High pressure CO2-controlled reactors: enzymatic chiral resolution in emulsions
Wenting Shang,
Xiaogang Zhang*,
Xiaoxi Yang and
Shujuan Zhang
Department of Chemistry, Renmin University of China, Beijing, 100872, P.R. China. E-mail: zhang_xg@ruc.edu.cn; Fax: +86-10-62515601; Tel: +86-10-62515601
First published on 23rd May 2014
Abstract
In this work we have reported the formulation of a CO2-based micelle stabilized by nontoxic TMN series surfactants. Enantioselection of racemic ibuprofen catalyzed by Candida antarctica lipase B (CALB) was used as a model reaction. The effect of reactive parameters, such as temperature, pH, pressure, and water content on reactive environment and conversion has been discussed. For the resolution of racemic ibuprofen in CO2-based micelles, the enzymatic activity reached a high level at 45 °C, with pressure 250 bar, pH 7.4, and water to surfactant ratio W0 25. In addition, the relatively long-chain length in TMN-10 could help the esterification and trans-esterification processes, which resulted in an efficient reaction rate in a CO2-based micelle system. Enzymatic catalysis has been conducted in a CO2-based system rather than in the conventional media to make the enzyme reaction greener. The better resolution efficiency in high pressure CO2-based micelles could be achieved within a relatively short period of time compared with other traditional reactive systems.
1 Introduction
With the growing demand for environmentally friendly processes working at ambient conditions, the use of biocatalysts in organic synthesis has become an interesting alternative to conventional chemical methods.1,2 Enzymes are eco-friendly biocatalysts that can catalyze chemical reactions under mild conditions, such as ambient temperature, pressure, and neutral pH.3,4 The use of enzymes generally circumvents the need for functional group activation and avoids protection and deprotection steps required in traditional organic syntheses.5From a biotechnological viewpoint, conducting enzymatic reactions in nonaqueous solvents offer new possibilities for producing valuable chemicals. Their versatility would be further expended if the enzymatic reactions could be performed in reverse micelles. Reverse micelles are thermodynamically stable water droplets dispersed in an organic phase by means of a surfactant. One of the most important properties of reverse micelles is their ability to entrap enzymes and other biomolecules in their water droplets. In addition, the micelles are dynamic structures, the substrates and products can be exchanged between water droplets and bulk organic solvent. Reverse micelles have wide applications in a variety of fields including chemical reactions,6–8 material synthesis,9–11 protein delivery,12–14 drug release15 and so on. A reversed micellar system is especially suitable for lipases, which are activated in the presence of a water–oil interface. These systems can provide a high interfacial area, with the enzyme anchoring on the aqueous side of the surfactant interface.
The water content which is necessary to favour synthesis reactions in organic media can be achieved by micro-encapsulation of the biocatalyst within reverse micelles. For reverse micelle system, its challenge is recovering the product from reverse micelles at large scale due to the presence of the surfactant and other components of the system, making the separation and purification of the product be more difficult, especially when it is to be used in foods, pharmaceuticals or other products that require either non-toxic or highly pure products.
High pressure carbon dioxide has been identified as a ‘green’ solvent with its potential applications for industrial use as it provides a clean, non-toxic, non-flammable and tunable solvent system which is easily removed to leave reaction products free from undesirable organic residues. Therefore, the combination of biocatalysis and carbon dioxide is extremely attractive. Enzymatic catalysis has been conducted in CO2-based system rather than in the conventional media to make enzyme reaction greener.
The first report on enzyme catalyzed reactions in high pressure CO2 was in 1985 by Randolph et al.,16 Hammond et al.,17 and in 1986 by Nakamura et al.18 Recently, a number of groups have explored the use of enzymes in high pressure CO2.19–24 However, it was reported that native enzymes were easily deactivated in CO2-based medium because CO2 molecules could react with ε-amino groups of lysine residues placed on the enzyme surface to form inhibitory carbamates.25 One attempt to prevent this phenomenon has been made by using reverse micelles to stabilize the enzyme in a water pool, and meantime maintain the benefit of high mass diffusivity of high pressure CO2. For the CO2-based systems, their applications are probably hindered by the weak solubility for many hydrophiles and proteins of interest.26 Recently, many of amphiphiles have been shown to form micelle in carbon dioxide media. For general case, the reactive rate in high pressure, especially supercritical fluid system is faster than that performed at atmospheric pressure because of the reduced mass-transfer.27,28 The extra advantages associated with use of CO2 as media for enzymatic reactions are not only related to its low environmental impact but also due to its chemical properties such as high diffusivity, tunable solubility with CO2 density, and easy separation of solvent from products.
