Enhanced room temperature electrocaloric effect in barium titanate thin films with diffuse phase transition
Lijie Wangab,
Jinbin Wang*ab,
Bo Li*ab,
Xiangli Zhongab,
Fang Wangab,
Hongjia Songab,
Yukui Zengab,
Dan Huangab and
Yichun Zhouab
aKey Laboratory of Low Dimensional Materials and Application Technology of Ministry of Education, Xiangtan University, Xiangtan 411105, China. E-mail: jbwang@xtu.edu.cn; bli@xtu.edu.cn
bFaculty of Materials, Optoelectronics and Physics, Xiangtan University, Xiangtan 411105, Hunan, China
First published on 17th April 2014
Abstract
Diffuse phase transition was found in barium titanate (BT) thin films with a thickness of 300 nm prepared by a sol–gel method. The electrocaloric effect of these BT thin films was also investigated under a sweep electrical field of 216.7 kV cm−1. Interestingly, it was found that the electrocaloric temperature change (ΔT) increased with a decrease in temperature when the temperature was below 310 K. The novel electrocaloric phenomenon observed in BT thin films with a diffuse phase transition is significant in studying the room temperature electrocaloric effect, which is promising in the refrigeration industry.
1. Introduction
Electrocaloric effect (ECE), a promising cooling method, has been investigated for many years. It is the temperature change (ΔT) under an adiabatic condition caused by an entropy change (ΔS) in polar materials upon the application or withdrawal of an electrical field, and has been widely studied in ferroelectric materials, which are promising functional materials.1–3 If materials with large ECE can be developed, the ECE may realize a solid-state cooling device for a broad range of applications such as on-chip cooling and regulating temperature for sensors and electronic devices.4 Refrigeration based on the ECE approach causes less harm to the environment and hence, may also provide an alternative to the substitute vapor-compression approach.5 It is known that a large number of devices work at around room temperature, while the current ECE that was reported in ferroelectric materials merely maximizes at the Curie temperature, which is often higher than the room temperature. For example, the giant ECE of 12 K in PbZr0.95Ti0.05O3 reported by Mischenko et al. occurs only at the temperature of 495 K.6 Thus, it is significant to research room temperature ECE in order to commercialize ECE cooling.Until now, many studies have been performed to investigate giant room temperature ECE.7–11 It is known that giant ECE only occurs around the Curie temperature of ferroelectric materials. Thus, finding new materials with a low Curie temperature and altering the Curie temperature of materials from a high temperature to the room temperature are effective methods to obtain giant ECE at room temperature. Recently, large ECE near room temperature was reported in a ferroelectric poly(vinylidene fluoride–trifluoroethylene) (P(VDF–TrFE)) copolymer working with the relaxor ferroelectric polymer of P(VDF–TrFE–chlorofluoroethylene) by Neese et al.7 Both the isothermal entropy change of more than 55 J K−1 kg−1 and the adiabatic temperature change of more than 12 K are quite large compared with those of other ferroelectric materials such as PMN-PT, PZT, BST, and BZT.12–18 Peng et al. also reported a giant room temperature ECE in Pb0.8Ba0.2ZrO3 with ΔT = 45.3 K and ΔS = 46.9 J K−1 kg−1 at 598 kV cm−1. In their work, barium was doped into lead zirconate to alter its phase transition point, which is caused by the coexistence of anti-ferroelectric and ferroelectric phases.19
Barium titanate (BT), as a lead-free ferroelectric material, is environment friendly and is the most widely used ferroelectric material. ECE in BT has been studied quite extensively in various forms including thin films, bulk ceramics and single crystals by many researchers. In addition, large ΔT and ΔS have been reported in BT ceramics at temperatures near the ferroelectric–paraelectric (FE–PE) transition. However, the room temperature ECE in BT is much lower than that at higher temperatures. In this work, we enhanced the room temperature ECE of BT thin films by changing its phase transition point to around room temperature by obtaining BT thin films with a pseudo cubic phase instead of a tetragonal phase.
