Photoresponsive superhydrophobic surfaces from one-pot solution spin coating mediated by polydopamine†
Jing Zhangab,
Weidong Zhang*a,
Nianchen Zhoub,
Yuyan Wenga and
Zhijun Hu*a
aCenter for Soft Condensed Matter Physics and Interdisciplinary Research & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, China. E-mail: zhijun.hu@suda.edu.cn; zhangweidong@suda.edu.cn
bDepartment of Polymer Science and Engineering, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China
First published on 29th May 2014
Abstract
Smart stimuli-responsive superhydrophobic surfaces with reversibly switchable wettability have received considerable attention due to their various potential applications. We demonstrate here a new but versatile approach for preparing photoresponsive superhydrophobic surfaces. Fluorinated azobenzene derivatives or polymers are immobilized onto silica surface by taking advantage of the remarkable adhesive ability of polydopamine. By simply spin coating the silica particles onto silicon wafer, superhydrophobic surfaces can be obtained with a water contact angle greater than 150° and low contact angle hysteresis (<10°). In addition, reversible change water contact angles on the surfaces can occur upon irradiation with alternate UV and visible light.
1 Introduction
Smart stimuli-responsive superhydrophobic surfaces with reversibly switchable wettability have received considerable attention due to their great potential applications ranging from self-cleaning materials to microfluidic devices.1–5 The reversible change in wettability can be achieved either by incorporating a stimuli-responsive material onto a micro/nano-structured surface or by creating micro/nano hierarchical structures on the surface of a stimuli-responsive material. Various external stimuli, such as temperature,6 pH,7 solvent,8 light9 and electric field,10 have been explored to trigger the conformation or composition change of stimuli-sensitive materials and thus the wettability. Additionally, the existing toolbox for immobilization of functional molecules onto surfaces including methods such as chemical grafting,11 self-assembled monolayer formation,12 Langmuir–Blodgett deposition,13 and layer-by-layer assembly,14 are exploited to construct smart stimuli-responsive superhydrophobic surfaces. The surface modification approaches, however, are typically developed case by case and depend on the relationship between material properties and immobilized molecules.Recently, polydopamine (PD) coating, inspired by the adhesive properties of marine mussels, has been demonstrated to be a simple and versatile approach for surface modification.15 PD is spontaneously formed by pH-induced, oxidative polymerization of dopamine-hydrochloride in alkaline solutions. Interestingly, the versatile catechol units in PD are able to functionalize virtually any material surfaces, regardless of their chemical functionality or surface energy,16–20 as a result of multiple binding mechanism, including π–π interactions, coordination bonds, and covalent bonds.21 In this respect, besides the surface modification, one can expect that the catechol units remained on the PD surface can be further explored to immobilize other species. This would pave the versatile and easy way to smart responsive surfaces.22 In addition, during the oxidative polymerization of dopamine-hydrochloride in alkaline solutions, micro/nano-aggregates of PD or oligomers of dopamine can be formed due to π–π bonding and van der Waals forces.23 The aggregates which form in solution and attach to functionalized surfaces would result in significant increases in surface roughness. This is an advantage for preparing superhydrophobic surfaces since micro/nano hierarchical structures are needed to increase the surface roughness.