There is an increasing trend towards the use of optically pure enantiomers for drugs and agrochemicals because they are more effective and have fewer side effects compared with their racemic mixtures. Ibuprofen, 2-(4-isobutylphenyl)propionic acid, is a racemic carboxylic acid, is a widely used nonsteroidal anti-inflammatory drug that belongs to the family of 2-arylpropionic acid derivatives. Due to its high medical activity and low toxicity, ibuprofen is one of the most popular non-prescription medicines in the world. The pharmaceutical activity of ibuprofen is dependent on the chirality of the compound. Only the S-isomer exhibits an anti-inflammatory property and is valuable.29 Enzyme kinetic separation was reported as one of the best ways to obtain pure (S)-ibuprofen.30 Utilization of lipase in the resolution of ibuprofen received great interests because of the mild reaction condition, high enantioselectivity, less side reactions and environment pollution of enzyme-catalyzed reactions. However, the biological catalytic processes for chiral separation often taken a very long reaction time, and stability could not be a good guarantee, recycling and reuse were also encountered many challenges.
In this paper, the CO2-based micelles with the TMN series surfactants, which were related to poly(ethylene glycol)-2,6,8-trimethyl-4-nonyl ethers, were used as reactive medium. Enantioselection of racemic ibuprofen catalyzed by Candida antarctica lipase B (CALB) was used as a model reaction. The better resolution efficiency in high pressure CO2-based micelles could be achieved within a relatively shorter period of time comparing with other reactive systems. The effect of reactive parameters, such as temperature, pH, pressure, and water content on reactive environment and conversion has been discussed.
2 Materials and method
2.1 Materials
Racemic ibuprofen was supplied by Xian Lang Hong Biotechnology Co. Ltd. Propanol was supplied by Sinopharm Chemical Reagent Co., Ltd. The standard reference of (S)- and (R)-ibuprofen were purchased from Sigma. Ibuprofen propyl ester was synthesized using the method of Patricia de et al.31 in our lab. Racemic(R,S)-ibuprofen and propanol were dropped into a round-bottom flask containing hydrochloric acid as the catalysis and the solution was refluxed for 6 hours. After the reaction, ibuprofen propyl ester was leached by adding right amount of n-hexane. From the reaction mixture ibuprofen propyl ester was extracted and purified by repeated extraction with n-hexane; ibuprofen could be detected in the product as tested by thin-layer chromatography (TLC). Candida antarctica lipase B (CALB) (1000 units per mg solid) was purchased from Sigma (China). CALB was used as received. Non-ionic surfactants Tergitol®TMN-3 (99% 5b-C12E3, n = 2.98, Mw = 335), Tergitol®TMN-6 (90% 5b-C12E8, 10 wt% water, n = 8.32, Mw = 552), Tergitol®TMN-10 (90% 5b-C12E12, 10 wt% water, n = 11.55, Mw = 694) were purchased from Sigma-Aldrich and were used as received. The chemicals were of G.R. grade and used without further purification. Carbon dioxide (99.995% purity) was purchased from Beijing Analytical Instruments Inc. Double distilled water was used throughout.2.2 Enzymatic dynamic kinetic resolution in CO2-based micelle system
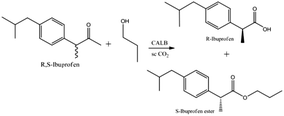
The apparatus in which the chiral resolution of racemic ibuprofen catalyzed by CALB (shown in Scheme 1) in CO2-based micelle was carried out was shown in Fig. 1. In typical experiments, racemic ibuprofen (20.6 mg), propanol (18 μl), sodium phosphate buffer (25 μl, pH = 7.4) mixed with surfactant TMN-10 (35 μl) and 3 mg CALB solution were loaded in a high-pressure cell with a working volume of 8 ml. The cell was sealed and then heated to 45 °C with stirring. CO2 was introduced slowly into the cell until to desired pressure. The mixture was stirred at 600 rpm during the reaction, carried out for 36 hours. After reaction, the cell was cooled to 0 °C and the gas stream was then vented slowly to ambient pressure through the traps containing 10 ml n-hexane. Thereafter, the cell was opened, and the remaining residue was extracted with another 20 ml of n-hexane. The mixture of the two solutions was then filtered through a water-soluble membrane in order to remove the lipase. The resulting solution was conducted by HPLC spectroscopy analysis. All experiments were performed three times to calculate the mean x, stand deviation s, and confidence interval of mean Δx of 95%.