Hydrothermally stable macro-meso-microporous materials: synthesis and application in heavy oil cracking†
Hongtao Liua,
Kun Wanga,
Yonggang Shia,
Xionghou Gaob,
Honghai Liub,
Baojie Wangb and
Chunyan Xu*a
aState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: xucy@mail.buct.edu.cn
bPetrochemical Research Institute, Petrochina Company Limited, Beijing 100195, P. R. China
First published on 18th June 2014
Abstract
Hydrothermally stable hierarchical materials with macro-meso-micropores were synthesized by combination of precursor assembly and PS/P123 dual templates. Precursor assembly aims at the formation of meso-micropores and improving the hydrothermal stability, and PS microspheres aim at the introduction of macropores. Moreover, worm-like mesopores vertical to the surface of PS microspheres were present, which achieve the full interconnection of macro-meso-micropores. The resulting aluminosilicates with hierarchical pores and high hydrothermal stability showed excellent catalytic cracking properties for heavy oil.
Heavy oil fluid catalytic cracking (FCC) has received remarkable improvements, in which the design and fabrication of hierarchical pores capable of the successive cracking of heavy oil molecules have raised considerable attention.1–4 From the viewpoint of industrial applications, kaolin, MCM-41, and USY have been employed to perform heavy oil FCC due to their hierarchical pore systems.5 However, two key problems remain unresolved for heavy oil FCC catalysts: (1) the disordered macropores of the kaolin matrix are not fully interconnected with the mesopores and micropores of zeolites; (2) hydrothermal stability of mesoporous zeolites is low, leading to collapse of catalysts in the severe conditions. Therefore, how to design hydrothermally stable materials with a macro-meso-micropore system still remains a great challenge.
During the past decades, considerable efforts have been focused on fabrication of hierarchical pores with high hydrothermal stability. An important approach is to introduce microporous zeolite precursors into the walls of mesophases.6–8 For example, the authors of the present investigation have prepared hydrothermally stable mesophase by assembling Beta and Y precursors with P123.9–13 Unfortunately, macropores are still absent in these materials, and so forth, which limit their practical application. Therefore, one effective solution to this problem is to develop a novel preparation strategy for the immobilization of macropores into the meso-microporous materials.
Polystyrene (PS) is one type of important materials which represents a large versatility in terms of its particle size and the ability to build up 3D-organized opening structures.14–16 PS microspheres have been widely used in the field of catalysis17 and sensor.18 This inspires us to challenge the goal of fabricating hierarchical pores with high hydrothermal stability via the combination of PS microspheres and precursors assembly technique. This combination will show the following advantages: (1) hydrothermal stability will be improved by precursors assembly method; (2) the interconnected macro-meso-micropores can be achieved by the introduction of PS microspheres.
In the present investigation, we report the synthesis of hydrothermally stable materials with macro-meso-micropores. In this strategy, PS microspheres with uniform particle size were synthesized by emulsion polymerization. PS/P123 dual templates are introduced into Y precursors and they can template the formation of the hierarchical materials. Therefore, precursor assembly aims at the formation of meso-micropores and improving the hydrothermal stability, and PS microsphere aims at the formation of macropores. A combination of PS microspheres and Y precursors assembly is believed to be the key in obtaining hydrothermally stable materials with macro-meso-micropores.
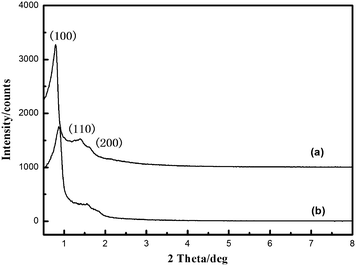
The XRD pattern (Fig. 1) of mesoporous stable aluminosilicates (denoted as MSA) exhibits three well resolved diffraction peaks with at 2 theta value of 0.82, 1.39 and 1.61, which can be indexed as the (100), (110) and (200) diffraction peaks. The ordered mesostructure can be seen clearly in TEM images. These results indicated that mesopores with high crystallinity have been obtained in the dual templates procedure. Interestingly, MSA after hydrothermal treatment in 100% water vapor at 800 °C for 2 h (denoted as A-MSA) still exhibits a well-resolved (100) diffraction peak, indicating the high hydrothermal stability. These results revealed that PS/P123 dual templates method is a good strategy for synthesizing mesopores with high hydrothermal stability.