Herein, we report the first example of fabricating photo-responsive superhydrophobic surface by taking advantage of the remarkable adhesive ability of PD and of the micro/nano-aggregates forming ability during PD formation. In order to verify this concept, fluorinated azobenzene derivatives and polymers are used in this study because of their well-known rapid and reversible trans–cis photoisomerization which results in a large change in both geometry and dipole moments.24,25 The large change in dipole moments can lead to photoswitchable wettability of azobenzene modified surfaces.26
2 Experimental
Materials
Dopamine hydrochloride was purchased from Adamas Reagent Co., Ltd and used as received. Silica particles of ∼430 nm were prepared by Stober method.27 Typically, 5 mL tetraethoxysilane (TEOS) was dispersed in 20 mL ethanol with stirring for 30 min. 10 mL water, 20 mL ethanol, 5 mL ammonia water were introduced in 100 mL flask with stirring for 20 min. The TEOS solution was dropwisely added to the flask with constantly stirring for 5 h at room temperature. Finally, the silica spheres were obtained after washing by ethanol and centrifugation for several times. The morphology of the silica particles were observed by field emission scanning electron microscopy (Hitachi, S-4700), operating at 15 kV. The synthesis and characterization of the fluorinated azobenzene derivatives and polymers were described in the ESI.†Methods
Dopamine hydrochloride (2 mg mL−1) was dissolved in buffered aqueous solution (Tris–HCl) at pH 8.5. Silica particles (0.1 g) with diameter of about 430 nm were added to dopamine solution (30 mL). The solution was stirred overnight at room temperature to generate silica/PD composite particles. After washing with deionized water for several times, the composite particles were dispersed in solution containing the molecules (10 mg mL−1) to be immobilized at room temperature. The size evolution of silica and composite particles was monitored by dynamic light scattering (DLS) with ZETA SIZER Nano Series (NANO-ZS90). Then the silica/PD composite particles with immobilized molecules were spin-coated on silicon wafer under 1000 rpm (30 s). The as-prepared thin films were annealed at 120 °C for 24 h in vacuum oven. The surface appearance were observed by field emission scanning electron microscopy (SEM, Hitachi, S-4700), operating at 15 kV, and transmission electron miscopy (TEM, HT7700, Hitachi). X-ray photoelectron spectrometry (XPS) spectra were recorded on an ESCALABMKLL spectrometer (VG Scientific) by using an Al K X-ray source (1200 eV). Sessile water droplet contact angle values were acquired on an OCA 20 optical contact angle meter (Dataphysics Company) at ambient temperature. 5 μL deionized water was dropped on the samples using an automatically dispense controller, and the contact angles were determined automatically by using the Laplace–Young fitting algorithm.3 Results and discussion
The general procedures for preparing the photo-responsive superhydrophobic surface are described in Scheme 1. Firstly, silica particles are dispersed in dopamine solution at pH of 8.5. After 24 hours, PD coated silica (silica/PD) particles are obtained due to pH-induced, oxidative polymerization of dopamine-hydrochloride.15,28 Secondly, the fluorinated azobenzene derivatives or polymers are immobilized onto the surface of silica/PD particles by dispersing the silica/PD particles in the solution and by virtue of the immobilization ability of PD. Finally, the hybrid silica particles mediated by PD are spin coated onto flat substrates such as silicon wafers. In order to verify the versatility of the approaches, three samples including the fluorinated azobenzene derivative 4-trifluoromethoxy[4-(6-hydroxyhexoxy)phenyl] azobenzene (TPA), homopolymer poly (4-trifluoromethoxyphenyl[4-hexoxyl(2-methylacrylate)phenyl] azobenzene) (PTHPA) and block copolymer poly(4-trifluoromethoxyphenyl[4-hexoxyl(2-methylacrylate)phenyl] azobenzene-b-4-vinylpyridine) (PTHPAm-b-P4VPn) have been tested in this study.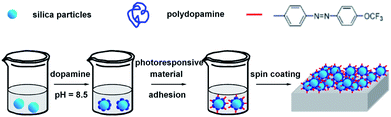 | ||
| Scheme 1 Schematic drawing of the general procedures for preparing photoresponsive superhydrophobic surfaces. | ||
The size evolution of silica particles dispersed in solution is monitored by dynamic light scattering (DLS) experiments. As shown in Fig. 1, the mean diameter of the initial silica particles is about 430 nm. After modification with PD, the diameter of the silica/PD is increased to about 500 nm, indicating that the PD which is not monolayer is successfully absorbed on the silica surfaces. When the fluorinated azobenzene derivative TPA or polymers are immobilized onto the surfaces of silica/PD particles, the mean diameter of the particles is further increased to about 530 nm, meaning that the photoresponsive are successfully immobilized onto the silica/PD particles.