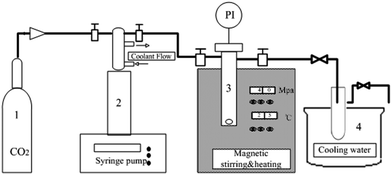 | ||
| Fig. 1 Schematic diagram of reaction apparatus. (1) CO2 reservoir tank; (2) high pressure syringe pump; (3) reaction vessel, pressure and temperature control system; (4) cooling water. | ||
2.3 HPLC analysis
The analysis of both enantiomers of ibuprofen was conducted by an Agilent 1100 high performance liquid chromatography (HPLC) instrument equipped with a OD-H chiral column (5 μm, 4.6 mm × 250 mm) and a UV detector. The wavelength of the detector and the environment of the column oven were set up at 254 nm and 40 °C respectively during the measurement. The column is capable of separating the (R)- and (S)-enantiomers of both acid and ester derivatives. The HPLC was performed with n-hexane–isopropyl alcohol (98/2, HPLC grade, Fisher Scientific Co.) as the mobile phase at a flow rate of 0.5 ml min−1. The concentration of the (R)- and (S)-ibuprofen ester was determined periodically, and the ees of the substrate was calculated by eqn (1).
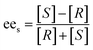 | (1) |
3 Results and discussion
The asymmetric synthesis of esters was the most active area of research; the several lipases combined with the unique properties of high pressure CO2 have successfully permitted the chiral resolution of a large number of racemates.32–35In this study, we compared the chiral resolution of racemic ibuprofen catalyzed by CALB performed in CO2-based and hexane-based micelle reactive systems. Fig. 2 showed that in the case of resolution of racemic ibuprofen catalyzed by enzyme, reactive equilibrium could be easily achieved in high pressure CO2-based than in atmosphere hexane-based micelle. The reason might be attributed to the faster mass transfer effects in high pressure CO2-based micelle. From the comparison results of enantio-separation for racemic ibuprofen in different reactive systems shown in Table 1, we saw that the better resolution efficiency in high pressure CO2-based micelles could be achieved within a relatively shorter period of time. The results clearly demonstrated that the CO2-based micelle stabilized by TMN-10 could be used as a suitable reaction medium for the resolution of racemic ibuprofen. Fig. 2 also showed that the ees levelled out in CO2-based micelle after 36 h. The reaction time was designated as 36 h in following experiments.
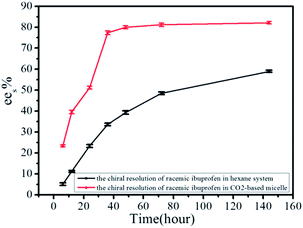 | ||
| Fig. 2 ees values of the chiral resolution of racemic ibuprofen in hexane-based and CO2-based micellar systems. | ||
The dependence of enzymatic activity on the different EO chain length in the TMN surfactant series were studied also (Fig. 3). For the TMN series surfactants, the ees values were increased with an increase in the chain length of EO in TMN. Possibly, the EO groups in TMN surfactant were favourable for stabilization of the enzymes. In addition, the relative long-chain length in TMN-10 could help the esterification and trans-esterification processes, which resulted in efficient reactive rate in micelle system.
Temperature, pH, pressure and water content were the most important environmental factors affecting enzymatic catalysis in CO2-based micelle system, particularly their activity, enantioselectivity and stability. The effects of these factors on the chiral resolution of racemic ibuprofen in CO2-based micelle were systematically investigated.