2. Experimental
BT thin films were fabricated by a sol–gel method. Ba(OAc)2 was dissolved in glacial acetic acid at the temperature of 50 °C in a beaker called solution A. Butyl titanate and 2-methoxyethanol working as the solvent were added to another beaker and stirred for 30 minutes. Then, solution B was obtained when acetyl acetone and formamide were added into the beaker. Here, acetyl acetone works as a catalyzer to accelerate the dissolution of butyl titanate and formamide is used for promoting film formation and preventing the generation of crackles. Solution B was transferred into solution A, and this solution was stirred for 10 hours at room temperature. The final concentration of the synthesized BT sol was 0.3 M.The BT thin film was deposited on the Pt/TiO2/SiO2/Si substrate by spin coating of a BT precursor solution, which was aged for a week at the temperature of 20 °C in a cooled incubator (ES252), at the spinning speed of 800 rpm min−1 for 5 seconds and 3000 rpm min−1 for 40 seconds. In order to pyrolyze the organics, the wet film was stepwise heated to 180 °C, 350 °C and 500 °C, holding for 3 minutes at each step. Then, the film was annealed for 5 minutes at 750 °C to crystallize the BT; this short annealing time is to obtain a small crystal size film. These processes were repeated 6 times to obtain the final film with a thickness of 300 nm.
The thickness of the BT film was measured by a film thickness measurement device (Filmetrics F50). The BT film structure was monitored by X-ray diffraction (XRD) on a diffractometer (D/MAX-2550V, Rigaku, Tokyo, Japan) using Cu Kα radiation in the 2θ range of 15° to 60°. Pt top electrodes with a diameter of 100 μm were sputtered through a mechanical mask. Electric dependences of the polarization hysteresis loop (P(V)) and leakage current (I–t) were obtained by ferroelectric test systems with the model of P-WS (made by Radiant Technologies, Inc.) at a frequency of 100 Hz after the samples were coated with the Pt electrode. Temperature was controlled with the aid of a Peltier element. The dielectric permittivity and the dielectric loss were measured by a semiconductor measurement machine (Agilent B1500A) at the frequency of 10 kHz and 100 kHz with 50 mV ac amplitude.
3. Results and discussion
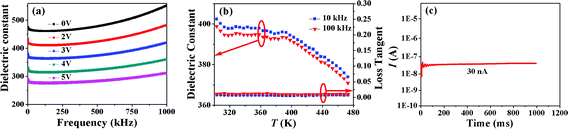
The XRD pattern of the BT thin film deposited on the Pt/TiO2/SiO2/Si substrate is displayed in Fig. 1. It is clear from the pattern that the thin film was polycrystalline. The XRD pattern was carefully analyzed and marked. The BT thin film has a cubic phase according to the XRD pattern peaks because they fit well with the characteristic peaks of cubic phase BT. The peaks at 2θ of 40° and 46° are Pt(111) and Pt(200), respectively. Generally, cubic phase BT is paraelectric and has no ferroelectricity. However, as shown in Fig. 2, the ferroelectricity is clearly shown in the prepared BT thin films. Hence, we infer that the BT thin films obtained here have a pseudo cubic phase. Maybe, the formation of the pseudo cubic phase in the BT thin film is due to the short time annealing, which is only 5 minutes and is not long enough for the crystals to grow.18The dielectric property and the leakage current of the BT thin film were investigated, and the results are shown in Fig. 3. The relationship between the dielectric constant and the frequency at different bias voltages is displayed in Fig. 3(a). It is obvious that the values of the dielectric constant at 0 V are larger than 450, which indicates that the film is dense and has few defects. In addition, the dielectric constant decreases with increasing bias voltage, and we find that the sensitivity of dielectric constant to the frequency becomes weak when the bias voltage increases. This phenomenon gives us the flexibility to control the dielectric constant by changing the bias voltage. The decrease in the dielectric constant under the bias voltage is caused by the polarization in the film, which is generated by the extra electric field imposed by the bias voltage. Temperature dependences of the dielectric constant and the dielectric loss (loss tangent) at 10 kHz and 100 kHz are shown in Fig. 3(b). It is clear that the dielectric constant has almost no change in the temperature range of 313 K to 393 K. This result has already been reported by