Simultaneous influence of Zn2+/Mg2+ on the luminescent behaviour of La2O3:Tm3+–Yb3+ phosphors
Astha Kumari,
Anurag Pandey,
Riya Dey and
Vineet Kumar Rai*
Laser and Spectroscopy Laboratory, Department of Applied Physics, Indian School of Mines, Dhanbad-826004, Jharkhand, India. E-mail: vineetkrrai@yahoo.co.in; rai.vk.ap@ismdhanbad.ac.in; Tel: +91-326-223-5404
First published on 23rd April 2014
Abstract
The structural characterizations of La2O3 phosphors doped/codoped with Tm3+ and Yb3+ ions synthesized by the urea assisted solution combustion technique were performed using X-ray diffraction analysis, Fourier transform infrared spectroscopy and scanning electron microscopy. Codoping with Zn2+/Mg2+ ions causes an increase in the particle size and aggregation for the La2O3:Tm3+–Yb3+ phosphor. The upconversion (UC) study under 980 nm excitation shows four UC emission bands centred around 476 nm (1G4 → 3H6), 653 nm (1G4 → 3F4), 702 nm (3F2 → 3H6) and 795 nm (1G4 → 3H5). The effect of codoping with Zn2+ and Mg2+ ions in the Tm3+–Yb3+ codoped La2O3 phosphor was investigated. Decay curve analysis of the prepared phosphors was done to understand the mechanism of the upconversion emission intensity variation due to codoping with Zn2+/Mg2+ ions. The purity of colour emitted from the sample does not show any change with variations in pump power density.
1. Introduction
Owing to their excellent luminescent behaviour, luminescence materials have found wide applications in the field of optical data storage, lasers, temperature sensors, detection of infrared radiation, colour displays, biomedical diagnostics, upconverters, optical amplifiers, telecommunication, plasma display panels, high definition televisions (HDTVs), light emitting diodes, cathode ray tubes (CRTs), finger print detection, scintillators, etc.1–8 In these materials, luminescence takes place due to energy transition within the energy levels of rare earth ions.1 Rare earth (RE) ions are characterized by a partially filled 4f shell that is shielded by completely filled outer 5s2 and 5p6 orbitals, which causes sharp luminescence, followed by intra 4f shell transitions. The energy levels of the rare earth ions are therefore largely insensitive to the host environment in which they are placed and that leads to their radiative transitions in solid hosts which resemble those of the free ions.9 Lanthanide elements when incorporated into crystalline or amorphous hosts, exist in a triply ionized state, or occasionally, a doubly ionized oxidation state.The upconversion emission from the singly thulium doped luminescent materials has been observed by many researchers10–14 but stronger luminescence was observed when thulium was codoped with ytterbium.12,15,16 This is because of the high absorption cross-section and favourable energy level structure of Yb3+ ions for being excited to the higher energy level upon 980 nm excitation.17,18 The selection of appropriate host material is important in the preparation of micro/nanocrystalline luminescent materials with efficient optical properties. Of the various solid host materials, oxides and fluorides are mostly preferred because of their low cut-off phonon frequencies which results in lesser energy loss due to non-radiative relaxation channels.19 La2O3, a solid material, is expected to be suitable for the preparation of highly luminescent phosphor materials due to its low cost, good chemical durability and thermal stability, low phonon frequency (∼400 cm−1) etc.20,21 Furthermore, La2O3 has been considered as an attractive host material for codoping of triply ionised lanthanides owing to its comparable ionic radius. It is known that the codoping with rare-earth ions increases the pump efficiency and hence the intensity of the upconversion (UC) emission, but the effect of codoping with non-rare earth ions has rarely been studied. Researchers have studied the effects of co-doping with zinc and magnesium individually on the electrical, structural, electrochemical and photoluminescence properties of rare earth doped/codoped luminescent materials. The UC luminescence enhancement of about ∼37 times for the green band at ∼551 nm in the Nd3+–Yb3+–Zn2+ codoped Y2O3 phosphor upon excitation at 980 nm has been observed by Dey et al.22 The effect of magnesium doping on the structural as well as optical properties of Zn2GeO4:Mn phosphor has been reported by Anoop et al.23 The enhancement of about ∼60 times in the green UC intensity has been observed due to codoping with Mg2+ ions in the Er3+–Yb3+ codoped CaAl12O19 phosphor.24 But the effects of codoping by a combination of non-rare earth ions like zinc and magnesium on the UC luminescence have not been reported until now to the best of our knowledge.
Here, we report the synthesis and characterization of the prepared phosphors. The near infrared (NIR) to visible frequency UC emission of the Tm3+–Yb3+ codoped La2O3 phosphors and the effects of Zn2+–Mg2+ codoping on the UC emissions arising from the thulium ions have been studied. XRD, FTIR, pump power dependence and decay curve analysis have been studied in order to obtain information on the mechanism involved in the frequency of UC emissions. The effect of the variations of pump power density on the colour emitted from the developed phosphors has also been examined.