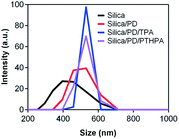 | ||
| Fig. 1 DLS diagrams of the initial silica, silica/PD, silica/PD/TPA and silica/PD/polymer particles, indicating the size evolution before and after PD and photoresponsive molecules modification. | ||
The morphological evolution of silica particles spin cast onto silicon wafer probed by scanning electron microscopy (SEM) confirms the successful modification of silica particles by the PD and the immobilization of fluorinated azobenzene derivatives or polymers. As shown in Fig. 2a. The surface of the silica particles is very smooth. Due to the low adhesion ability of silica particles on silicon wafer, only discontinuous silica film can be obtained. After modification with PD, some roughness is created on the surfaces of silica particles, as shown in Fig. 2b. Recently researches indicates that the absorption of dopamine on surfaces are very complicated and a large amount of dopamine remains unpolymerized as a self-assembled stable complex of (dopamine)2/5,6-dihydroxyindole (DHI), which is tightly bound within PD.21 The layer of PD is formed through at least three different pathways, physical non-covalent self-assembly of dopamine, DHI and covalent polymerization of dopamine. As a result, the roughness of the surface like hierarchical structures of lotus leaf can easily be fabricated by the PD treatment. The thickness of PD absorbed on the surface of silica particles is about 70 nm, as measured with transmission electron microscopy (TEM) images shown in Fig. 3. In addition, multilayer silica/PD particles can be easily obtained by the spin coating method. After the fluorinated azobenzene derivative TPA is immobilized onto the surfaces of silica/PD particle, the morphology of the particle has little change and still remains rough (Fig. 3b). The hybrid silica particles mediated by fluorinated azobenzene derivative are spin coated onto the silicon wafer and the surface also has little change. One example of silica/PD/TPA systems is shown in Fig. 2c. Additionally, multilayer coating of silica/PD/TPA particles is easily obtained by the spin coating process.
 | ||
| Fig. 3 TEM photographs of (a) PD coated silica particles, and (b) fluorinated azobenzene derivative TPA modified silica particles, indicating the surface roughness increases of silica particles. | ||
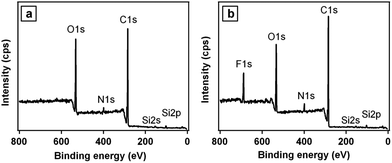
In order to further prove the successful adsorption process, the chemical composition of the microparticles surfaces at each step of surface modification is determined by X-ray photoelectron spectroscopy (XPS). The Si 2s, Si 2p, O 1s, C 1s and N 1s peaks are discernible in the wide scan spectrum of the silica/PD particles (Fig. 4a), indicating the formation of PD films on the silica particles. Similarly, the appearance of a strong F 1s signal at binding energy of about 690 eV indicates that the fluorinated azobenzene molecules are adhered on the rough surface of silica/PD particles (Fig. 4b). These results indicates that PD treated particles can adhere the functionalized materials.
The surface modification of silica particles leads to dramatic changes in water contact angles (CAs). Fig. 5 shows the evolution of water CA of modified silica particles spin coated on silicon wafers. The surface composed of silica/PD particles is extremely hydrophilic, wherein the water CA is 6 ± 2° (Fig. 5a) due to the hydrophilic nature of PD. After modification with fluorinated azobenzene derivative TPA, the surface composed of silica/PD/TPA particles becomes hydrophobic, wherein the water CA is 115 ± 2° (Fig. 5b). After annealing at 120 °C, the surface is expected to rearrange and the CF3 groups to migrate to the particle–air surfaces.29 The water CAs would thus be further increased due to the interplay of hierarchical structure and lower surface free energy.30 Indeed, the annealed samples become superhydrophobic with a water CA of 151 ± 1° (Fig. 5c) and a small contact angle hysteresis (7°, advancing CA 154°, receding CA 147°).
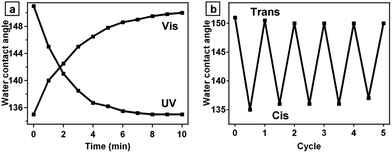
An azobenzene molecule can undergo reversible trans–cis isomerization upon exposed to alternate UV and visible light irradiation. The trans isomer of azobenzene has a nearly zero dipole moment, whereas the dipole moment of the cis isomer is around 3.0 debye.31 The large change in dipole moment of azobenzene molecules after photoisomerization can cause the change of water CA. As shown in Fig. 6a, when the surface composed of silica/PD/TPA is irradiated with UV light (λ = 365 nm, 15 mW cm−2), the CA slowly decreases from 151 ± 1° to 135 ± 1° with a hysteresis of about 14° (advancing CA 144°, receding CA 130°). If the samples are exposed to visible light (λ > 420 nm), the water CAs recover to their initial levels due to the back cis-to-trans isomerization. After irradiation for about 10 min with UV or visible light, the CA reaches saturation. In addition, this phenomenon can be repeated for many switching cycles (Fig. 6b).
The successful immobilization of functional materials onto the PD coated silica particles is not limited to azobenzene derivative TPA with low molecular weight. The immobilization of polymers such as homopolymer PTHPA and block copolymer PTHPAm-b-P4VPn is also valid. As shown in Fig. 7, superhydrophobic surfaces are obtained from the polymer modified silica particles mediated by PD. The water CAs can be tuned with alternate UV and visible light irradiation, similar to that of silica/PD/TPA surfaces. In addition, the stability of the polymers is much higher than the small molecules of TPA, as shown in the thermal gravimetric analysis in the ESI (Fig. S3†).
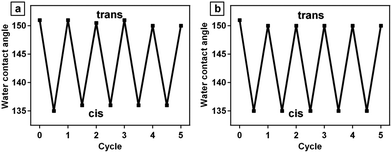 | ||
| Fig. 7 Water contact angle changes of surfaces composed of (a) silica/PD/PTHPA particles and (b) silica/PD/PTHPAm-b-P4VPn particles upon exposed to alternate UV and visible light irradiation. | ||
We have to point out that the change of water CAs is less than 20° upon alternate UV and visible light irradiation. This value is relatively small compared to the results reported previously32 in porous thin films modified with fluorinated azobenzene molecules. In the porous thin films, the air trapped in the pores provides a cushion at the film–water interface (Cassie–Baxter mode) which prevents the penetration of water droplets into the inner space.33 After UV irradiation, the water would fill the inner space (Wenzel mode), therefore the thin films becomes more hydrophilic.34 In our case of silica particles, although some nanostructures are created after the PD coating, the roughness of the hybrid silica surface is still relatively low, which is not sufficient to be tuned from Cassie–Baxter to Wenzel contact mode upon photoirradiation. Nevertheless, the change of water CAs on the surface of hybrid silica particles is much higher than that of azobenzene-modified silicon wafer mediated by PD, wherein only 99° of CA and minor changes of about 10° was observed (Fig. S4 in the ESI†). In order to make comparison, we prepared sample that the azobenzene derivative TPA was immobilized on the PD-modified silicon wafer and we found that the water contact angle of the sample surface is 99 ± 1°. After UV irradiation, the CA decreases from 99 ± 1° to 88 ± 1°. The wettability of the surface can be reversibly switched under alternate UV and visible light irradiation (ESI†).
4 Conclusions
In conclusion, we introduced a general integration approach to obtain photoresponsive superhydrophobic surface by PD treatment. PD not only makes the rough surface, but also adhere smart material onto the surface. By simply spin coating the hybrid particles onto substrates, photoresponsive surfaces can be obtained. We believe that our general approaches for immobilization of functional materials can make materials structure and function to the desired application.Acknowledgements
We gratefully acknowledge the support of the National Natural Science Foundation of China (no. 91027040, 21074084, 21104051), the National Basic Research Program of China (no. 2012CB821500), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, the Natural Science Foundation of Jiangsu Province of China (no. BK2010213) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- F. Xia and L. Jiang, Adv. Mater., 2008, 20, 2842–2858 CrossRef CAS.
- H. S. Lim, S. G. Lee, D. H. Lee, D. Y. Lee, S. Lee and K. Cho, Adv. Mater., 2008, 20, 4438–4441 CrossRef CAS.
- B. Xin and J. Hao, Chem. Soc. Rev., 2010, 39, 769–782 RSC.
- K. Liu, X. Yao and L. Jiang, Chem. Soc. Rev., 2010, 39, 3240–3255 RSC.
- X. Liu, Y. Liang, F. Zhou and W. Liu, Soft Matter, 2012, 8, 2070–2086 RSC.
- T. Sun, G. Wang, L. Feng, B. Liu, Y. Ma, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 357–360 CrossRef CAS PubMed.
- X. Yu, Z. Q. Wang, Y. G. Jiang, F. Shi and X. Zhang, Adv. Mater., 2005, 17, 1289–1293 CrossRef CAS.
- G. K. Jennings and E. L. Brantley, Adv. Mater., 2004, 16, 1983–1994 CrossRef CAS.