Mahbub et al. and is explained by the diffuse phase transition due to the small crystal size, which means that many grain boundaries have a spinning effect during the phase change.20–23 Here, diffuse phase transition stands for the phase change from the pseudo cubic phase to the cubic phase.24 A peak around room temperature is inferred in Fig. 3(b) with a phase change from the orthorhombic phase to the pseudo cubic phase. In addition, the dielectric constant begins to decrease beyond 393 K, which implies that the phase transition from the pseudo cubic phase to the cubic phase almost ends. Similar results have been reported by Qiao et al.25 Loss tangents at both 10 kHz and 100 kHz displayed in Fig. 3(b) are quite small, which indicates low energy loss. Leakage current was investigated near the room temperature in the maximum electrical field imposed on the BT film, and the result is displayed in Fig. 3(c). The observed transients persist up to 1000 milliseconds, beyond which no breakdown occurs even after repetitive testing.19 In contrast, beyond 200 milliseconds, breakdown occurs in the PZT thin film. It can be seen that 30 nA is the upper bound for the steady-state leakage current. This value yields negligible Joule heating (<10−3 K) and does not affect P(V), which are the electric dependences of the polarization hysteresis loop of the BT thin film, because the currents of hundreds of microamperes are required to switch the measured polarizations at 100 Hz.6,19
Temperature dependent electrical hysteresis were measured roughly after every 5 K in the temperature range of 395 K to 300 K because measurements from a high temperature to a low temperature can minimize reductions in polarization due to fatigue.6 Representative plots of P(V) are shown in Fig. 4. It is obvious that polarization decreases when the temperature increases, which is common for ECE ferroelectric materials. The ferroelectric hysteresis loops in Fig. 4(a) and (b) are symmetric, which means good ferroelectricity. However, the ferroelectricity decays with an increase in temperature, which can be seen in Fig. 4(c) and (d). The decay of the ferroelectricity of BT thin films with an increase in temperature can be easily found in the insets of each plot, which are the magnification of the blue square frames. The decay of ferroelectricity is due to the phase transition from the pseudo cubic phase to the cubic phase.
 | ||
| Fig. 4 (a–d) Ferroelectric hysteresis loop of BT thin film at selected temperature; the insets of each plot show the magnification of the blue square frames. | ||
Reversible adiabatic changes in temperature ΔT for a material of density ρ with heat capacity C are given by
 | (1) |
More interesting, a salient phenomenon occurs around 300 K, which implies that ΔT increases with a decrease in temperature. This is attributed to the phase change from the orthorhombic phase to the pseudo cubic phase, and the phase transition is displayed in Fig. 3(b). ΔT of a BT thin film at 300 K is about 0.46 K under an external electrical field of 216.7 kV cm−1. It is almost equal to that of P(VDF–TrFE) working with P(VDF–TrFE–chlorofluoroethylene), which is 2 K under an external electrical field of 100 MV m−1.7 This result has never been reported in BT materials and is significant in the research of giant room temperature ECE.
4. Conclusions
BT thin films with a diffuse phase transition from the pseudo cubic phase to the cubic phase have been prepared by a sol–gel method. The enhanced room temperature ECE has been found in these thin films because of a phase transition from the pseudo cubic phase to the orthorhombic phase. The results indicate that we can achieve giant room temperature ECE by controlling the phase change of the ECE materials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 11272274, 11372266 and 11032010), the Hunan Provincial Natural Science Foundation of China (no. 12JJ1007), the Specialized Research Fund for the Doctoral Program of Higher Education (no. 20114301110004) and the Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201143).References
- J. F. Scott, Annu. Rev. Mater. Res., 2011, 41, 229 CrossRef CAS.
- M. Valant, Prog. Mater. Sci., 2012, 57, 980 CrossRef CAS PubMed.
- X. Li, S. G. Lu, X. Z. Chen, H. Gu, X. S. Qian and Q. M. Zhang, J. Mater. Chem. C, 2013, 1, 23 RSC.