2. Experimental
2.1 Material synthesis
The Tm3+, Tm3+–Yb3+, Tm3+–Yb3+–Zn2+ and Tm3+–Yb3+–Zn2+–Mg2+ doped/codoped La2O3 phosphors have been prepared by a combustion route. The compositions taken for the synthesis of these phosphor powders are as follows:| (100 − p − q)La2O3 + pTm2O3 + qYb2O3 | (1) |
| (100 − p − q − r)La2O3 + pTm2O3 + qYb2O3 + rZnO | (2) |
| (100 − p − q − r − s)La2O3 + pTm2O3 + qYb2O3+ rZnO + sMgCO3 | (3) |
2.2 Characterization
For the structural study, X-ray diffraction (XRD) analysis and scanning electron microscopy (SEM) have been done using Bruker D8 advanced X-ray diffractometer and HITACHI (S-3400N) scanning electron microscope, respectively. To study the presence of impurities in the samples, Fourier transform infrared (FTIR) spectra of the samples were recorded in the 400–4000 cm−1 spectral region on a Perkin Elmer Spectrum RX1 spectrophotometer. The UC emission spectra of the phosphor powders were recorded through a monochromator attached with a photomultiplier tube (PMT) upon excitation with 980 nm continuous wave (CW) diode laser. The lifetime analysis was performed by chopping the 980 nm laser beam by using a mechanical chopper and measurements were recorded with the help of a quick start digital oscilloscope. All the measurements were performed at room temperature.3. Results and discussion
3.1. XRD analysis
The powder XRD patterns of the Tm3+–Yb3+; Tm3+–Yb3+–Zn2+ and Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphor samples were obtained between the range of 20 and 90 degrees (Fig. 1). All the XRD patterns have the same baselines and patterns, which shows that the crystal structure and phase of these prepared materials have not been altered due to codoping with rare-earth as well as non-rare-earth elements. A large number of intense diffraction peaks for different 2θ values of ∼26.71°, ∼29.6°, ∼30.55°, ∼39.94°, ∼46.66°, ∼52.72°, ∼55.53°, ∼63.04°, ∼72.88°, ∼75.94°, ∼79.42°, ∼81.37°, ∼84.37°, ∼85.72° corresponding to the diffraction from the (100), (002), (011), (012), (110), (103), (112), (202), (203), (211), (114), (122), (015), (300) planes, respectively, were observed. The observed structure of the prepared phosphors was indexed suitably with JCPDS file no. 74-1144 that shows the hexagonal phase of the La2O3 with lattice parameters a = b = 3.93 Å and c = 6.12 Å.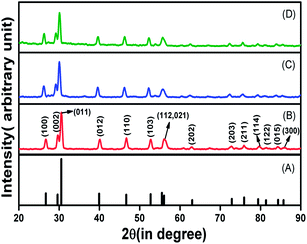 | ||
| Fig. 1 XRD pattern of (A) JCPDS file no. 74-1144, (B) Tm3+–Yb3+, (C) Tm3+–Yb3+–Zn2+, and (D) Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors. | ||
The average crystallite size of synthesized phosphors was calculated using the Debye–Scherrer equation:20
d = 0.89λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (4) |
In addition to the Debye–Scherrer method, the crystallite size of the prepared materials was also estimated with the help of Williamson–Hall analysis which satisfies the equation25
β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = (0.89λ/d) + 4ε θ = (0.89λ/d) + 4ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (5) |
The strain present in the material and the crystallite size could be extracted from the slope and the intercept, respectively, of the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ versus β
θ versus β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ plot. The Williamson–Hall plots for Tm3+–Yb3+, Tm3+–Yb3+–Zn2+ and Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors are shown in Fig. 2.
θ plot. The Williamson–Hall plots for Tm3+–Yb3+, Tm3+–Yb3+–Zn2+ and Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors are shown in Fig. 2.
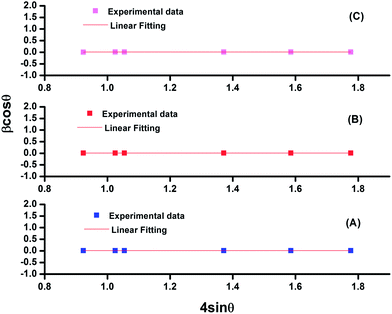 | ||
| Fig. 2 Williamson–Hall plot for (A) Tm3+–Yb3+, (B) Tm3+–Yb3+–Zn2+, and (C) Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors. | ||
The values of the crystallite size as calculated by Williamson–Hall analysis are 15.65 nm, 16.81 nm and 28.29 nm for La2O3:Tm3+–Yb3+, La2O3:Tm3+–Yb3+–Zn2+ and La2O3:Tm3+–Yb3+–Zn2+–Mg2+ phosphors, respectively. Also, by analysing the results it can be concluded that the crystallite size calculated by both methods (Debye–Scherrer Method and Williamson–Hall method) for all three samples are comparable and a slight increase in the crystallite size is observed as we move from La2O3:Tm3+–Yb3+ to La2O3:Tm3+–Yb3+–Zn2+–Mg2+ via La2O3:Tm3+–Yb3+–Zn2+ phosphors. The crystallite size in each case was found to be in the nanometer range, which indicates that the developed phosphors are nanocrystalline in nature.
