A hierarchical porous graphene/nickel anode that simultaneously boosts the bio- and electro-catalysis for high-performance microbial fuel cells†
Yan Qiaoabc,
Xiao-Shuai Wuabc,
Cai-Xia Maabc,
Hong Heabc and
Chang Ming Li*abc
aFaculty of Materials and Energy, Southwest University, Chongqing 400715, P.R. China
bInstitute for Clean Energy & Advanced Materials, Southwest University, Chongqing 400715, P.R. China
cChongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies, Chongqing 400715, P.R. China. E-mail: ecmli@swu.edu.cn
First published on 25th April 2014
Abstract
The microbial fuel cell (MFC) is an extremely attractive green energy source, which has gained considerable research interest, since it cleans the environment by using wastewater and/or organic wastes as fuels while harvesting electricity; however, a MFC has much lower power density than conventional fuel cells. In this study, a hierarchical porous graphene/nickel (G/Ni) composite electrode with a hierarchical porous structure over distributed pore sizes of 20 nm to 50 μm is developed by a freeze-drying assisted self-assembly process for MFC anodes. Since a MFC anode involves both electro- and bio-catalytic processes, the beauty of this G/Ni anode is its unique nanostructure that provides a high active surface area for efficient electrocatalysis, while its macroporous structure with strong biocompatibility allows bacteria growing in the pores to have high biocatalyst loading to boost biocatalysis. Thus, this binder-free hierarchical porous G/Ni anode delivers a maximum power density of 3903 mW m−2 in Shewanella putrefaciens (S. putrefaciens) MFCs, which is ∼13-fold higher than that of conventional MFC carbon cloth anode. Considering the low cost of porous Ni and the low weight percentage of graphene (5 wt%), this composite electrode offers great promise for practical, high-performance, cost-effective and mass-manufacturable MFCs.
1 Introduction
A microbial fuel cell (MFC), which can simultaneously decompose organic matter, is a promising clean energy source to produce electricity. It has great potential applications in many fields such as biosensors,1–3 wastewater treatment4–6 and electronic power sources for space shuttles.7 However, the poor long-term stability and low power density limit its practical applications. There are many factors that can affect a MFC's performance, including cell design, proton exchange material and electrode materials;8–11 however, the main limitation of MFC is the sluggish reaction kinetics of the anode for both electro- and bio-catalysis.12 To achieve high performance, rational design and delicate fabrication of an anode with unique nanostructures is very critical to meet the demands of both bio- and electro-catalysis.13,14To increase the performance of an anode, different porous electrode materials have been widely used. Carbon-based porous anodes such as carbon paper,15 carbon cloth,16 carbon nanotube17 and carbon foam18 are frequently employed in MFCs. Nevertheless, these electrodes have limited active surface areas, and the pores in these materials can be easily clogged by rapidly growing microbial cells, which can block their “food” supply. Recently, a number of new porous materials with nano components or three-dimensional structures, including polyaniline/mesoporous TiO2 nanocomposite,19 carbon nanotube,20 graphene-coated sponges21 and reduced graphene oxide-covered nickel foam anode,22 have been developed to improve MFC performance. Since the size of a bacterial cell is around 1–2 μm, the mesoporous electrode cannot allow them to grow in the pores to increase the biocatalyst loading for fast kinetics. The reported graphene/nickel foam material22 possesses a three-dimensional porous structure but with the pore sizes ranging between 100 and 500 μm, mainly determined by the Ni foam structure, which can provide more room for cell adhesion, but cannot provide a large active surface area for electrocatalytic reactions. In another study, graphene/nickel foam composite material was used for super capacitance,23 in which the nickel foam pores were completely filled with graphene hydrogel but lacked macro-pores (>10 μm) for cell adhesion in the pores. Thus, we expect that a hierarchical porous-structured electrode with both macro- and meso-pores could promote cell growth inside pores for high loading of biocatalysts while providing a large active surface area for high electrocatalytic process. For high performance MFCs, it is very important to develop a novel MFC anode with an appropriate porous structure and excellent electronic conductivity to simultaneously boost both electro- and bio-catalysis.
In this work, we developed a hierarchical porous graphene aerogel/nickel (G/Ni) electrode without any binders or conducting additives. The electrodes were optimized with different graphene loading for the best electrochemical behaviors and discharging profiles were examined. To the best of our knowledge, for the first time, this hierarchical porous graphene, which allows for growth of bacteria inside pores and possesses good conductivity to simultaneously promote both bio- and electro-catalytic process, has been fabricated and applied in MFCs.