- X. J. Feng, L. Feng, M. H. Jin, J. Zhan, L. Jiang and D. Zhu, J. Am. Chem. Soc., 2004, 126, 62–63 CrossRef CAS PubMed.
- S. Millefiorini, A. H. Tkaczyk, R. Sedev, J. Efthimiadis and J. Ralston, J. Am. Chem. Soc., 2006, 128, 3098–3099 CrossRef CAS PubMed.
- H. Heinz, R. A. Vaia, H. Koerner and B. L. Farmer, Chem. Mater., 2008, 20, 6444–6456 CrossRef CAS.
- M. Min, G. S. Bang, H. Lee and B.-C. Yu, Chem. Commun., 2010, 46, 5232–5234 RSC.
- K. Morigaki, Z.-F. Liu, K. Hashimoto and A. Fujishima, J. Phys. Chem., 1995, 99, 14771–14777 CrossRef CAS.
- J. B. Han, D. P. Yan, W. Y. Shi, J. Ma, H. Yan, M. Wei, D. G. Evans and X. Duan, J. Phys. Chem. B, 2010, 114, 5678–5685 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Mesersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- S. M. Kang, I. You, W. K. Cho, H. K. Shon, T. G. Lee, I. S. Choi, J. M. Karp and H. Lee, Angew. Chem., Int. Ed., 2010, 49, 9401–9404 CrossRef CAS PubMed.
- I. You, S. M. Kang, S. Lee, Y. O. Cho, J. B. Kim, S. B. Lee, Y. S. Nam and H. Lee, Angew. Chem., Int. Ed., 2012, 51, 6126–6130 CrossRef CAS PubMed.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244–4258 RSC.
- B. H. Kim, D. H. Lee, J. Y. Kim, D. O. Shin, H. Y. Jeong, S. Hong, J. M. Yun, C. M. Koo, H. Lee and S. O. Kim, Adv. Mater., 2011, 23, 5618–5622 CrossRef CAS PubMed.
- S. Hong, Y. S. Na, S. Choi, I. T. Song, W. Y. Kim and H. Lee, Adv. Funct. Mater., 2012, 22, 4711–4717 CrossRef CAS.
- S. M. Kang, N. S. Hwang, J. Yeom, S. Y. Park, P. B. Messersmith, I. S. Choi, R. Langer, D. G. Anderson and H. Lee, Adv. Funct. Mater., 2012, 22, 2949–2955 CrossRef CAS PubMed.
- S. Hong, J. Kim, Y. S. Na, J. Park, S. Kim, K. Singha, G.-I. Im, D.-K. Han, W. J. Kim and H. Lee, Angew. Chem., Int. Ed., 2013, 35, 9187–9191 CrossRef PubMed.
- K. Ichimura, S. Oh and M. Nakagawa, Science, 2000, 288, 1624–1626 CrossRef CAS.
- L. M. Siewierski, W. J. Brittain, S. Pettash and M. D. Foster, Langmuir, 1996, 12, 5838–5844 CrossRef CAS.
- H. S. Lim, W. H. Lee, S. G. Lee, D. Lee, S. Jeon and K. Cho, Chem. Commun., 2010, 46, 4336–4338 RSC.
- W. Stober and A. Fink, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- J. Sagert, C. Sun and J. H. Waite, Chemical Subtleties of Mussel and Polychaete Holdfasts, Springer-Verlag, Berlin, Germany, 2006, pp. 125–140 Search PubMed.
- A. M. Granville, S. C. Boyes, B. Akgun, M. D. Foster and W. J. Brittain, Macromolecules, 2005, 38, 3263–3270 CrossRef CAS.
- L. Feng, S. H. Li, Y. S. Li, H. J. Li, L. J. Zhang, J. Zhai, Y. L. Song, B. Q. Liu, L. Jiang and D. B. Zhu, Adv. Mater., 2002, 14, 1857–1860 CrossRef CAS.
- G. S. Hartley and R. J. W. le Fèvre, J. Chem. Soc., 1939, 531–535 RSC.
- H. S. Lim, J. T. Han, D. Kwak, M. Jin and K. Cho, J. Am. Chem. Soc., 2006, 128, 14458–14459 CrossRef CAS PubMed.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546–551 RSC.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis of materials and experimental detail. See DOI: 10.1039/c4ra03469d |
| This journal is © The Royal Society of Chemistry 2014 |