3.2 FTIR Spectroscopy
The FTIR spectra of the prepared materials annealed at 800 °C temperature were recorded in the 400–4000 cm−1 region (Fig. 3). The presence of different impurities in the prepared materials was traced by matching the vibrational frequencies corresponding to each impurity in the FTIR spectrum. From the FTIR spectrum of the prepared samples it can be concluded that functional groups like –OH, –CH and –NO are present in all the samples. The peaks observed around 3613 cm−1 and 2382 cm−1 are due to asymmetric stretching vibration of O–H. The peaks around 2930 cm−1 and 1475 cm−1 are due to C–H and N–O stretching vibrations, respectively. It is also observed from the FTIR spectrum that the presence of O–H impurity observed around 3613 cm−1 was reduced in the case of La2O3:Tm3+–Yb3+–Zn2+–Mg2+ phosphor compared to other cases. The peak around 650 cm−1 is due to the lattice vibration of La–O.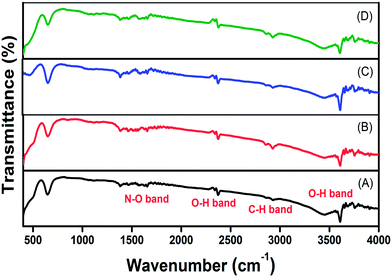 | ||
| Fig. 3 FTIR spectrum of (A) Tm3+, (B) Tm3+–Yb3+, (C) Tm3+–Yb3+–Zn2+, and (D) Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors. | ||
3.3 SEM Image
The surface morphology and crystallinity of solid host materials are important parameters which determine the emission characteristics of phosphors. The microstructural analysis of La2O3:Tm3+–Yb3+ and La2O3:Tm3+–Yb3+–Zn2+–Mg2+ samples was performed with the help of SEM examination (Fig. 4). From Fig. 4 it is clear that the aggregation and particle sizes of the La2O3:Tm3+–Yb3+ phosphors increase with codoping Zn2+/Mg2+ ions. The SEM image shows that the particles are not uniformly distributed throughout the surface, which is mainly caused by the inhomogeneous distribution of temperature during the synthesis of the material by the combustion technique. Also, it is clearly visualized that the particles are in the agglomerated form with their size in the micrometer range.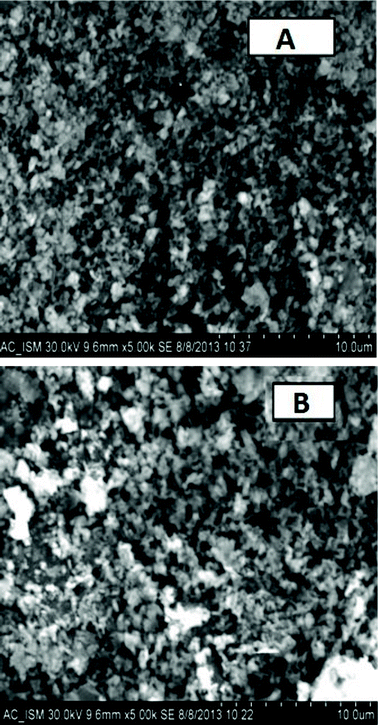 | ||
| Fig. 4 SEM images of (A) Tm3+–Yb3+ codoped La2O3 phosphor and (B) Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphor. | ||
3.4 Upconversion emissions study of La2O3:Tm3+–Yb3+ phosphor
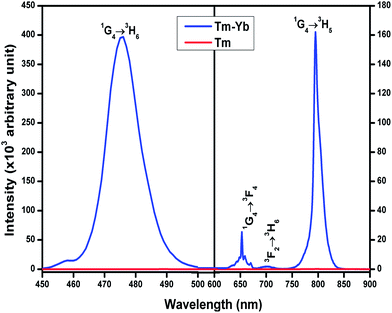
The room temperature frequency upconversion spectra of Tm3+/Tm3+–Yb3+ doped/codoped La2O3 phosphor powders upon excitation with 980 nm CW diode laser were recorded in the 450–900 nm range (Fig. 5). From Fig. 5, it is clear that no UC emission was observed under a 980 nm diode laser excitation from singly Tm3+ doped La2O3 phosphor. But in the case of La2O3:Tm3+–Yb3+ phosphor, four UC emission bands were observed around 476 nm, 653 nm, 702 nm and 795 nm corresponding to the 1G4 → 3H6, 1G4 → 3F4, 3F2 → 3H6 and 1G4 → 3H5 transitions, respectively.To get the optimum UC intensity, the concentration of Yb3+ ions was varied from 1.0 to 5.0 mol% with the Tm3+ ions concentration fixed at 0.1 mol%. The maximum UC emission intensity was observed for the 0.1 mol% Tm3+ + 3.0 mol% Yb3+ combination. The light emitted from the phosphor appears blue which could be detectable by the naked eye. The required NIR photons for the origin of these UC bands can be explained with the help of the pump power dependence study.