Fig. 2(1) gives the nitrogen adsorption–desorption isotherms of MSA and A-MSA samples. The isotherm of the MSA shows the representative characteristics of type IV adsorption isotherm with a sharp inflection at relative pressure 0.7 < P/P0 < 0.85 and a well defined hysteresis loop, pointing out the existence of mesopores. Moreover, the isotherm of the MSA rises sharply at relative pressure P/P0 < 0.05, indicating a characteristic of micropores. MSA reflects some mesopores with a diameter of 7.4 nm (resulted from P123 template), 3.8 nm (resulted from the agglomeration of particles), and 35.7 nm (resulted from PS microspheres). In addition, the micropores are characterized by Smic in the materials although they can not be detected in pore size distribution curve (Fig. 2(2)). These results indicated the existence of macro-meso-micropores, which are fully consistent with those of TEM studies.
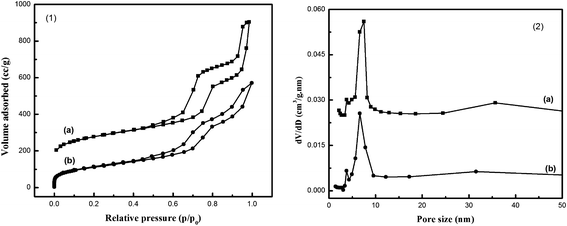 | ||
| Fig. 2 (1) N2 adsorption–desorption isotherms and (2) pore size distribution curves of (a) MSA, and (b) A-MSA. | ||
Interestingly, MSA after hydrothermal treatment in 100% water vapor at 800 °C for 2 h shows a similar feature with that of MSA. However, 3.8–6.6–32 nm were observed in pore size distribution curve (Fig. 2(2)). From Table 1, it can be seen that high surface area and pore volume (58% and 71%) were retained for A-MSA, indicating that the hierarchical materials have high hydrothermal stability. For comparison, B-MAS-4 obtained in Tan's investigation retained 23.5% surface area and 47.2% pore volume after the same hydrothermal treatment.8
| Samples | SBET (m2 g−1) | SMIC (m2 g−1) | SMES (m2 g−1) | VBJH (cc g−1) | VMIC (cc g−1) | VMES (cc g−1) |
|---|---|---|---|---|---|---|
| a MSA: mesoporous stable aluminosilicates. A-MSA: MSA after hydrothermal treatment in 100% water vapor at 800 °C for 2 h. | ||||||
| MSA | 638 | 102 | 536 | 1.243 | 0.038 | 1.105 |
| A-MSA | 373 | 46 | 327 | 0.885 | 0.025 | 0.860 |
From Fig. S1a† it can be seen that PS microspheres with a diameter of about 50 nm were prepared. From Fig. S1b,† it can be seen that macropores (the macropores were marked by the red lines in Fig. S1†) with a diameter of about 50 nm were present, and the mesoporous shell of the macropores is not hexagonal arrays, but wormhole-like. It suggests that the introduction of PS microspheres may result this deformation of mesopores. In this synthesis strategy, PS microspheres were introduced into Y precursors and acted as the core of systems.19,20 Therefore, the negatively charged PS will have a strong electrostatic interaction with the positively charged P123 micelles. As a direct result of these, P123 micelles would be attached on the surface of PS microspheres in disorder and in all directions. Therefore, the distorted P123 micelles rather than hexagonal arrays were fixed soon on the surface of PS. Finally, the worm-like mesopores rather than the parallel hexagonal mesopores are present. Interestingly, the worm-like mesopores are vertical to the surface of PS microspheres, which achieve the full interconnection of macro-meso-micropores. The vertical pores would have better the mass transfer of reactants and products than the hexagonal pores paralleling with the surface of PS.
Interestingly, MSA after hydrothermal treatment in 100% water vapor at 800 °C for 2 h still has the similar worm-like mesopores and macropores. These results combined with those of BET reveal the high hydrothermal stability of the resulting materials.
The FT-IR spectra of MSA sample are shown in Fig. S2.† The band at 572 cm−1 which is attributed to the double six-member rings indicated that precursors containing zeolite Y primary and secondary building units have been incorporated into the walls of MSA.21–24
Solid state 27Al-NMR shows a single sharp signal at 50 ppm (Fig. S3†), indicating that tetrahedrally coordinated Al species have been introduced into the walls of mesophases. This is desired because only tetrahedrally coordinated Al can be ion exchanged and contributed to the Brønsted acidic centers.
The main characteristics of MSA are the present of ordered macropores and interconnected hierarchical pores. These advantages are supposed to have superior catalytic cracking properties for large molecules, such as heavy oil compared with kaolin matrix. To study the catalytic properties of MSA, two catalysts including USY and MSA are prepared and evaluated in a microreactor with Daqing heavy oil as feed stocks. From Table 2 it can be seen that Cat-2 including MSA has a heavy cycle oil yield of 14.3%, much lower than that (16.5%) of Cat-1. This is because the presence of ordered macropores can precracking the heavy oil more effectively than kaolin matrix. Interestingly, the yields of light oil fraction of the Cat-1 and Cat-2 are 41.2% and 47.5%, respectively. Moreover, that coke yields for Cat-1 and Cat-2 are 11.3% and 9.7%, respectively. For comparison, the catalytic properties of commercial DO-75 are also studied in the same unit. It can be seen that the catalytic properties of Cat-1 and Cat-2 are better than those of DO-75. Moreover, the authors of the present investigation have prepared mesoporous aluminosilicates (denoted as LFs) by combination of precursors assembly and pH-adjusting method and the yield of light fraction oil of LF-3 is 67.19%.9 It is generally accepted that the activity of catalysts also depends on the acidity. In Cat-2, 5% MSA replaces 5% kaolin in Cat-1. However, the yield of dry gas of Cat-2 is lower than that of Cat-1 despite the fact that the acidity of MSA is stronger than that of kaolin. Therefore, it is reasonably deduced that pore structure in stead of acidity predominate in the cracking of heavy oil molecules. These can be ascribed to the fact that the interconnected macro-meso-micropores provide better diffusion of reactants and products.
| Catalyst samples | Dry gas (wt%) | Liquefied gas (wt%) | Light fraction oil (wt%) | Heavy cycle oil (wt%) | Coke (wt%) |
|---|---|---|---|---|---|
| a Cat-1: prepared from kaolin (65%), alumina gel (10%), and USY (25%). Cat-1: prepared from kaolin (60%), alumina gel (10%), MSA (5%), and USY (25%). DO-75: commercial catalyst from Lanzhou Petrochemical Company. | |||||
| Cat-1 | 2.7 | 28.3 | 41.2 | 16.5 | 11.3 |
| Cat-2 | 2.2 | 26.3 | 47.5 | 14.3 | 9.7 |
| DO-75 | 3.0 | 25.4 | 39.1 | 17.0 | 15.5 |
Conclusions
The hierarchical materials with uniform macro-meso-micropores were obtained by combination of precursors assembly and PS/P123 dual templates. It was suggested that the high hydrothermal stability was inherited from precursors assembly, and the macro-meso-micropores are obtained from PS/P123 dual templates. The worm-like mesopores vertical to the surface of PS microspheres achieve the full interconnection of triple pores. This change in the pore systems is benefit for the mass transfer of reactants and products than the hexagonal pores paralleling with the surface of PS. When used in heavy oil catalytic cracking, the catalyst including MSA showed excellent catalytic cracking properties.Acknowledgements
The authors acknowledge the financial supports from the Natural Science Foundation of China (Grant no. 20606003), Petrochina Limited Company (Grant nos. 11-02-01-1 and 2012A-2102-01), and PetroChina Innovation Foundation (Grant no. 2013D-5006-0403).Notes and references
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS PubMed.
