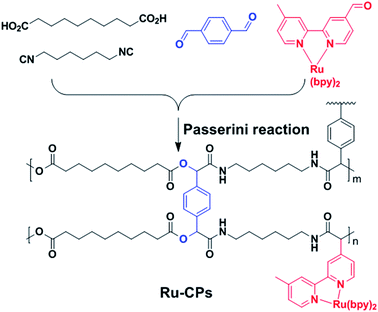
Synthesis of cross-linked polymers via multi-component Passerini reaction and their application as efficient photocatalysts†
Wenhai Linab,
Tingting Sunab,
Min Zhengc,
Zhigang Xie*a,
Yubin Huanga and
Xiabin Jinga
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun 130022, P. R. China. E-mail: xiez@ciac.ac.cn; Tel: +86-431-85262779
bUniversity of Chinese Academy of Science, Beijing 10039, P. R. China
cState key of laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, 3888 East Nanuhu Road, Changchun, Jilin 130033, P. R. China
First published on 29th May 2014
Abstract
One-step synthesis of cross-linked polymers containing Ru complexes (Ru-CPs) is demonstrated via multi-component Passerini reaction. Ru-CPs were shown to be highly effective and recyclable heterogeneous photocatalysts for oxidation of thioanisole and benzyl amine.
The development of photocatalysts for organic reactions driven by visible light is gaining increasing interest from organic chemists because of their mild conditions for substrate activation and the potential to mediate thermodynamically uphill reactions by harvesting energy from sunlight.1–5 Metal complexes such as [Ru(bpy)3]2+ (bpy = 2,2′-bipyridine) and organic dyes have been used as photocatalysts for a number of organic transformations.6,7 These photocatalysts display high catalytic activity and selectivity for many organic reactions as a result of the homogeneous reaction environment. Like other precious metal catalyzed reactions, it is highly desirable to develop recyclable heterogeneous photocatalytic systems.8 The reuse of such heterogeneous photocatalyst not only eliminates contamination of the organic products by trace amounts of heavy metals but also reduces processing and waste disposal costs in large scale production.
Cross-linked polymers (CPs) represent a new class of robust and porous materials and have shown great promise in gas storage and chemical sensing.9–14 Our previous work has demonstrated that CPs could serve as a platform for incorporating molecular catalytic modules into stable and reusable heterogeneous catalyst systems.15,16 However, synthesis of CPs usually needs harsh reaction conditions, like high temperature and pressure, or even a large amount of catalysts.17,18 Therefore, developing of simple and mild reactions for preparing CPs is still a big challenge in this field.
Passerini three-component reaction could combine isocyanides, aldehydes, and carboxylic acids into the ester and amide linkages via an atom-economic way. This reaction has been employed to synthesize diverse monomers and functional polymers due to the mild conditions without catalysts and tolerance to many functional groups.19–22 For example, Li et al. used this reaction to make various linear functional poly(ester–amide).23–26 Up to now, no report using Passerini reaction to make branched or cross-linked polymers was found. In this work, we successfully incorporated Ru complexes into CPs simply through multi-component Passerini reaction. The resulting Ru-CPs are highly stable and exhibit a high conversion rate and selectivity that are comparable to those of homogeneous counterparts in catalyzing the visible-light-driven oxidation of sulfides and benzyl amine. Moreover, the Ru-CPs photocatalysts can be readily recovered by filtration and reused without a decrease in catalytic efficiency.
The synthetic route to Ru-CPs is shown in Scheme 1. The [(bpy)2Ru(fmbpy)] (PF6)2 complex was prepared by the reaction of formy-5′-methyl-2,2′bipyridine (fmbpy) with Ru(bpy)2Cl2 at 90 °C overnight according to literature methods.27,28 Copolymerization of the [(bpy)2Ru(fmbpy)] (PF6)2, 1,6-di-isocyanohexane, sebacic acid and terephthalaldehyde was achieved through Passerini reaction in dichlomethane at room temperature for 4 days (ESI†). The resulting yellow solids were washed with various solvents to afford the Ru-CPs in 90% yield. The Ru-CPs were characterized by thermogravimetric analysis (TGA), inductively coupled plasma mass spectrometry (ICP-MS), infrared spectroscopy (IR), transmission electron microscopy (TEM), and solid-state 13C cross polarization/magic angle spinning nuclear magnetic resonance (CP/MAS/NMR) spectroscopy. A control experiment was run in the absence of Ru complexes to afford the cross-linked polymers (CPs) without catalytic modules.