It is well known that for extremely small upconversion rates the number of photons involved in populating the upconversion emitting level can be calculated directly from the slope of the logarithmic plot of intensity versus pump power. But, as we increase the pump power to a higher value the upconversion luminescence caused by ‘n’ photon absorption is found to obey the relationship IUC < Pn.26
We know that UC emission intensity (IUC) is directly proportional to pump excitation power (P) in the low pump power region by the relationship,27
| IUC ∝ Pk | (6) |
The slopes corresponding to 476 nm and 653 nm bands are calculated to be ∼1.93 and ∼2.08, which shows that both the blue and red emissions are due to two photon absorption processes.
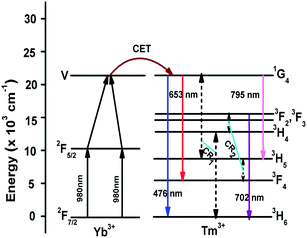
The origin of the UC emission bands can be explained with the help of an energy level diagram as shown in Fig. 6. The UC emissions observed from Tm3+–Yb3+ codoped La2O3 phosphor are due to the cooperative energy transfer (CET) from Yb3+ to Tm3+ ions only, as no UC emission is observed in the singly doped Tm3+:La2O3 phosphor. The Yb3+ ions present in the 2F7/2 ground state, after absorption of 980 nm NIR photon, get excited to the 2F5/2 manifold. In the 2F5/2 excited state, the excited donor Yb3+ ion transfers its excitation energy to the acceptor Yb3+ ions in the 2F5/2 state and these acceptor ions are further excited to the virtual level (V). The energy of the virtual level ‘V’ of Yb3+ ions is in resonance with the 1G4 (∼21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 cm−1) level of Tm3+ ions. So, the Yb3+ ions in the virtual level transfer their excitation energy to the Tm3+ ions in ground state. The ground state Tm3+ ions then get excited to the 1G4 level. Finally, the Tm3+ ions in 1G4 level transit downward to the 3H6, 3F4 (∼5765 cm−1) and 3H5 (∼8258 cm−1) levels, emitting blue, red and NIR emissions around 476 nm, 653 nm and 795 nm, respectively. The 3H4 (∼12
015 cm−1) level of Tm3+ ions. So, the Yb3+ ions in the virtual level transfer their excitation energy to the Tm3+ ions in ground state. The ground state Tm3+ ions then get excited to the 1G4 level. Finally, the Tm3+ ions in 1G4 level transit downward to the 3H6, 3F4 (∼5765 cm−1) and 3H5 (∼8258 cm−1) levels, emitting blue, red and NIR emissions around 476 nm, 653 nm and 795 nm, respectively. The 3H4 (∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556 cm−1) and 3F2 (∼15
556 cm−1) and 3F2 (∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 cm−1) levels of Tm3+ ion are populated by the cross-relaxation energy transfer mechanisms, CR1 and CR2 viz. (1G4, 3H6 → 3H5, 3H4) and (3H5, 3H4 → 3F4, 3F2) respectively. After that, the Tm3+ ions in the 3F2 level decay radiatively to the 3H6 level giving a photon at ∼702 nm corresponding to the 3F2 → 3H6 transition. The 3F3 (∼14
240 cm−1) levels of Tm3+ ion are populated by the cross-relaxation energy transfer mechanisms, CR1 and CR2 viz. (1G4, 3H6 → 3H5, 3H4) and (3H5, 3H4 → 3F4, 3F2) respectively. After that, the Tm3+ ions in the 3F2 level decay radiatively to the 3H6 level giving a photon at ∼702 nm corresponding to the 3F2 → 3H6 transition. The 3F3 (∼14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 627 cm−1) level is populated by non-radiative relaxation from the 3F2 level followed by the emission of a phonon. The emission peak intensity ratio between the blue and red emissions was ∼30 and that between the blue to NIR emissions was ∼5. This large UC emission intensity corresponding to the 1G4 → 3H6 transition is basically due to the direct cooperative energy transfer (CET) from the Yb3+ ions to the Tm3+ ions followed by the absorption of two NIR photons.
627 cm−1) level is populated by non-radiative relaxation from the 3F2 level followed by the emission of a phonon. The emission peak intensity ratio between the blue and red emissions was ∼30 and that between the blue to NIR emissions was ∼5. This large UC emission intensity corresponding to the 1G4 → 3H6 transition is basically due to the direct cooperative energy transfer (CET) from the Yb3+ ions to the Tm3+ ions followed by the absorption of two NIR photons.