- L. Mañosa, A. Planes and M. Acet, J. Mater. Chem. A, 2013, 1, 4925 Search PubMed.
- C. R. Bowen, H. A. Kim, P. M. Weaver and S. Dunn, Energy Environ. Sci., 2014, 7, 25 CAS.
- A. S. Mischenko, Q. Zhang, J. F. Scott, R. W. Whatmore and N. D. Mathur, Science, 2006, 311, 1270 CrossRef CAS PubMed.
- B. Neese, B. Chu, S. G. Lu, Y. Wang, E. Furman and Q. M. Zhang, Science, 2008, 321, 821 CrossRef CAS PubMed.
- M. A. Hamad, AIP Adv., 2012, 3, 032115 CrossRef PubMed.
- B. Li, J. B. Wang, X. L. Zhong, F. Wang and Y. C. Zhou, J. Appl. Phys., 2010, 107, 014109 CrossRef PubMed.
- G. Singh, I. Bhaumik, S. Ganesamoorthy, R. Bhatt, A. K. Karnal, V. S. Tiwari and P. K. Gupta, Appl. Phys. Lett., 2013, 102, 082902 CrossRef PubMed.
- B. Li, J. B. Wang, X. L. Zhong, F. Wang, Y. K. Zeng and Y. C. Zhou, RSC Adv., 2013, 3, 7928 RSC.
- J. H. Qiu, J. N. Ding, N. Y. Yuan and X. Q. Wang, Chin. Phys. B, 2013, 22, 017701 CrossRef.
- X. S. Qian, S. G. Lu, X. Li, H. Gu, L. C. Chien and Q. Zhang, Adv. Funct. Mater., 2013, 23, 2894 CrossRef CAS.
- X. Q. Liu, T. T. Chen, Y. J. Wu, X. M. Chen and M. Valant, J. Am. Ceram. Soc., 2013, 19, 1 Search PubMed.
- M. A. Hamad, Philos. Mag. Lett., 2013, 93, 346 CrossRef CAS.
- A. K. Axelsson, F. Le Goupil, L. J. Dunne, G. Manos, M. Valant and N. M. Alford, Appl. Phys. Lett., 2013, 102, 102902 CrossRef PubMed.
- X. Hao, Z. Yue, J. Xu, S. An and C. W. Nan, J. Appl. Phys., 2011, 110, 064109 CrossRef PubMed.
- Z. Feng, D. Shi and S. Dou, Solid State Commun., 2011, 151, 123 CrossRef CAS PubMed.
- B. Peng, H. Fan and Q. Zhang, Adv. Funct. Mater., 2013, 23, 2987 CrossRef CAS.
- M. M. Vijatović Petrović, J. D. Bobić, J. Banys and B. D. Stojanović, Mater. Res. Bull., 2013, 48, 3766 CrossRef PubMed.
- R. Mahbub, T. Fakhrul and M. F. Islam, Procedia Eng., 2013, 56, 760 CrossRef CAS PubMed.
- T. Badapanda, V. Senthil, S. Panigrahi and S. Anwar, J. Electroceram., 2013, 31, 55 CrossRef CAS.
- O. Namsar, A. Watcharapasorn and S. Jiansirisomboon, Ceram. Int., 2012, 38, 827 CrossRef PubMed.
- D. Y. Lu and D. D. Han, Ceram. Int., 2013, 39, 9727 CrossRef CAS PubMed.
- L. Qiao and X. Bi, Appl. Phys. Lett., 2008, 92, 062912 CrossRef PubMed.
- X. Moya, E. S. Taulats, S. Crossley, D. G. Alonso, S. K. Narayan, A. Planes, L. Manosa and N. D. Mathur, Adv. Mater., 2013, 25, 1360 CrossRef CAS PubMed.
- Y. Bai, G. P. Zheng, K. Ding, L. Qiao, S. Q. Shi and D. Guo, J. Appl. Phys., 2011, 110, 094103 CrossRef PubMed.
- Y. Bai, G. Zheng and S. Shi, Appl. Phys. Lett., 2010, 96, 192902 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |