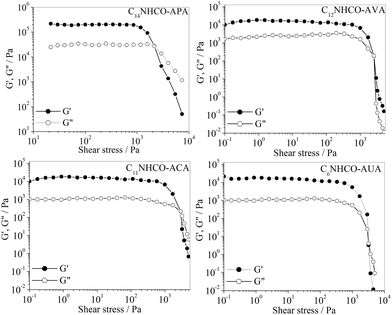 | ||
| Fig. 3 Variation of the storage modulus (G′) and loss modulus (G′′) of the p-Ph(Me)2 organogels of the amphiphiles (0.15 M) with the shear stress at 298 K. | ||
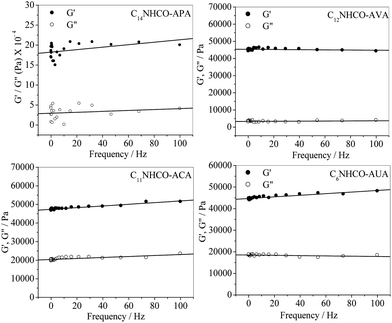 | ||
| Fig. 4 Variation of the storage modulus (G′) and loss modulus (G′′) of the p-Ph(Me)2 organogels of the amphiphiles (0.15 M) with the frequency at 298 K. | ||
Conclusions
As with 3-N-(n-tetradecylcarbamoyl)aminopropionic acid, its positional isomers C12NHCO-AVA, C11NHCO-ACA, and C6NHCO-AUA were also observed to exhibit an efficient water-induced organogelation in different organic solvents in the presence of a small amount of H2O. These gelators have better gelation abilities than N,N′-dialkyl urea derivatives already reported in the literature.16 The gelator having urea linkage in the middle of the hydrocarbon chain has lower CGC value and hence greater gelation ability than the other amphiphiles. Their gelation abilities and the gel-to-sol transition temperatures (Tgs) and mechanical strengths (σy) of the organogels increase in the order C14NHCO-APA < C12NHCO-AVA < C11NHCO-ACA < C6NHCO-AUA. This means that these properties of the organogels are functions of intermolecular interactions, such as van der Waals and H-bonding interactions between gelator molecules. The results suggest that for optimum gelation ability a balance between the van der Waals forces and H-bonding interactions is essential for this class of gelator molecules. The gel morphology, however, does not change with the molecular structure of the amphiphiles.Acknowledgements
The authors acknowledge Indian Institute of Technology, Kharagpur, for financial support of this work. We thank Mr Kiran Patruni, Department of Agricultural Science, IIT Kharagpur, for his help with the rheological measurements.Notes and references
- J. C. MacDonald and G. M. Whitesides, Chem. Rev., 1994, 94, 2383 CrossRef CAS.
- D. M. Wood, B. W. Greenland, A. L. Acton, F. Rodríguez-Llansola, C. A. Murray, C. J. Cardin, J. F. Miravet, B. Escuder, I. W. Hamley and W. Hayes, Chem.–Eur. J., 2012, 18, 2692 CrossRef CAS PubMed.
- G. O. Lloyd and J. W. Steed, Soft Matter, 2011, 7, 75 RSC.
- Z. Qi, C. Wu, P. M. de Molina, H. Sun, A. Schulz, C. Griesinger, M. Gradzielski, R. Haag, M. B. Ansorge-Schumacher and C. A. Schalley, Chem.–Eur. J., 2013, 19, 10150 CrossRef CAS PubMed.
- C. Dou, C. Wang, H. Zhang, H. Gao and Y. Wang, Chem.–Eur. J., 2010, 16, 10744 CrossRef CAS PubMed.
- N. Zweep, A. Hopkinson, A. Meetsma, W. R. Browne, B. L. Feringa and J. H. van Esch, Langmuir, 2009, 25, 8802 CrossRef CAS.
- M. O. M. Piepenbrock, N. Clarke and J. W. Steed, Soft Matter, 2011, 7, 2412 RSC.
- G. O. Lloyd, M. O. M. Piepenbrock, J. A. Foster, N. Clarkec and J. W. Steed, Soft Matter, 2012, 8, 204 RSC.
- C. C. Lu and S. K. Su, Supramol. Chem., 2009, 21, 572 CrossRef CAS.
- J. Rubio, V. Martí-Centelles, M. I. Burguete and S. V. Luis, Tetrahedron, 2013, 69, 2302 CrossRef CAS PubMed.
- N. Goyal, S. Cheuk and G. Wang, Tetrahedron, 2010, 66, 5962 CrossRef CAS PubMed.
- K. Hanabusa, K. Shimura, K. Hirose, M. Kimura and H. Shirai, Chem. Lett., 1996, 885 CrossRef CAS.
- J. Van Esch, S. D. Feyter, R. M. Kellogg, F. C. D. Schryver and B. L. Feringa, Chem.–Eur. J., 1997, 3, 1238 CrossRef CAS.
- T. Seki, T. Fukuchi and K. Ichimura, Langmuir, 2002, 18, 5462 CrossRef CAS.
- T. Kobayashi and T. Seki, Langmuir, 2003, 19, 9297 CrossRef CAS.
- M. George, G. Tan, V. T. John and R. G. Weiss, Chem.–Eur. J., 2005, 11, 3243 CrossRef CAS PubMed.
- G. Wang and A. D. Hamilton, Chem. Commun., 2003, 310 RSC.
- A. Pal and J. Dey, Langmuir, 2011, 27, 3401 CrossRef CAS PubMed.
- The hydrocarbon chain lengths of the amphiphiles were obtained from the energy minimized (using MM2 force field of ChemDraw Ultra 8.0 software) structures of the amphiphiles; the distance between the β-carbon and the –CH3 group was considered. The dihedral angles of the energy minimized structures were also obtained using the same software.
| This journal is © The Royal Society of Chemistry 2014 |