3.5 Effect of Zn2+ and Mg2+ codoping in La2O3:Tm3+–Yb3+ phosphor
From Fig. 7 it is clearly observed that the UC intensity of all the bands is enhanced by the addition of Zn2+ and Mg2+ ions in the La2O3:Tm3+–Yb3+ phosphor. The variations in UC intensity with different doping concentrations of Zn2+ and Mg2+ ions were investigated. The enhancement of ∼2 times for the blue UC bands was observed with the codoping of 15.0 mol% Zn2+ ions in the Tm3+–Yb3+ codoped La2O3 phosphor. It was also observed that the intensities of UC bands are further increased when the Mg2+ ion is incorporated into the Tm3+–Yb3+–Zn2+ codoped La2O3 phosphor. The optimum concentration of Mg2+ ion is found to be 5.0 mol%. Enhancements of about 36 times, 34 times and 11 times in the intensity of UC peaks at ∼476 nm, ∼653 nm and ∼795 nm have been observed respectively with the codoping of 5.0 mol% Mg2+ ions compared to that of Tm3+–Yb3+ codoped La2O3 phosphor. The intensity of upconversion emission bands lying in the blue, red and NIR regions is increased with increasing concentration of Zn2+ and Mg2+ ions up to 15.0 mol% and 5.0 mol% respectively. The enhancement in the UC intensity is caused by the creation of the oxygen vacancies due to codoping with the Zn2+/Mg2+ ions in the La2O3 phosphor. These oxygen vacancies act as sensitizers for energy transfer due to the mixing of charge transfer states.28 This enhancement in the UC emission intensity may also be explained by the broadening in the absorption bands, followed by the overlap between the defect states produced by the codopants with the rare earth ions charge transfer states,29 which is beyond the scope of the present study.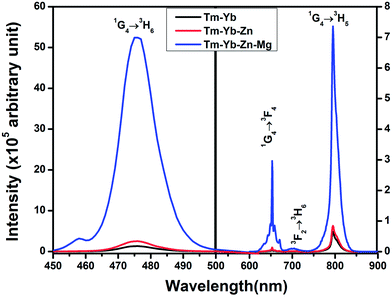 | ||
| Fig. 7 The upconversion emission spectra of Tm3+–Yb3+, Tm3+–Yb3+–Zn2+ and Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors. | ||
The UC emission peak positions remain unaltered with the codoping of Zn2+/Mg2+ ions. The non-occurrence of the spectral shift and the presence of a sharp UC emission peak are due to the effect of the shielding of 4f electrons by the outer 5s and 5p orbitals. The UC intensity for all of the bands is quenched beyond the optimum concentration of Zn2+ and Mg2+ ions. The reduction in the UC intensity is most probably due to imperfect atomic arrangement in the La2O3 phosphor. These defects present in solid host material may lead to quenching in the emission intensity.
The ionic radii of La3+, Tm3+, Yb3+, Zn2+ and Mg2+ are 103 pm, 88 pm, 86.8 pm, 74 pm and 72 pm, respectively. The ionic radii of Tm3+, Yb3+, Zn2+ and Mg2+ are smaller than that of La3+ (which has largest ionic radius compared to other rare earth ions). Also, there is a valency mismatch between Zn2+/Mg2+ and La3+ ion. Therefore, the excess codoping of Zn2+/Mg2+ ions can introduce distortion and hence defects into the La2O3 host. Thus, when the codoping concentration of Zn2+/Mg2+ ions is beyond the optimum value, the UC emission intensity is reduced.
Although many researchers have demonstrated that the enhancement in luminescence intensity is caused by Mg2+ incorporation, no one has reported such a large enhancement with Mg2+ codoping to the best of our knowledge.24,29,30 An enhancement of about 10 times in the red UC band by codoping of Mg2+ ions in CaAl12O19:Er3+:Yb3+ phosphor has been observed by Singh et al.24 A photoluminescence enhancement by a factor of 1.7 and 1.3 times for the red emission band in Eu3+ doped Y2O3 phosphor due to codoping with Mg2+ and Al3+ ions has been observed by Chong et al.29 Pan et al. have reported enhancement of more than 3 times in luminescence efficiency of CaAl12O19:Mn4+ phosphor due to the codoping of Mg2+ ions.30
From the FTIR spectrum (Fig. 3), it is clear that the presence of –OH impurity around 3613 cm−1 which acts as a quencher is reduced for La2O3:Tm3+–Yb3+–Zn2+–Mg2+ phosphor compared to other samples. This also supports why the UC emission intensity is significantly enhanced in the La2O3:Tm3+–Yb3+–Zn2+–Mg2+ phosphor. Therefore, such a large enhancement in UC luminescence intensity, as observed in La2O3:Tm3+–Yb3+–Zn2+–Mg2+, has made the present material a promising candidate as a good blue upconverter.