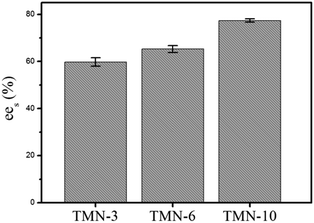 | ||
| Fig. 3 ees values of the chiral resolution of racemic ibuprofen in CO2-based micelle involved TMN surfactants with different EO chain length. | ||
3.1 The effect of temperature
The reactive temperature was an important factor that affects conversion of the substrate and the enzyme activity. For different reaction systems, the optimum reactive temperature was determined by experiment. In the CO2-based reverse micelle system, the dependence of temperature on the yield was measured at 250 bar. Fig. 4 showed the conversion and ees values of racemic ibuprofen ester under the temperature range of 25–50 °C at 36 hour of the reaction. The results demonstrated that the conversion of the substrate and ees increased as the reaction temperature increased until it reached an optimum reaction temperature of 45 °C. The ees and conversion of the racemic ibuprofen obtained at this temperature were 85% and 55% respectively. However, the ees and conversion decreased rapidly when the temperature was further elevated. A sharp decay in conversion and ees at 50 °C was mainly due to the exposure of the enzyme above its optimum temperature for a long time. The accumulated heat could result in partial deactivation and denaturation of enzyme structure. Temperature had a positive effect on kinetics, but it had also a negative effect on enzyme activity. Both effects could be counteracted each other, and resulted in the optimum temperature. This result agreed with other reported findings that the stability of enzyme decreased with the elevated temperatures, although the initial reaction rate was high.37 Therefore, based on the results obtained, the reactive temperature of 45 °C was selected for the resolution of racemic ibuprofen, for which both rapid reactive rate and high enzyme activity were essential for an optimum operation. The actual desired temperature for a reaction was a temperature at which the enzyme exhibited a constant activity over a period of time.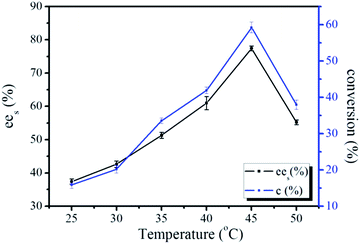 | ||
| Fig. 4 The conversion and ees values of the chiral resolution of racemic ibuprofen in CO2-based micelle with TMN-10 at different temperatures. | ||
3.2 The effect of pH
In the enzymatic reaction, the pH effect on lipase activity was also carefully investigated. In most enzymatic resolution processes, it has been suggested that the variation in pH of buffer solution might influence the chiral selectivity, since the conformation of an enzyme depended on its ionization state.38,39 For general case, the lipase might contain both positively and negatively charged groups, these ionizable groups constituted part of the active sites and often involved in acid–base catalysis. For the CO2-based micelle system, the measurement of pH inside the water pool was difficult; the values of pH mentioned here referred to that of the buffer before it was solubilized in the micelles. In fact, the buffer solution played a great role in pH control for the CO2-based micelle.40 The results for the pH profile were shown in Fig. 5. It is observed that the pH profile would no longer be bell-shaped fashion; the catalytic activity of CALB was relatively low in a pH 7 buffer solution. The enzymatic activity increased to a maximum value at pH 7.4 and quickly dropped with an increase in the pH value.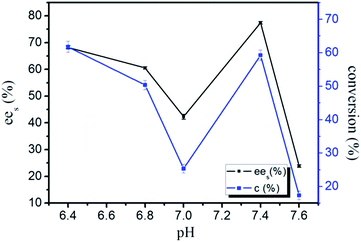 | ||
| Fig. 5 The conversion and ees values of the chiral resolution of racemic ibuprofen in CO2-based micelle with TMN-10 at different pH. | ||
3.3 The effect of pressure
The effect of pressure on enantioselection was investigated by the lipase-catalyzed resolution of ibuprofen at pressures ranging from 70 to 300 bar with maintaining the temperature at 45 °C. As shown in Fig. 6, the ees values decreased from 66% to 52% when pressure was increased from 70 to 120 bar, and then increased from 52% to the maximum value 78% with the pressure increasing from 120 to 250 bar. A further raise of pressure started to decline the ees. The conversion profiles followed similar trends with the ees changes with pressure, just a small rise at 80 bar.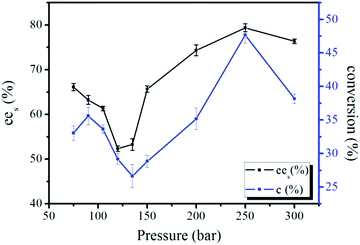 | ||
| Fig. 6 The conversion and ees values of the chiral resolution of racemic ibuprofen in CO2-based micelle with TMN-10 at different pressure ranges. | ||
Generally, density change with pressure was very sensitive near the critical region for CO2-based systems. A large change in density significantly changed the interactions between CO2 and enzyme, causing the formation of carbamates from CO2 and free amine groups on the surface of the enzyme.25 The interactions might gradually change the conformation of the enzyme in response to pressure, and further resulting in a continuous change in enantioselectivity.41–43 The effect of pressure on enzymatic reaction in CO2-based systems had been the subject of investigation in several studies. However, the influence of pressure on the biocatalysis reactions was still not fully understood due to contradictory results.44,45 The rising pressure was accompanied by an increase of the solvating power of high pressure CO2, which meant the increase of the solubility of the substrates in CO2. Due to this solvation effect, the partitioning of the substrates between the high pressure CO2 phase of the reverse micelle and the immediate vicinity of the enzyme was changed. The enzyme environment was depleted with regard to the substrates, which caused a decline in the reaction rate.46 When the partitioning of the substrates shifted to the supercritical phase, a further increase of pressure could no longer result in a further depletion in the enzyme environment, i.e. the reactive rate could no longer be affected by the rise of pressure. For micelle reactive system, the extent of interfacial area offered more reactive opportunity with the forming of the micelles. The solubility of water in high pressure CO2 is raised drastically when the pressure is larger than 100 bar.47 According to the phase diagrams of water–CO2 microemulsion formed by TMN series surfactants in the domain of CO2,48 the reactive system tended to form microheterogeneous CO2-based micelles with the pressure increasing from 150 to 300 bar. Significantly increased interfacial areas favored the substrates approached the interfacial regions in the micelles, accelerating the reaction rate. Therefore, the ees values and conversion increased when the pressure was larger than 150 bar. The ees and conversion reached a maximum at 250 bar, where the interfacial areas of the microreactors reached a steady state. With further increase of the pressure at constant volume, the diluting effect caused a decrease in conversion at higher CO2 pressure. A common rule could not be found to identify how pressure affected the activity of enzyme. From a standpoint of reaction rate, pressure could affect the reaction rate by changing the rate constant directly. An increase in pressure resulted in enhancing fluid density, and improved solvating power of the fluid.
3.4 The effect of water
Water concentration was a key factor which played a significant role in the enzyme-catalyzed reactions in CO2-based micelles. Control of the water content was important for optimizing the activity in enzymatic systems. The amount of water required to retain catalytic activity was enzyme dependent and varied for different reactive systems. The size of the water pool could be tailored by controlling the W0 value (water to surfactant ratio) to make the micelle mimic a biological system or any other restricted environment. In common with many micelles, water-in-CO2 micelle showed a spherical droplet structure for which the droplet radius was directly proportional to W0 value,49 and the microenvironment around the enzyme could be tuned directly by simply changing the W0 value. The results shown in Fig. 7 demonstrated that increasing the amount of water was expected to increase the enzymatic activity of the lipase in the CO2-based micelle. The ees values and conversion gradually decreased as the W0 was increased up to 25, after which further increasing the W0 could result in the decrease of the ees and conversion. Generally, a minimum amount of water in the enzyme vicinity was necessary for maintaining catalytic activity, i.e. the enzyme needed to be sufficiently hydrated in micelles. If the water content in micelle was too high, the micelle system might be separated two phases; the increased humidity might lead to enzyme deactivation.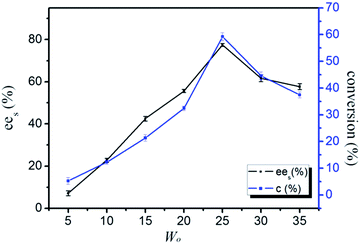 | ||
| Fig. 7 The conversion and ees values of the chiral resolution of racemic ibuprofen in CO2-based micelle with TMN-10 at different water contents. | ||
4 Conclusions
The chiral resolution of racemic ibuprofen catalyzed by CALB in CO2-based micelle was explored. The experimental results demonstrated that CO2-based micelle was used instead of the conventional oil micelle to improve the greenness of the enzyme reactions. For the CO2-based micelle with surfactant TMN series, the ees was enhanced with an increase in the chain length of EO in TMN. The effects of reaction parameters such as temperature, pH, pressure, and water content on the reactive behavior were discussed. The combination of enzyme with high pressure CO2 represented a promising “green” reaction system for bioconversions. In such combination, the amount of less green substances such as surfactants was very small. Studies along these are now in progress.Acknowledgements
The authors are grateful to the National Nature Science Foundation of China (Grant no. 20876169) for financial support.Notes and references
- R. Azerad, Curr. Opin. Biotechnol., 2001, 12, 533–534 CrossRef CAS.
