Chunfeng Wang, Guowei Zhou, Delan Xu, Bin Sun, Yan Zhang and Fengjiao Chen
RSC Adv., 2014,4, 37270-37273
DOI:
10.1039/C4RA04294H,
Communication
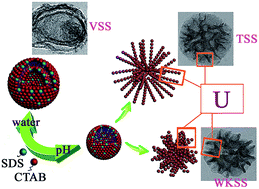
The synthesis of vesicular, walnut-kernel-, and tremella-like silica spheres (VSS, WKSS, and TSS) composed of hierarchical or U-shaped mesoporous structures by a pH-based approach was proposed. Spherical bodies with textural mesopores having sizes ranging from 30 to 40 nm were formed as a result of the diameter of the U-shaped silica skeleton.