Decay curves analysis corresponding to the 1G4 → 3H6 (476 nm) blue transition of Tm3+ ions in the La2O3:Tm3+–Yb3+, La2O3:Tm3+–Yb3+–Zn2+ and La2O3:Tm3+–Yb3+–Zn2+–Mg2+ phosphors powder samples was performed (Fig. 8). The calculated values of decay time for the blue emission from the decay curves were found to be ∼1.78 ± 0.47 ms, ∼1.13 ± 0.12 ms, and ∼0.98 ± 0.06 ms for the La2O3:Tm3+–Yb3+, La2O3:Tm3+–Yb3+–Zn2+ and La2O3:Tm3+–Yb3+–Zn2+–Mg2+ phosphors, respectively. This observed variation in decay times of emitting level also support the increase in radiative transition probability of emitting state due to the incorporation of Zn2+ and Mg2+ ions into the La2O3:Tm3+–Yb3+ phosphor. The decrease in decay time results in an increase of the radiative transition probability of emitting state. The reason behind this increase in radiative transition probability due to Zn2+ and Mg2+ ions codoping is the sensitizing effect caused by the creation of oxygen vacancies, as discussed earlier.
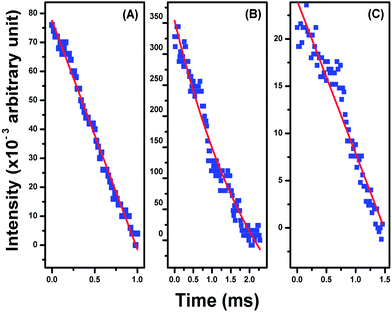 | ||
| Fig. 8 The fluorescence decay curves corresponding to the 1G4 → 3H6 (476 nm) blue transition of thulium ion in (A) Tm3+–Yb3+, (B) Tm3+–Yb3+–Zn2+ and (C) Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors. | ||
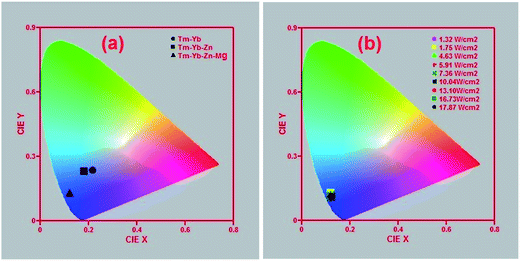
To compare the colours emitted by the Tm3+–Yb3+, Tm3+–Yb3+–Zn2+ and Tm3+–Yb3+–Zn2+–Mg2+ codoped phosphors, the CIE colour coordinates corresponding to these three samples at 10.04 W cm−2 power density were calculated and represented in Fig. 9(a), from which it is clearly visible that all these three phosphors are emitting blue colour but with the incorporation of Zn2+ and Mg2+ ions the colour is tuned towards the more bluish region. This indicates that the purity of the colour emitted by the sample is improved by codoping with Zn2+ and Mg2+ ions. In order to check the tunability of the colour emitted from the Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphor, the CIE colour coordinates of the sample were calculated at different excitation power densities as shown in Fig. 9(b), which shows that the present material is an efficient blue emitter. Based on the observed results, we can say that the present phosphor can be used as an efficient NIR to blue upconverter. It is observed from Fig. 9(b) that the blue colour emitted by the sample is independent of the pump power density, so it can be used in the fabrication of display devices. Also, the intense blue light emitted from the Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphor may be used in photodynamic therapy (PDT) for the diagnosis and localization of cancer cells.7
To examine the quality of white light, we calculated the Colour Correlated Temperature (CCT) values at different excitation powers densities using McCany empirical formula31 which is expressed as,
| CCT = −449n3 + 3525n2 − 6823n + 5520.33 | (7) |
The calculated values of CCT corresponding to different colour coordinates are presented in Table 1. The colour correlated temperatures for different power densities fall in the region of ∼4171 K to ∼5512 K. So, from the results it can be concluded that the CCT values lie in the cold white light region.
| Power densities (W cm−2) | CIE (x, y) coordinates | CCT value (K) | |
|---|---|---|---|
| x coordinate | y coordinate | ||
| 1.32 | 0.12 | 0.13 | 5848.70 |
| 1.75 | 0.12 | 0.13 | 5848.70 |
| 4.63 | 0.12 | 0.12 | 5093.34 |
| 5.91 | 0.12 | 0.12 | 5093.34 |
| 7.36 | 0.12 | 0.12 | 5093.34 |
| 10.04 | 0.12 | 0.11 | 4170.64 |
| 13.10 | 0.12 | 0.11 | 4170.64 |
| 16.73 | 0.12 | 0.11 | 4170.64 |
| 17.87 | 0.12 | 0.11 | 4170.64 |
4. Conclusion
The Tm3+–Yb3+, Tm3+–Yb3+–Zn2+ and Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphors have been successfully prepared by the combustion method. The XRD patterns of the synthesized materials shows that these phosphors exhibit the hexagonal phase. The crystallite sizes of the materials calculated by Scherrer's formula as well as by the Williamson–Hall analysis show that the phosphor materials are nanocrystalline. The SEM images of the prepared materials reveal agglomerations within the materials with particle size in the micrometer range. The SEM analysis shows an increase in the particle size due to the introduction of Zn2+/Mg2+ ions in the codoped phosphors. The impurities present in the prepared materials are confirmed by FTIR analysis. The analysis of the upconversion spectra and the decay curve collectively reveals that the maximum enhancement of the blue UC band is observed for the Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphor upon excitation with a 980 nm NIR laser source. The CIE colour coordinates corresponding to the Tm3+–Yb3+–Zn2+–Mg2+ codoped La2O3 phosphor does not show any significant change with variations in the pump power density. As a result, the present phosphor can be used in making blue light upconverters, display devices and devices for the diagnosis and localization of cancer cells in photodynamic therapy.Acknowledgements
The authors are thankful to the University Grants Commission (UGC), New Delhi, India and Indian School of Mines, Dhanbad, India for providing financial support.References
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag Berlin Heidelberg, 1994, ISBN-13: 978-3-540-58019-5 Search PubMed.
- V. K. Rai, A. Pandey and R. Dey, Photoluminescence study of Y2O3:Er3+-Eu3+-Yb3+ phosphor for lighting and sensing applications, J. Appl. Phys., 2013, 113, 83104 CrossRef PubMed.
- G. Wang, W. Qin, L. Wang, G. Wei, P. Zhu and R. Kim, Intense ultraviolet upconversion luminescence from hexagonal NaYF4:Yb3+/Tm3+ microcrystals, Opt. Express, 2008, 16, 11907–11914 CrossRef CAS.
- B. M. Tissue, Synthesis and Luminescence of Lanthanide Ions in Nanoscale Insulating Hosts, Chem. Mater., 1998, 10, 2837–2845 CrossRef CAS.
- V. K. Rai, Temperature sensors and optical sensors, Appl. Phys. B, 2007, 88, 297–303 CrossRef CAS PubMed.
- S. K. Singh, K. Kumar and S. B. Rai, Multifunctional Er3+–Yb3+ codoped Gd2O3 nanocrystalline phosphor synthesized through optimized combustion route, Appl. Phys. B, 2009, 94, 165–173 CrossRef CAS.
- D. L. Yang, H. Gong, E. Y. B. Pun, X. Zhao and H. Lin, Rare-earth ions doped heavy metal germanium tellurite glasses for fiber lighting in minimally invasive surgery, Opt. Express, 2010, 18, 18997–19008 CrossRef CAS PubMed.
- R. S. Niwdbala, H. Feindt, K. Kardos, T. Vail, J. Burton, B. Bielska, S. Li, D. Milunic, P. Bourdelle and R. Vallejo, Detection of analytes by immunoassay using up-converting phosphor technology, Anal. Biochem., 2001, 293, 22–30 CrossRef PubMed.
- K. Binnemans, Lanthanide-Based Luminescent Hybrid Materials, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- C. Zhao, Q. Zhang, G. Yang and Z. Jiang, Laser-diode-excited blue upconversion in Tm3+/Yb3+-codoped TeO2-Ga2O3-R2O (R = Li, Na, K) glasses, J. Fluoresc., 2008, 18, 87–91 CrossRef CAS PubMed.
- H. J. Lin and Y. S. Chang, Blue-Emitting Phosphor of YInGe2O7 Doped with Tm3+ Ions, Electrochem. Solid-State Lett., 2007, 10, J79–J82 CrossRef CAS PubMed.
- H. Guo, N. Dong, M. Yin, W. Zhang, L. Lou and S. Xia, Visible Upconversion in Rare Earth Ion-Doped Gd2O3 Nanocrystals, J. Phys. Chem. B, 2004, 108, 19205–19209 CrossRef CAS.
- J. Silver, M. I. Martinez-Rubio, T. G. Ireland, G. R. Fern and R. Withnal, Yttrium Oxide Upconverting Phosphors. Part 4: Upconversion Luminescent Emission from Thulium-Doped Yttrium Oxide under 632.8 nm Light Excitation, J. Phys. Chem. B, 2003, 107, 1548–1553 CrossRef CAS.
- H. Zhang, X. Fu, S. Niu, G. Sun and Q. Xin, Photoluminescence of nanocrystalline YVO4:TmxDy1−x prepared by a modified Pechini method, Mater. Lett., 2007, 61, 308–311 CrossRef CAS PubMed.