- B. M. Nestl, S. C. Hammer, B. A. Nebel and B. Hauer, Angew. Chem., Int. Ed., 2014, 53, 3070–3095 CrossRef CAS PubMed.
- J. Kim, J. W. Grate and P. Wang, Trends Biotechnol., 2008, 26, 639–646 CrossRef CAS PubMed.
- K. M. Koeller and C.-H. Wong, Nature, 2001, 409, 232–240 CrossRef CAS PubMed.
- R. A. Sheldon, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef CAS.
- M. Pileni, J. Phys. Chem., 1993, 97, 6961–6973 CrossRef CAS.
- D. Goswami, J. K. Basu and S. De, Crit. Rev. Biotechnol., 2013, 33, 81–96 CrossRef CAS PubMed.
- R. N. Mitra, A. Dasgupta, D. Das, S. Roy, S. Debnath and P. K. Das, Langmuir, 2005, 21, 12115–12123 CrossRef CAS PubMed.
- J. Zhang and B. Han, Acc. Chem. Res., 2012, 46, 425–433 CrossRef PubMed.
- W. Shang, X. Kang, H. Ning, J. Zhang, X. Zhang, Z. Wu, G. Mo, X. Xing and B. Han, Langmuir, 2013, 29, 13168–13174 CrossRef CAS PubMed.
- M. A. Hillmyer, Science, 2007, 317, 604–605 CrossRef CAS PubMed.
- C. Müller-Goymann, Eur. J. Pharm. Biopharm., 2004, 58, 343–356 CrossRef PubMed.
- N. Nasongkla, X. Shuai, H. Ai, B. D. Weinberg, J. Pink, D. A. Boothman and J. Gao, Angew. Chem., Int. Ed., 2004, 116, 6483–6487 CrossRef.
- C. M. Stegmann, D. Seeliger, G. M. Sheldrick, B. L. de Groot and M. C. Wahl, Angew. Chem., Int. Ed., 2009, 121, 5309–5312 CrossRef.
- J. P. Tan, S. H. Kim, F. Nederberg, E. A. Appel, R. M. Waymouth, Y. Zhang, J. L. Hedrick and Y. Y. Yang, Small, 2009, 5, 1504–1507 CrossRef CAS PubMed.
- T. W. Randolph, H. Blanch, J. Prausnitz and C. Wilke, Biotechnol. Lett., 1985, 7, 325–328 CrossRef CAS.
- D. Hammond, M. Karel, A. Klibanov and V. Krukonis, Appl. Biochem. Biotechnol., 1985, 11, 393–400 CrossRef CAS.
- K. Nakamura, Y. M. Chi, Y. Yamada and T. Yano, Chem. Eng. Commun., 1986, 45, 207–212 CrossRef CAS.
- S. V. Kamat, E. J. Beckman and A. J. Russell, J. Am. Chem. Soc., 1993, 115, 8845–8846 CrossRef CAS.
- T. Mori and Y. Okahata, Chem. Commun., 1998, 2215–2216 RSC.
- K. Chaudhary, S. V. Kamat, E. J. Beckman, D. Nurok, R. M. Kleyle, P. Hajdu and A. J. Russell, J. Am. Chem. Soc., 1996, 118, 12891–12901 CrossRef.
- T. Matsuda, T. Harada and K. Nakamura, Green Chem., 2004, 6, 440–444 RSC.
- H. R. Hobbs, B. Kondor, P. Stephenson, R. A. Sheldon, N. R. Thomas and M. Poliakoff, Green Chem., 2006, 8, 816–821 RSC.
- T. Matsuda, T. Harada and K. Nakamura, Curr. Org. Chem., 2005, 9, 299–315 CrossRef CAS.