2 Experimental
2.1. Preparation of graphene oxide and electrode
The graphene oxide (GO) used in this work was prepared from graphite by using a modified Hummers method.24 In a typical preparation, 1 g of graphite and 0.6 g of sodium nitrate (NaNO3) were mixed with 35 mL of concentrated sulfuric acid (H2SO4) in a 250 mL flask. After the mixture was placed in an ultrasonic bath for 15 minutes and stirred for 30 min in an ice bath, 4.5 g of potassium permanganate (KMnO4) was added with vigorous stirring. Note that the rate of addition should be carefully controlled to keep the reaction temperature lower than 20 °C. After continuously stirring the mixture at room temperature overnight, 36 mL of deionized (DI) water was slowly added with vigorous agitation. After the diluted suspension was stirred at 50 °C for one day, 12 mL of 30 wt% H2O2 was added to the mixture. The mixture was then washed several times by rinsing and centrifugation with 5 wt% HCl and DI water. Chemical exfoliation of the thus-obtained graphite oxide was carried out in an ultrasonic bath to obtain the final GO colloid.The G/Ni composite electrode can be prepared by immersing a piece of nickel foam (1 cm × 2 cm × 1.8 mm, mass per unit area 30 mg cm−2) in a whirlpool of GO colloid suspension with different concentration (2.0 mg mL−1, 4.0 mg mL−1, 6.0 mg mL−1 and 8.0 mg mL−1) (Scheme 1). The resultant GO/Ni composite film is then immersed in ascorbic acid solution (10.0 mg mL−1) overnight and subsequently freeze-dried for 24 h. The obtained G/Ni composites are denoted as G-NiI (2.0 mg mL−1), G-NiII (4.0 mg mL−1), G-NiIII (6.0 mg mL−1), and G-NiIV (8.0 mg mL−1).
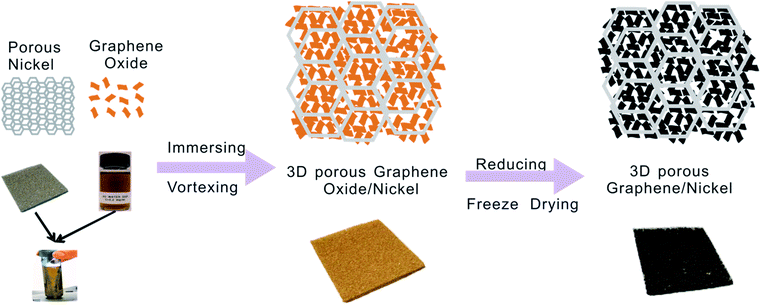 | ||
| Scheme 1 Schematic illustration of the fabrication process of hierarchical porous graphene/nickel composite electrode. | ||
2.2. Material characterization
Thermogravimetric analysis was performed from 50 °C to 450 °C by using a Q50 TGA system (TA Instruments-Waters LLC, America) to investigate the percentage of graphene present in the electrode. Scanning electron microscopy (SEM, JSM-6510LV, Japan) was used to examine the morphology of as-prepared electrode materials. For characterization, the discharged bacteria-absorbed anodes were immersed in 4% polyoxymethylene for 12 h and then sequentially dehydrated with ethanol (30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100%), and all the samples were dried in vacuum at room temperature overnight before SEM measurements.2.3. Analysis of electrochemical behaviors
The electrochemical analyses were conducted in an anode limited half-cell with SCE as the reference electrode and a titanium plate as the counter electrode. Cyclic voltammograms (CVs) were recorded and electrochemical impedance spectroscopy (EIS) experiments were conducted by using a potentiostat (CHI660E, Shanghai Chenhua, China). The electrochemical impedance measurements for the G/Ni composite electrodes were performed over a frequency range of 0.1 Hz to 100 kHz at −0.45 V with a perturbation signal of 10 mV. Unless otherwise stated, the voltammograms were recorded with a scan rate of 30 mV s−1. All tests were conducted at room temperature.2.4. MFC operation