- I. Etchart, I. Hernandez, A. Huignard, M. Berard, M. Laroche, W. P. Gillin, R. J. Curry and A. K. Cheetham, Oxide phosphors for light upconversion; Yb3+ and Tm3+ co-doped Y2BaZnO5, J. Appl. Phys., 2011, 109, 63104 CrossRef PubMed.
- A. Patra, S. Saha, M. A. R. C. Alencar, N. Rakov and G. S. Maciel, Blue upconversion emission of Tm3+–Yb3+ in ZrO2 nanocrystals: role of Yb3+ ions, Chem. Phys. Lett., 2005, 407, 477–481 CrossRef CAS PubMed.
- V. Petit, J. L. Doualan, P. Camy, V. Menard and R. Moncorge, CW and tunable laser operation of Yb3+ doped CaF2, Appl. Phys. B, 2004, 78, 681–684 CrossRef CAS PubMed.
- D. A. Simpson, W. E. K. Gibbs, S. F. Collins, W. Blanc, B. Dussardier, G. Monnom, P. Peterka and G. W. Baxter, Visible and near infra-red up-conversion in Tm3+/Yb3+ co doped silica fibers under 980 nm excitation, Opt. Express, 2008, 16, 13781–13799 CrossRef CAS.
- T. Riuttamäki, Upconverting Phosphor Technology: Exceptional Photoluminescent Properties Light Up Homogeneous Bioanalytical Assays, doctoral thesis, Department of Biochemistry and Food Chemistry/Biotechnology, University of Turku, Turku, Finland, 2011, ISBN: 978-951-29-4742-3.
- S. K. Singh, A. K. Singh, D. Kumar, O. Prakash and S. B. Rai, Efficient UV-visible up-conversion emission in Er3+/Yb3+ co-doped La2O3 nano-crystalline phosphor, Appl. Phys. B, 2010, 98, 173–179 CrossRef CAS.
- X. Zhang, P. Yang, D. Wang, J. Xu, C. Li, S. Gai and J. Lin, La(OH)3:Ln3+ and La2O3:Ln3+ (Ln = Yb/Er, Yb/Tm, Yb/Ho) Microrods: Synthesis and Up-conversion Luminescence Properties, Cryst. Growth Des., 2012, 12, 306–312 CAS.
- R. Dey, V. K. Rai and A. Pandey, Green upconversion emission in Nd3+–Yb3+–Zn2+:Y2O3 phosphor, Spectrochim. Acta, Part A, 2012, 99, 288–291 CrossRef CAS PubMed.
- G. Anoop, K. M. Krishna and M. K. Jayaraj, The Effect of Mg Incorporation on Structural and Optical Properties of Zn2GeO4:Mn Phosphor, J. Electrochem. Soc., 2008, 155, J7–J10 CrossRef CAS PubMed.
- V. Singh, V. K. Rai, I. J. Lee, I. Ledoux-Rak, K. Al-Shamery, J. Nordmann and M. Haase, Infrared, visible and upconversion emission of CaAl12O19 powders doped with Er3+, Yb3+ and Mg2+ ions, Appl. Phys. B, 2012, 106, 223–228 CrossRef CAS.
- K. Venkateswarlu, A. C. Bose and N. Rameshbabu, X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson–Hall analysis, Phys. B, 2010, 405, 4256–4261 CrossRef CAS PubMed.
- M. Pollnau, D. R. Gamelin, S. R. Luthi, H. U. Gudel and M. P. Hehlen, Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 3337–3346 CrossRef CAS.
- K. Zheng, L. Wang, D. Zhang, D. Zhao and W. Qin, Power switched multiphoton upconversion emissions of Er3+ in Yb3+/Er3+ codoped β-NaYF4 microcrystals induced by 980 nm excitation, Opt. Express, 2010, 18, 2934–2939 CrossRef CAS PubMed.
- H. K. Yang, K. S. Shim, B. K. Moon, B. C. Choi, J. H. Jeong, S. S. Yi and J. H. Kim, Luminescence characteristic of YVO4:Eu3+ thin film phosphors by Li doping, Thin Solid Films, 2008, 516, 5577–5581 CrossRef CAS PubMed.
- M. K. Chong, K. Pita and C. H. Kam, Photoluminescence of sol–gel-derived Y2O3:Eu3+ thin-film phosphors with Mg2+ and Al3+ co-doping, Appl. Phys. A, 2004, 79, 433–437 CrossRef CAS PubMed.
- Y. X. Pan and G. K. Liu, Enhancement of phosphor efficiency via composition modification, Opt. Lett., 2008, 33, 1816–1818 CrossRef CAS.
- A. K. Parchur, A. I. Prasad, S. B. Rai and R. S. Ningthoujam, Improvement of blue, white and NIR emissions in YPO4:Dy3+ nanoparticles on co-doping of Li+ ions, Dalton Trans., 2012, 41, 13810–13814 RSC.
| This journal is © The Royal Society of Chemistry 2014 |