The morphology of the Ru-CPs was examined by TEM. As shown in Fig. 1a and b, particles on the order of several micrometers in size displayed rather rough surfaces and appeared to be the aggregates of much smaller particles with dimensions of around 60 nm. CP/MAS/NMR spectroscopy in Fig. 1c gave all the carbon signals from 20 to 180 ppm. For example, the signals from 120 to 145 ppm and at 75 ppm are assigned to the13C atoms of the phenyl and methine, respectively. More important, the signals for carbon atoms of carbonyl appeared around 175 ppm, indicating that the cross-coupling reaction had happened. The absence of the carbon–hydrogen stretching peak of the –CHO around 2820 and 2720 cm−1 in the IR spectra of the Ru-CPs and CPs suggested that most of the aldehyde groups in the starting materials have been consumed in the cross-linked polymers, and a high degree of polymerization was achieved (Fig. 2a).
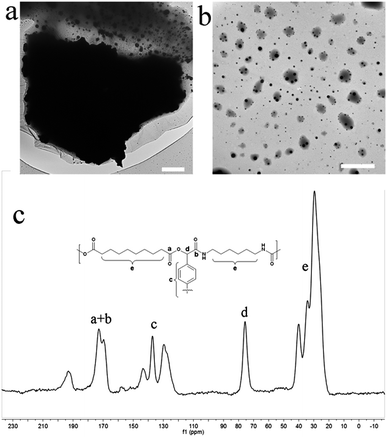 | ||
| Fig. 1 TEM image (a) and (b), scale bars are 2 μm; and the solid-state 13C NMR spectrum (c) of Ru-CPs. | ||
The Ru-CPs are insoluble in water and all of the organic solvents we tested. Both Ru-CPs and CPs are thermal stable in air up to 300 °C, as revealed by TGA (Fig. 2b). Ru catalyst loading for Ru-CPs was determined by ICP-MS to be 3.9 wt%. As shown in Fig. S1,† there is no N2 uptake for Ru-CPs, indicating the nonporous nature. The UV-Vis spectra of Ru-CPs suspension of methanol is shown in Fig. 2c, a typical absorbance band of Ru complexes was observed at 452 nm, indicating that the catalytic Ru complexes were incorporated into the polymers.
With the Ru complexes covalently integrated into the cross-linked polymers, we believed Ru-CPs could act as solid-state photosensitizer by using the 3MLST states of the Ru complexes. Fluorescence measurements were performed on a suspension of the Ru-CPs in methanol. As shown in Fig. 2d (red solid line), one broad band centered at 603 nm was observed which is originated from 3MLST to MS transitions, which could be quenched by oxygen. A quenching experiment was carried out to confirm that the 3MLST state of the phosphors in the Ru-CPs can be quenched by oxygen. As shown in Fig. 2d, the intensity of phosphorescence increased after degassing using nitrogen for 5 min. The quenching results suggested that good photocatalytic performance of Ru-CPs should be expected.
Catalytic activities of the Ru-CPs were evaluated with thioanisole as the substrate and methanol as the solvent. The reaction was carried out in the presence of air with a 24 W household fluorescent lamp as the visible light source. The concentration of the substrates was 12.4% (v/v). The molar ratio of the catalyst (Ru) to substrates was 0.001. The reaction was stopped after 24 h by filtering off the Ru-CPs catalyst, and then the precipitate was washed with methanol three times and dried under vacuum. Conversions of the thioanisole were determined by integrating the signals of the reactant (δ = 2.48 ppm) and the product (δ = 2.74 ppm) in the crude reaction mixtures (Fig. S2†).29
As shown in Table 1, the Ru-CPs are highly effective photocatalysts for the oxidation of thioanisole, as a conversion of 92% was obtained. No sulfone was detected by 1H NMR spectroscopy, which demonstrates the high degree of selectivity of this reaction. The high conversion and selectivity are comparable to those obtained with the use of their homogeneous counterparts Ru(bpy)32+. We also examined the scope of the substrates for the oxidation reaction using Ru-CPs. Several sulfides were efficiently oxidized under ambient condition, and the corresponding sulfoxides were produced with high conversions. We studied the recyclability and reusability of the Ru-CPs catalyst. Ru-CPs could be readily recovered from the reaction mixtures via simple filtration. The recovered Ru-CPs showed no decrease of conversion for oxidation of thioanisole after recycling three times (Table 1, entry 3–5). Furthermore, UV-Vis spectra of the supernatant showed no characteristic sign of Ru complexes leaching into the solution. This result supports the heterogeneous nature of the Ru-CPs photocatalysts.
| Entry | Catalyst | Substrate | Conversionb (%) |
|---|---|---|---|
| a All of the reactions were run at room temperature for 24 h.b Conversions were determined by 1H NMR spectroscopy. | |||
| 1 | Ru(bpy)32+ | Thioanisole | 92 |
| 2 | Ru-CPs | Thioanisole | 92 |
| 3 | Ru-CPs, reuse 1 | Thioanisole | 91 |
| 4 | Ru-CPs, reuse 2 | Thioanisole | 92 |
| 5 | Ru-CPs, reuse 3 | Thioanisole | 85 |
| 6 | CPs | Thioanisole | <5 |
| 7 | Ru-CPs | Phenyl ethyl sulfide | 55 |
| 8 | Ru(bpy)32+ | Phenyl ethyl sulfide | 56 |
| 9 | Ru-CPs | Methyl p-tolyl sulfide | 90 |
| 10 | Ru(bpy)32+ | Methyl p-tolyl sulfide | 92 |
We have also demonstrated the applicability of the Ru-CPs photocatalysts in oxidation of benzyl amine in chloroform. Oxidation of benzyl amine under visible light using homogenous photocatalysts had been reported.30,31 Here, we used this reaction to study the versatility of Ru-CPs in photocatalysis. As show in Fig. 3, Ru-CPs efficiently catalyzed the oxidation of benzyl amine in chloroform with 90% conversion (Fig. S3†), which showed similar catalytic activity with Ru(bpy)32+. We also showed that Ru-CPs could be recovered and reused for oxidation of benzyl amine without drop in the conversion. These results indicate the generality of the phosphorescent Ru-CPs in catalyzing photodriven organic transformations.
In summary, Ru complexes have been integrated into cross-linked polymers via multi-component Passerini reaction. This facile reaction is convenient for the synthesis of various functional polymeric materials. The formed cross-linked polymeric materials are stable in various solvents and were shown to be highly active and recyclable heterogeneous photocatalyst in oxidation of thioanisole and benzyl amine. This work highlights the potential of using multi-component Passerini reaction as a mild method for developing highly efficient heterogeneous photocatalyst for a number of organic transformations.
This work was supported by the National Natural Science Foundation of China (Project no. 21104075, 21201159 and 91227118).
Notes and references
- J. Liu, Q. Liu, H. Yi, C. Qin, R. Bai, X. Qi, Y. Lan and A. Lei, Angew. Chem., Int. Ed., 2014, 53, 502–506 CrossRef CAS PubMed.
- F. R. Petronijevic, M. Nappi and D. W. Macmillan, J. Am. Chem. Soc., 2013, 135, 18323–18326 CrossRef CAS PubMed.
- D. Prasad Hari, T. Hering and B. Konig, Angew. Chem., Int. Ed., 2014, 53, 725–728 CrossRef CAS PubMed.
- J. B. Xia, C. Zhu and C. Chen, J. Am. Chem. Soc., 2013, 135, 17494–17500 CrossRef CAS PubMed.
- J. Zhang, L. Wang, Q. Liu, Z. Yang and Y. Huang, Chem. Commun., 2013, 49, 11662–11664 RSC.
- D. M. Schultz and T. P. Yoon, Science, 2014, 343, 1239176 CrossRef PubMed.
- L. Quan, W. Lin, T. Sun, Z. Xie, Y. Huang and X. Jing, Catal. Lett., 2013, 144, 308–313 CrossRef PubMed.