Classic H-shaped MFCs were constructed using two 100 mL glass flasks (Fig. S1†). A Nafion 117 membrane that can separate the anodic and cathodic chambers was clamped in a 3.5 cm diameter tubular junction window. The anode (2 cm2) was immersed in LB medium containing 10 g L−1 sodium chloride, 10 g L−1 tryptone and 5 g L−1 yeast extract. S. putrefaciens (ATCC, BBA-1097), which is the bacteria used in this MFC, was grown at 30 °C for 10 h in lysogeny broth (LB) medium and harvested by centrifuging at 4 °C (6000 rpm, 5 min). Nitrogen was purged into the suspension for 30 min to remove oxygen from the cell before every test. The external load resistor for the discharge experiments was 1.45 kΩ, and the cathodic electrolyte was prepared using 50 mM potassium ferricyanide in 0.1 M phosphate buffered saline (pH 7.4).3 Results and discussion
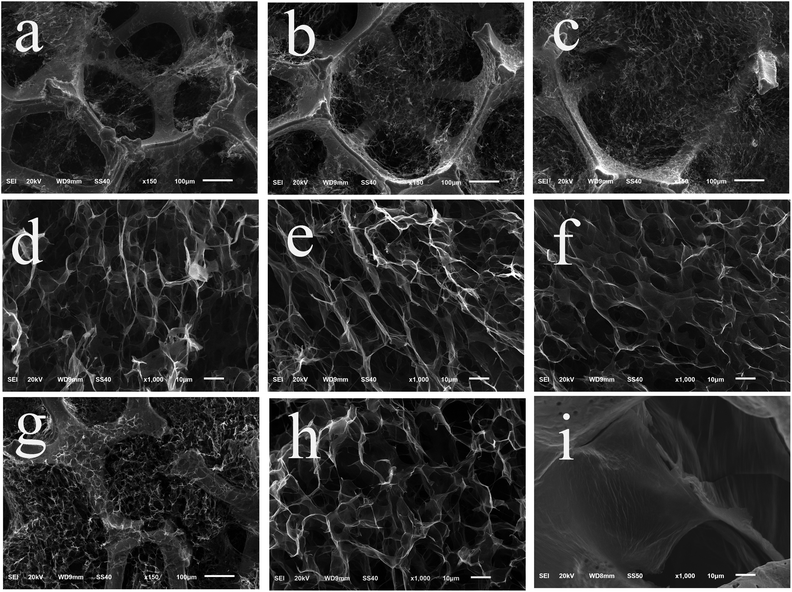
The SEM images of G/Ni composites are displayed in Fig. 1. The SEM images show that the reduced graphene oxide (rGO) nanosheets have a self-assembled porous aerogel structure and fill the pores of the nickel foam (Fig. 1a–h). The pore size of the graphene aerogel is in a range of 10–50 μm. Note that quite a few pores of nickel foam are not filled by graphene aerogel in G/NiI and G/NiII (Fig. 1a–c and g), while almost all pores are completely filled in G/NiIII and G/NiIV by graphene aerogel. Furthermore, in G/NiIII and G/NiIV, the graphene aerogel has more uniform pore structure and smaller pore size, suggesting that with the increasing loading amount of graphene, the pore size of the aerogel decreases. The high magnification micrographs show a rough surface of graphene aerogel (Fig. S2†). The morphology of vacuum-dried G/Ni composite (Fig. 1i) reveals that the graphene nanosheets stack into thick layers and do not have a hierarchical porous structure similar to the freeze-dried ones.The XRD pattern of G/NiIV was measured and compared with the patterns of freeze-dried GO and rGO (Fig. S3a†). The results reveal that the GO aerogel in the composite electrode is partially reduced. The TGA results (Fig. S3b†) show that the weight percentage of graphene in the composite increases with increasing GO concentration. The maximum value is around 8%, which is obtained in the G/NiIV composite. In addition, the pore-size distribution of the graphene aerogel in G/NiIII was examined with N2 adsorption/desorption methods. The result shows that the dominant mesopores in the aerogel have a size of ∼20 nm (Fig. S4†). According to the physical characterization results, the G/Ni composite electrode has a hierarchical porous structure that can provide large surface area for bacteria adhesion and growth for bioelectrocatalytic reactions.
The electrochemical behaviors of the composite electrodes were investigated in phosphate buffer, phosphate buffer with bacterial cell suspension and LB medium with cell suspension. The G/Ni electrodes show double layer capacitor behaviors, and the capacitive current increases with the loading of graphene aerogel (Fig. S5a†). The proportion of the increased capacitance is identical to that of the increased loading of graphene aerogel, thus indicating that the surface of the graphene aerogel in the composite electrode can be fully accessed by the electrolyte. In Fig. S5b,† a redox pair at ∼−0.45 V is observed on each CV curve of G/Ni electrodes but not on plain nickel foam, clearly symbolising an important fact that direct electron transfer between bacterial cell and the electrode is achieved through the graphene nanosheets. From Fig. 2a a well-defined redox pair can be observed on each curve of G/Ni anodes, and no redox peak can be found in the curve of plain nickel foam. Among the four composite anodes, the G/NiIII and G/NiIV anodes achieve much higher redox peak current and capacitive current. This could be contributed by the more filled graphene aerogel for more electrocatalytic active sites and charge storage. The EIS analysis was conducted in a half-cell with S. putrefaciens cell cultures in LB medium as electrolyte. The Nyquist plots (Fig. 2b) show that the G/NiIII and G/NiIV have the smallest charge transfer resistance. These findings are consistent with the CV results. Fig. 1 reveals that increasing graphene amount not only increases filling in the Ni frame but also raises the density of the pores in the graphene aerogel, which apparently could increase the electrode surface area for convenient electron transfer. However, too much graphene loading can decrease the pore size to block the bacterial cells swimming into the pores. This could be why the peak current of G/NiIII anode is higher than that of G/NiIV and has the highest electrocatalytic performance.
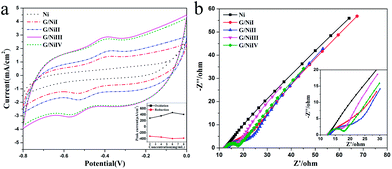 | ||
| Fig. 2 Cyclic voltammograms (a) and Nyquist plots (b) conducted in S. putrefaciens cells suspended in LB medium. | ||
To further investigate the effect of G/Ni nanostructures on the catalytic enhancement mechanism in MFCs, G/Ni electrodes were also made by a vacuum-drying process with the same graphene loading as that in G/NiIII. The electrocatalytic behavior of G/NiIII is compared with the vacuum dried one as shown in Fig. 3a and b, in which the CV curve (Fig. 3a) of G/NiIII illustrates a well-defined pair of redox peaks while the vacuum dried electrode only shows a very weak pair. The capacitive current of freeze-dried G/NiIII is also higher than that of the vacuum-dried one. For the EIS analysis (Fig. 3b), the freeze-dried electrode possesses a faster interfacial charge transfer rate. Apparently, the poorer electrocatalytic performance of the vacuum-dried electrode is mainly due to its lack of graphene aerogel hierarchical porous structure in Ni pores compared to that produced by the freeze-drying process (Fig. 1).
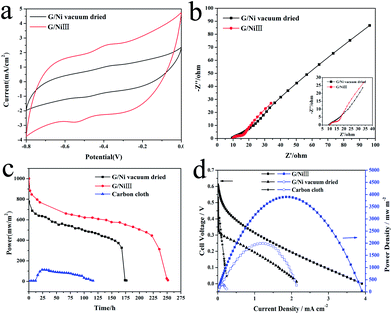 | ||
| Fig. 3 Cyclic voltammograms (a) and Nyquist plots (b) of vacuum-dried and freeze-dried G/Ni electrode, discharge performance (c), power output and polarization curve (d) of MFCs. | ||
The performances of G/NiIII, vacuum-dried G/Ni and carbon cloth anode-based MFCs were examined in a batch mode (Fig. 3c). The results show that G/NiIII has a higher power density and a much longer discharge life (250 h) than that utilizing a vacuum-dried anode (175 h) and carbon cloth (100 h). The drop of the power was due to the exhaustion of the substrates. As the three MFCs have the same substrate concentration, the longest discharge life indicates that the freeze-dried electrode also has highest energy conversion efficiency for higher Coulombic rate due to the highest bio- and electro-catalytic activity. The power density curves and polarization curves (Fig. 3d) show that the G/NiIII anode delivers a maximum power density of 3903 mW m−2, which is around 2-fold higher than that of the vacuum-dried anode (1991 mW m−2), around 13-fold higher than that of carbon cloth anode (303.5 mW m−2) and also higher than reported for mediator-less MFCs with graphene-based anode25,26 and three-dimensional anodes.27,28 These results demonstrate that the hierarchical porous graphene aerogel/nickel foam anode could greatly enhance the power output performance of MFCs.
To completely understand the catalytic enhancement of the nanostructured electrode, the morphologies of the electrodes were examined after discharge measurements. It is found that most of the bacterial cells are adhered in graphene aerogel (Fig. 4) instead of the Ni frame (Fig. S6†). Fig. 4a–c clearly show the morphologies at different regions of the G/NiIII anode. A very interesting observation is that the cells not only grow on the outside surface of the graphene gel but also live in the pores by adhering on the pore inside surface. The cells accumulate together to form an integrated biofilm network that can connect different layers of graphene nanosheets through the macropores. This not only significantly increases the loading of the biocatalyst (bacteria cells), but also facilitates the cell-to-cell electron communication for fast electron transport, strongly indicating that the bacterial cells can grow in the pores to significantly increase the biocatalyst loading. It should also be noted that the cell density on the vacuum-dried G/Ni electrode surface after discharge (Fig. 4d) is much lower than that of the freeze-dried one, in which only few separated cells live on the vacuum-dried electrode surface and no networked biofilm is observed. This indicates that the hierarchical porous structure has higher cell-affinity for more bacterial growth inside and outside pores. It is possible that the freeze-dried graphene aerosol with unique pore structure, size distribution and high biocompatibility allows cells to grow outside and inside pores for high biocatalyst loading while offering a large porous surface for electron transfer/transport, thus simultaneously boosting the bio- and electro-catalysis for high performance MFCs.
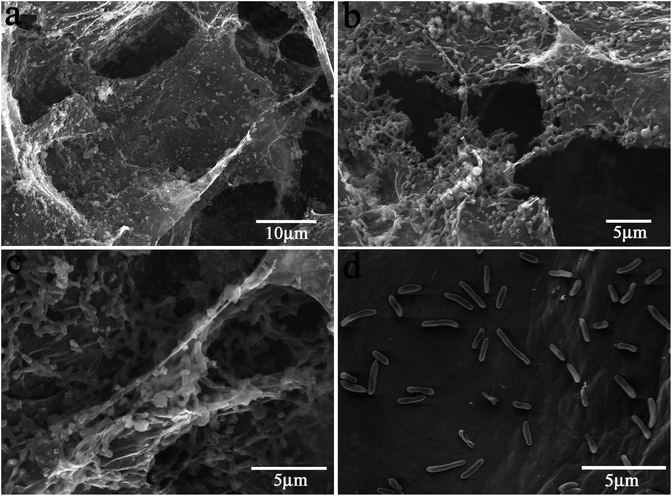 | ||
| Fig. 4 SEM micrographs of S. putrefaciens cells adhered on the G/Ni anode electrode surface (a–c: G/NIII anode d: vacuum-dried G/Ni anode). | ||
4 Conclusions
In brief, a novel hierarchical porous graphene aerogel/nickel foam anode fabricated by growing graphene aerogel on nickel foam without any binders or conducting additives was developed to simultaneously boost both electro- and bio-catalytic processes for a high performance MFC. The freeze-dried anode delivered a maximum power density of 3903 mW m−2 in S. putrefaciens MFCs, which is around 13-fold higher than that of the conventional carbon cloth-MFC anode. The unique hierarchical structure with appropriate macro-/nano-pore distribution and enhanced biocompatibility not only allows bacterial growth on both the outside and inside surfaces of the pores, but also generates a biofilm to increase the electron transfer/transport between bacteria and graphene, thus simultaneously boosting the bio- and electro-catalytic processes for a high power MFC. This study provides great promise to fabricate high power MFCs for practical applications.Acknowledgements
We gratefully acknowledge the financial support from the Fundamental Research Funds for the Central Universities (XDJK2012C090), National Natural Science Foundation of China (no. 31200102), Institute for Clean Energy & Advanced Materials, Southwest University, Chongqing, P.R. China, Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies and Chongqing Science and Technology Commission (cstc2012gjhz90002), and National Program of College Students Innovation and Entrepreneurship Training (no. 201310635039). The authors would like to also thank Tingting Zhou and Cuicui Yang for the help in the experiments.Notes and references
- I. S. Chang, J. K. Jang, G. C. Gil, M. Kim, H. J. Kim, B. W. Cho and B. H. Kim, Biosens. Bioelectron., 2004, 19, 607–613 CrossRef CAS
.
- I. S. Chang, H. Moon, J. K. Jang and B. H. Kim, Biosens. Bioelectron., 2005, 20, 1856–1859 CrossRef CAS PubMed
.
- J. M. Tront, J. D. Fortner, M. Ploetze, J. B. Hughes and A. M. Puzrin, Biosens. Bioelectron., 2008, 24, 586–590 CrossRef CAS PubMed
.
- J. Sun, Y. Hu, Z. Bi and Y. Cao, J. Power Sources, 2009, 187, 471–479 CrossRef CAS PubMed
.
- R. A. Rozendal, H. V. M. Hamelers, K. Rabaey, J. Keller and C. J. N. Buisman, Trends Biotechnol., 2008, 26, 450–459 CrossRef CAS PubMed
.
- B. E. Logan, Water Sci. Technol., 2005, 52, 31–37 CAS
.
- D. R. Lovley, Nat. Rev. Microbiol., 2006, 4, 497–508 CrossRef CAS PubMed
.
- H. S. Park, B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. K. Park and H. I. Chang, Anaerobe, 2001, 7, 297–306 CrossRef CAS
.
- C. S. Butler and R. Nerenberg, Appl. Microbiol. Biotechnol., 2010, 86, 1399–1408 CrossRef CAS PubMed
.
- S. E. Oh and B. E. Logan, Appl. Microbiol. Biotechnol., 2006, 70, 162–169 CrossRef CAS PubMed
.
- G. C. Gil, I. S. Chang, B. H. Kim, M. Kim, J. K. Jang, H. S. Park and H. J. Kim, Biosens. Bioelectron., 2003, 18, 327–334 CrossRef CAS
.
- Y. Qiao, S.-J. Bao and C. M. Li, Energy Environ. Sci., 2010, 3, 544–553 CAS
.
- Y. Qiao and C. M. Li, J. Mater. Chem., 2011, 21, 4027–4036 RSC
.
- Y. Zhang, G. Mo, X. Li, W. Zhang, J. Zhang, J. Ye, X. Huang and C. Yu, J. Power Sources, 2011, 196, 5402–5407 CrossRef CAS PubMed
.
- B. Min and B. E. Logan, Energy Environ. Sci., 2004, 38, 5809–5814 CAS
.
- S. Cheng, H. Liu and B. E. Logan, Energy Environ. Sci., 2006, 40, 2426–2432 CAS
.
- Y. Qiao, C. M. Li, S.-J. Bao and Q.-L. Bao, J. Power Sources, 2007, 170, 79–84 CrossRef CAS PubMed
.
- S. K. Chaudhuri and D. R. Lovley, Nat. Biotechnol., 2003, 21, 1229–1232 CrossRef CAS PubMed
.
- Y. Qiao, S.-J. Bao, C. M. Li, X.-Q. Cui, Z.-S. Lu and J. Guo, ACS Nano, 2008, 2, 113–119 CrossRef CAS PubMed
.
- X. Xie, M. Ye, L. Hu, N. Liu, J. R. McDonough, W. Chen, H. N. Alshareef, C. S. Criddle and Y. Cui, Energy Environ. Sci., 2012, 5, 5265–5270 CAS
.
- X. Xie, G. Yu, N. Liu, Z. Bao, C. S. Criddle and Y. Cui, Energy Environ. Sci., 2012, 5, 6862–6866 CAS
.
- H. Wang, G. Wang, Y. Ling, F. Qian, Y. Song, X. Lu, S. Chen, Y. Tong and Y. Li, Nanoscale, 2013, 7, 10283–10290 RSC
.
- J. Chen, K. Sheng, P. Luo, C. Li and G. Shi, Adv. Mater., 2012, 24, 4569–4573 CrossRef CAS PubMed
.
- J. Zhang, Z. Xiong and X. S. Zhao, J. Mater. Chem., 2011, 21, 3634–3640 RSC
.
- W. Guo, Y. Cui, H. Song and J. Sun, Bioprocess Biosyst. Eng., 2014 DOI:10.1007/s00449-014-1148-y
.
- C. Zhao, P. Gai, C. Liu, X. Wang, H. Xu, J. Zhang and J. J. Zhu, J. Mater. Chem. A, 2013, 1, 12587–12594 CAS
.
- Y. C. Yong, X. C. Dong, M. B. Chan-Park, H. Song and P. Chen, ACS Nano, 2012, 6, 2394–2400 CrossRef CAS PubMed
.
- J. Hou, Z. Liu, S. Yang and Y. Zhou, J. Power Sources, 2014, 258, 204–209 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03082f |
| This journal is © The Royal Society of Chemistry 2014 |