- D. Wang, Z. Liu, H. Wang, Z. Xie and Y. Tang, Microporous Mesoporous Mater., 2010, 132, 428 CrossRef CAS PubMed.
- S. Guldin, M. Kolle, M. Stefik, R. Langford, D. Eder, U. Wiesner and U. Steiner, Adv. Mater., 2011, 23, 3664 CrossRef CAS PubMed.
- K. Na, M. Choi and R. Ryoo, Microporous Mesoporous Mater., 2013, 166, 3 CrossRef CAS PubMed.
- H. Liu, X. Bao, W. Wei and G. Shi, Microporous Mesoporous Mater., 2003, 66, 117 CrossRef CAS PubMed.
- Y. Wan and D. Zhao, Chem. Rev., 2007, 107, 2821 CrossRef CAS PubMed.
- Q. Tan, X. Bao, T. Song, Y. Fan, G. Shi, B. Shen, C. Liu and X. Gao, J. Catal., 2007, 251, 69 CrossRef CAS PubMed.
- Q. Tan, Y. Fan, H. Liu, T. Song, G. Shi, B. Shen and X. Bao, AIChE J., 2008, 54, 1850 CrossRef CAS.
- H. Liu, L. Wang, W. Feng, L. Cao, X. Gao, H. Liu and C. Xu, Ind. Eng. Chem. Res., 2013, 52, 3618 CAS.
- H. Liu, J. Wang, W. Feng and C. Xu, J. Alloys Compd., 2013, 557, 223 CrossRef CAS PubMed.
- H. Liu, X. Zhao, P. Chen, X. Gao, H. Liu, L. Cao, C. Xu and Q. Tan, J. Porous Mater., 2014 DOI:10.1007/s10934-014-9783-x.
- J. Jin, L. Cao, Q. Hu, C. Xu, X. Gao, W. Feng, H. Liu and H. Liu, J. Mater. Chem. A., 2014, 2, 7853 CAS.
- H. Liu, K. Guo, X. Li, S. Liu, X. Gao, H. Liu, L. Cao, Y. Chen and C. Xu, Microporous Mesoporous Mater., 2014, 188, 108 CrossRef CAS PubMed.
- Z. Deng, M. Chen, S. Zhou, B. You and L. Wu, Langmuir, 2006, 22, 6403 CrossRef CAS PubMed.
- J. Yuan, D. Wan and Z. Yang, J. Phys. Chem. C, 2008, 112, 17156 CAS.
- H. Wan, Y. Long, H. Xu, K. Song, G. Yang and C. H. Tung, Langmuir, 2014, 30, 683 CrossRef CAS PubMed.
- T. Umegaki, A. Seki, Q. Xu and Y. Kojima, J. Alloys Compd., 2014, 588, 615 CrossRef CAS PubMed.
- W. Jiang, T. Li, F. Qu, L. Ding, Z. Shen and M. Yang, Sens. Actuators, B, 2013, 185, 658 CrossRef CAS PubMed.
- W. Song, H. Liu, K. Wang, J. Cao, C. Xu and Z. Zhang, Chin. J. Inorg. Mater., 2010, 15(2), 163 CrossRef.
- L. Jia, X. Sun, X. Ye, C. Zou, H. Gu, Y. Huang, G. Niu and D. Zhao, Microporous Mesoporous Mater., 2013, 176, 16 CrossRef CAS PubMed.
- S. Kotrel, J. Lunsford and H. Kno1zinger, J. Phys. Chem. B, 2001, 105, 3917 CrossRef CAS.
- X. Wang, X. Zhang, Y. Wang, H. Liu, J. Qiu, J. Wang, W. Han and K. Yeung, Chem. Mater., 2011, 23, 4469 CrossRef CAS.
- Y. Zhang, Y. Liu and Y. Li, Appl. Catal., A, 2008, 345, 73 CrossRef CAS PubMed.
- P. Prokešová, S. Mintova, J. Čejka and T. Bein, Microporous Mesoporous Mater., 2003, 64, 165 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra03752a |
| This journal is © The Royal Society of Chemistry 2014 |