- H. Xu, X. Chen, J. Gao, J. Lin, M. Addicoat, S. Irle and D. Jiang, Chem. Commun., 2014, 50, 1292–1294 RSC.
- Y. Li, Z. Sun, T. Sun, L. Chen, Z. Xie, Y. Huang and X. Jing, RSC Adv., 2013, 3, 21302 RSC.
- Q. Sun, X. Meng, X. Liu, X. Zhang, Y. Yang, Q. Yang and F. S. Xiao, Chem. Commun., 2012, 48, 10505–10507 RSC.
- Z. Ye, B. Tangeysh and B. B. Wayland, Chem. Commun., 2013, 49, 5372–5374 RSC.
- Q. Chen, X. Cao, H. Liu, W. Zhou, L. Qin and Z. An, Polym. Chem., 2013, 4, 4092–4102 RSC.
- X. Shi, M. Miao and Z. An, Polym. Chem., 2013, 4, 1950–1959 RSC.
- E. H. H. Wong, A. Blencowe and G. G. Qiao, Polym. Chem., 2013, 4, 4562–4565 RSC.
- C. Wang, Z. Xie, K. E. deKrafft and W. Lin, ACS Appl. Mater. Interfaces, 2012, 4, 2288–2294 CAS.
- Z. Xie, C. Wang, K. E. deKrafft and W. Lin, J. Am. Chem. Soc., 2011, 133, 2056–2059 CrossRef CAS PubMed.
- M. Ciftci, M. U. Kahveci, Y. Yagci, X. Allonas, C. Ley and H. Tar, Chem. Commun., 2012, 48, 10252–10254 RSC.
- Q. Sun, X. Meng, X. Liu, X. Zhang, Y. Yang, Q. Yang and F.-S. Xiao, Chem. Commun., 2012, 48, 10505–10507 RSC.
- R. Kakuchi, Angew. Chem., Int. Ed., 2014, 53, 46–48 CrossRef CAS PubMed.
- R. Kakuchi and P. Theato, ACS Macro Lett., 2013, 2, 419–422 CrossRef CAS.
- O. Kreye, T. Toth and M. A. Meier, J. Am. Chem. Soc., 2011, 133, 1790–1792 CrossRef CAS PubMed.
- A. Sehlinger, O. Kreye and M. A. R. Meier, Macromolecules, 2013, 46, 6031–6037 CrossRef CAS.
- X.-X. Deng, L. Li, Z.-L. Li, A. Lv, F.-S. Du and Z.-C. Li, ACS Macro Lett., 2012, 1, 1300–1303 CrossRef CAS.
- L. Li, A. Lv, X. X. Deng, F. S. Du and Z. C. Li, Chem. Commun., 2013, 49, 8549–8551 RSC.
- A. Lv, X.-X. Deng, L. Li, Z.-L. Li, Y.-Z. Wang, F.-S. Du and Z.-C. Li, Polym. Chem., 2013, 4, 3659 RSC.
- Y.-Z. Wang, X.-X. Deng, L. Li, Z.-L. Li, F.-S. Du and Z.-C. Li, Polym. Chem., 2013, 4, 444 RSC.
- M. Zheng, Z. Sun, Z. Xie and X. Jing, Chem.–Asian J., 2013, 8, 2807–2812 CrossRef CAS PubMed.
- M.-J. Li, C.-Q. Zhan, M.-J. Nie, G.-N. Chen and X. Chen, J. Inorg. Biochem., 2011, 105, 420–425 CrossRef CAS PubMed.
- W. Li, Z. Xie and X. Jing, Catal. Commun., 2011, 16, 94–97 CrossRef CAS PubMed.
- M. Rueping, C. Vila, A. Szadkowska, R. M. Koenigs and J. Fronert, ACS Catal., 2012, 2, 2810–2815 CrossRef CAS.
- F. Su, S. C. Mathew, L. Möhlmann, M. Antonietti, X. Wang and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657–660 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, TGA trace, XRD patterns, nitrogen adsorption–desorption isotherms and photo images. See DOI: 10.1039/c4ra02666g |
| This journal is © The Royal Society of Chemistry 2014 |