- J. Mesiano, E. J. Beckman and A. J. Russell, Chem. Rev., 1999, 99, 623–634 CrossRef PubMed.
- S. Cummings, K. Trickett, R. Enick and J. Eastoe, Phys. Chem. Chem. Phys., 2011, 13, 1276–1289 RSC.
- P. G. Jessop, T. Ikariya and R. Noyori, Chem. Rev., 1999, 99, 475–494 CrossRef CAS PubMed.
- J. Jin, Z. M. Cheng, J. G. Li and S. C. Wu, Chem. Eng. Sci., 2013, 100, 69–73 CrossRef CAS PubMed.
- M. C. Hillier and P. J. Reider, Drug Discovery Today, 2002, 7, 303–314 CrossRef.
- K. Williams, R. Day, R. Knihinicki and A. Duffield, Biochem. Pharmacol., 1986, 35, 3403–3405 CrossRef CAS.
- P. d. O. Carvalho, F. J. Contesini, R. Bizaco, S. A. Calafatti and G. A. Macedo, J. Ind. Microbiol. Biotechnol., 2006, 33, 713–718 CrossRef CAS PubMed.
- T. Matsuda, K. Watanabe, T. Harada, K. Nakamura, Y. Arita, Y. Misumi, S. Ichikawa and T. Ikariya, Chem. Commun., 2004, 2286–2287 RSC.
- E. Celia, E. Cernia, C. Palocci, S. Soro and T. Turchet, J. Supercrit. Fluids, 2005, 33, 193–199 CrossRef CAS PubMed.
- G. Paggiola, A. J. Hunt, C. R. McElroy, J. Sherwood and J. H. Clark, Green Chem., 2014, 16, 2107–2110 RSC.
- P. Lozano, Green Chem., 2010, 12, 555–569 RSC.
- D. Zhao, E. Xun, J. Wang, R. Wang, X. Wei, L. Wang and Z. Wang, Biotechnol. Bioprocess Eng., 2011, 16, 638–644 CrossRef CAS PubMed.
- M. Romero, L. Calvo, C. Alba, M. Habulin, M. Primožič and Ž. Knez, J. Supercrit. Fluids, 2005, 33, 77–84 CrossRef CAS PubMed.
- V. L. Schramm, Annu. Rev. Biochem., 2011, 80, 703–732 CrossRef CAS PubMed.
- J. M. Palomo, G. Fernández-Lorente, C. Mateo, M. Fuentes, R. Fernández-Lafuente and J. M. Guisan, Tetrahedron: Asymmetry, 2002, 13, 1337–1345 CrossRef CAS.
- P. K. Chan and G. T Rochelle, ACS Symp. Ser., 1982, 188, 75–98 CrossRef CAS PubMed.
- T. Matsuda, J. Biosci. Bioeng., 2013, 115, 233–241 CrossRef CAS PubMed.
- R. L. Silveira, J. Martinez, M. S. Skaf and L. Martinez, J. Phys. Chem. B, 2012, 116, 5671–5678 CrossRef CAS PubMed.
- J. C. Erickson, P. Schyns and C. L. Cooney, AIChE J., 1990, 36, 299–301 CrossRef CAS.
- C. Kohlmann, S. Leuchs, L. Greiner and W. Leitner, Green Chem., 2011, 13, 1430–1436 RSC.
- S. Kara, W. S. Long, M. Berheide, S. Peper, B. Niemeyer and A. Liese, J. Biotechnol., 2011, 152, 87–92 CrossRef CAS PubMed.
- C. Blattner, M. Zoumpanioti, J. Kröner, G. Schmeer, A. Xenakis and W. Kunz, J. Supercrit. Fluids, 2006, 36, 182–193 CrossRef CAS PubMed.
- K. Nakamura, Trends Biotechnol., 1990, 8, 288–292 CrossRef CAS.
- W. Ryoo, S. E. Webber and K. P. Johnston, Ind. Eng. Chem. Res., 2003, 42, 6348–6358 CrossRef CAS.
- M. Sagisaka, S. Iwama, S. Ono, A. Yoshizawa, A. Mohamed, S. Cummings, C. Yan, C. James, S. E. Rogers and R. K. Heenan, Langmuir, 2013, 29, 7618–7628 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |