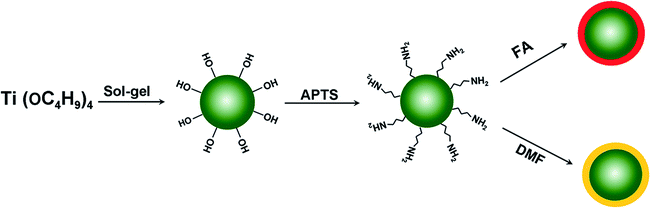
Formamide-modified titanium oxide nanoparticles with high electrorheological activity†
Jinghua Wu,
Ting Jin,
Fenghua Liu,
Jianjun Guo,
Yuchuan Cheng* and
Gaojie Xu*
Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo, 315201, P.R.China. E-mail: yccheng@nimte.ac.cn; xugj@nimte.ac.cn; Fax: +86-574-86685163; Tel: +86-574-86685162
First published on 23rd June 2014
Abstract
TiOx-based nanospheres modified by formamide (FA) as dielectric particles for electrorheological (ER) fluids were successfully synthesized through simple sol–gel hydrolysis and self-assembly. The suspension containing TiOx–FA displays superior ER activity, with a yield stress of 148 kPa (at 5 kV mm−1) under a DC electric field, which is 10 times that of ER fluids containing pure TiOx nanoparticles. More importantly, comparison between the FA and N,N-dimethylformamide (DMF) as the shell structure indicated that the ER performance was positively correlated with the dielectric constant of the polar molecule shell. The result represents a critical step towards an in depth understanding the enhancement effect of polar molecules. This study can afford a new strategy to achieve optimal performance in ER fluids.
Introduction
Electrorheological (ER) fluids are colloidal suspensions of highly polarizable particles in an insulating liquid medium that can switch from a liquid-like material to a solid-like material under the application of an electric field.1–4 This rheological change is reversible and can be achieved within several milliseconds. Coupled with sensors to trigger the electric field, the ER fluids can turn many devices, such as clutches, valves and dampers, into active mechanical elements capable of responding to environmental variations.5 Over the last decade, ER fluids have received persistent attention from academia and industry due to their favorable properties and potential application in electric-mechanical interfaces.6–15 However, their insufficient shear stress prevents the engineering application of ER fluids,16 highlighting the need for more practical materials.The discovery of giant ER (GER) fluids effect in some types of ER fluids, revealed that polar molecules or polar groups with a high dipole moment play a crucial role in ERF exhibiting a high yield stress.17,18 Unfortunately, ER fluids modified with some polar molecules or polar groups did not deliver high ER performance, implying that the molecular dipole moment is not the only key factor.19 There may be other factors contributing to the high ER activity than the dipole moment. In almost all of the ER fluids, the presence of water can effectively strengthen the ER activity. Orellana et al. reported that the ER activity is rather sensitive to the moisture content of the particles, which could be used to control the strength of the response of the ER fluid. Additionally, previous results have shown that water molecules on the surface of the particles can take on the role of the permanent dipoles used in GER fluids.20 In addition, Wen et al. also demonstrated that the presence of water molecules could effectively enhance the mechanical ER response by analyzing the frequency dependence of ER performance and dielectric properties with different water contents.21 Despite the superior ER activity, the water has underperformed as a practical dipole molecule due to its volatility and corrosivity to the ER device. However, the properties of water provide us with a useful inspiration: as a polar molecule, water possesses a rather high dielectric constant. It is possible that the synergy of the dielectric constant and molecular dipole moment promotes the ER performance. Therefore, the choice of polar molecules with high dielectric constant may be a prerequisite for obtaining ER materials with high ER activity.
Herein, to verify this concept, we evaluated the effect of polar molecules with different dielectric constants. Formamide (FA) was selected to modify the TiOx due to its high dielectric constant. N,N-Dimethylformamide (DMF) has a similar dipole moment and a markedly lower dielectric constant and was thus selected as a contrast. The results demonstrated that FA could enhance the ER activity more effectively than DMF could. Hence, the feasibility of the idea of employing polar molecules with a high dielectric constant to enhance ER performance was demonstrated.
Experimental
Materials
Tetrabutyl titanate (TBT, >98%), N,N-dimethylformamide (DMF, AR), formamide (FA, AR), toluene and acetic acid were purchased from Sinopharm Chemical Reagent Company (Shanghai, China). 3-Aminopropyl trimethoxysilane (APTS, >97%) was purchased from Aldrich. All of the reagents were of analytical grade and used without further purification. Silicone oils (η = 50 mPa s, 25 °C) were obtained from Hangping Company (Beijing China). Millipore-Q water (18.2 MΩ cm) was used in all cases.Preparation of TiOx particles and surface-modified TiOx particles
TiOx nanoparticles were synthesized via the simple hydrolysis process of TBT. First, 20 ml of TBT was dissolved in anhydrous ethanol at a volume ratio of TBT–ethanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, in which a small amount of acetic acid was added to prevent precipitation. Next, the diluted TBT solution was slowly added by dropping to a mixture of 100 ml of ethanol and 100 ml of de-ionized water had been loaded. An opalescent sol was produced instantly, and the mixture was continually stirred for 8 h and then aged for 10 h to ensure complete hydrolysis. The white precipitate was obtained by centrifugation, followed by washing with water and ethanol three times and then dried at 60 °C for 12 h and 120 °C for 4 h.
5, in which a small amount of acetic acid was added to prevent precipitation. Next, the diluted TBT solution was slowly added by dropping to a mixture of 100 ml of ethanol and 100 ml of de-ionized water had been loaded. An opalescent sol was produced instantly, and the mixture was continually stirred for 8 h and then aged for 10 h to ensure complete hydrolysis. The white precipitate was obtained by centrifugation, followed by washing with water and ethanol three times and then dried at 60 °C for 12 h and 120 °C for 4 h.
The TiOx nanoparticles were dispersed in a dilute solution of APTS in toluene (5 vol%) and stirred for 4 h at 25 ± 5 °C. The amino-coated TiOx nanoparticles were centrifuged and washed with ethanol. The precipitates were then dried in a vacuum at 60 °C for 12 h. Half of these amino-coated TiOx nanoparticles were dispersed in a dilute solution of FA in ethanol (5 vol%). After stirring for 4 h, the suspensions were centrifuged and washed with ethanol to obtain the FA-modified TiOx (TiOx–FA). The remaining amino-coated TiOx particles were dispersed in a dilute solution of N,N-dimethylformamide in ethanol. After stirring for 4 h, the suspensions were centrifuged and washed to obtain the N,N-dimethylformamide-modified TiOx (TiOx–DMF). The schematic shown in Fig. 1 illustrates our concept. The traditional sol–gel method was introduced to prepare TiOx particles. Next, the groups on the surface of the uniform TiOx nanospheres were charged by coating with homogenous APTS. This step is advantageous because it enhances the weight fraction of the FA or DMF on the surface of the TiOx particles due to the affinity of amino groups to the FA and DMF.
Preparation of ER fluids
Silicone oils were dried at 120 °C for 2 h before the experiment to prevent the influence of moisture. The density and dielectric constant (20 °C) of the silicone oil are 0.940 g cm−3 and 2.5, respectively. The ER suspension was prepared by dispersing the particles in silicone oil by grinding. The concentration of the ER fluids is denoted as the ratio of the nanoparticle weight to the total weight of the ER fluid.Characterization
The morphology of the samples was examined by a Hitachi S4800 field emission scanning electron microscope (FESEM). Transmission electron microscopy (TEM) characterizations were performed on a FEI Tecnai G2 F20 transmission electron microscope at an accelerating voltage of 200 kV. X-ray diffraction (XRD) patterns were obtained with a Bruker D8 Advance/Discover diffractometer using Cu Kα radiation. All of the measurements were obtained using a generator voltage of 40 kV and a current of 40 mA. The Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 6700 spectrometer using 32 averaged scans at a 4 cm−1 resolution. The solid samples were prepared as KBr pellets. The dielectric spectra of the ER fluids were measured using a Novocontrol Alpha-A analyzer within 10–106 Hz.The rheological properties were measured by a circular-type rheometer (Haake RS6000) with a circular-plate system (15 mm in diameter and a gap width of 1.0 mm). To obtain the dynamic yield stress, a stress–strain measurement was carried out at a very low shear rate, and the stress value at which the viscosity decreases abruptly is defined as the dynamic yield stress. The flow curves of the shear stress–shear rate were measured under controlled shear rate (CR) mode within a shear rate range of 0.1 to 100 s−1. Each shear rate was maintained for 10 s to obtain an equilibrium structure before collecting data. The maximum voltage of the DC high-voltage generator was 8 kV (SL 300 Spellman, USA). Experimental data were collected with the help of the software package Rheowin. All of the measurements were carried out at room temperature.
Results and discussion
Materials characteristics
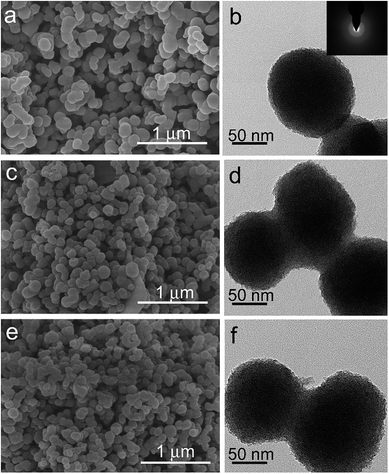
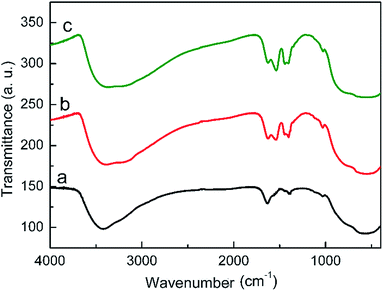
The morphology of the naked TiOx and modified TiOx samples was examined by SEM and TEM. Fig. 2a shows a typical SEM image of the naked TiOx particles. It can be seen that the as-prepared TiOx particles are approximately spherical with an average diameter of 150 ± 50 nm. The low-magnification SEM images (Fig. 2c and e) reveal that the TiOx–FA and TiOx–DMF particles still maintain a sphere-like appearance, similar to the raw TiOx particles. The results illustrate that the coating with polar molecules did not change the morphology of the TiOx particles. The TEM images (Fig. 2b, d and f) further validated the size-scale and shape of the three types of TiOx particles. Because the thickness of the layer of organic molecules on the surface of TiOx is on the molecule level, the core/shell structure is difficult to distinguish in the TEM images. The absence of crystalline diffraction peaks in the corresponding small-angle electron diffraction (SAED) pattern (inset of Fig. 2b) indicates that the samples are amorphous, which is consistent with the XRD patterns (Fig. S1, ESI†). The amorphous TiOx-based material was selected as the dispersed phase for the ER fluids due to its higher electrorheological activity relative to crystal titania, as there are abundant polar molecules or polar groups on the surface and it is easily modified by other polar groups.Fig. 3 shows the FTIR spectra of the as-prepared and modified TiOx samples. The broad band at approximately 3400 cm−1 is assigned to the stretching vibration mode of the structure (or surface) hydroxyl groups bonding to titanium atoms. For the TiOx particles, the band between 500 and 800 cm−1 corresponds to the Ti–O stretching vibration mode. Compared to the spectrum of naked TiOx, some new bands are found in the spectra of modified TiOx (curve b and c). The bands at 1420 and 1470 cm−1 can be attributed to the alkyl group distortion vibration. The bands at 1530 and 1216 cm−1 correspond to the amide II and amide III mode, respectively.
The band at 1620 cm−1 can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode. The emerging of these bands proves that the TiOx particles are indeed modified by the FA and DMF molecules. These polar molecules play a key role in the improvement of the ER effect. Being a strong hydrogen bond donor, the hydroxyl group can participate in inter-polymer hydrogen bonding interactions,22,23 which link the ER particles together effectively and create high yield stress without greatly enhancing the zero-field viscosity. Furthermore, the carbonyl group and amide group on the surface of the hybrid particles would promote surface activity and enhance the polarization.24,25
O stretching mode. The emerging of these bands proves that the TiOx particles are indeed modified by the FA and DMF molecules. These polar molecules play a key role in the improvement of the ER effect. Being a strong hydrogen bond donor, the hydroxyl group can participate in inter-polymer hydrogen bonding interactions,22,23 which link the ER particles together effectively and create high yield stress without greatly enhancing the zero-field viscosity. Furthermore, the carbonyl group and amide group on the surface of the hybrid particles would promote surface activity and enhance the polarization.24,25
Rheological properties of ER suspensions
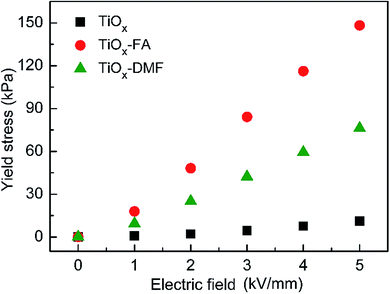
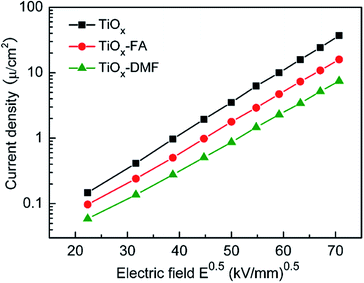
The static yield stresses of three TiOx ER fluids under different DC electric fields are shown in Fig. 4. It can be seen that the yield stress of the naked TiOx ER fluid is only 11 kPa at 5 kV mm−1. In contrast, the ER fluids containing core/shell structural TiOx nanoparticles display notable ER activity. The results indicate that both FA and DMF coating can enhance the ER performance of TiOx materials markedly. According to the polar-molecule-dominated model, polar molecules with large dipole moments are beneficial for obtaining high yield stress. Moreover, compared with the TiOx–DMF ER fluid, the TiOx–FA ER fluid possesses significantly higher yield stress. Although the molecular dipole moments of the FA and DMF were almost identical, the dielectric constant of FA is much larger than that of DMF (the dipole moment and dielectric constant of water, FA and DMF are shown in Table S1, ESI†). This shows that, in addition to the dipole moment, the dielectric properties of polar molecules have important effects on the ER activity. Under electric fields, the yield stress of TiOx–FA ER fluid can reach up to 148 kPa, and the dependence of the yield stress on the electric field presents linear behavior as the electric field exceeds a critical field Ec ≈ 1 kV mm−1. This result indicates that a saturated surface polarization layer is established, which is the distinct feature of GER fluids.26 In general, the static yield stress is proportional to the electric energy density PE, where E is the local electric field in the contact region between two adjacent particles. P refers to the polarizability, which is presumed in the saturation polarization to be a constant independent of E.27 In addition, the yield stress of the TiOx–FA ER fluid is also higher than that of the acetamide-modified TiOx in our previous study. Although the FA, DMF and acetamide possess similar structures and high dipole moments, the highest dielectric constant of the FA provides it with the highest yield stress. This further validates the importance of the dielectric constant in strengthening the ER activity.Leaking current density, leading to energy consumption, is another evaluation criterion for the application of ER fluid. Fig. 5 shows the current density as a function of applied electric field for three types of TiOx-based ER fluid. It can be seen that the leaking current densities of the TiOx–FA ER fluids and TiOx–DMF ER fluids were lower than that of the naked TiOx ER fluids. This can be attributed to the electrical insulating effect of the thin layer of organic molecules. Furthermore, a prominent feature is the near-linear dependence of the logarithm of the current density on E1/2, indicating the mechanism of activation over the Coulomb barrier.28
The relationship between the shear stress and the shear rate was also investigated. Fig. 6 shows the steady shear stress–shear rate curve under various electric fields for the TiOx–FA and TiOx–DMF ER fluids. In the absence of an electric field, both suspensions behave as Newtonian fluids, with shear stress increasing linearly with shear rate. When the electric field is applied, the suspensions exhibit strong increases in shear stress and act as plastic materials presenting yield stresses. It is known that the rheological behavior of an ER suspension under an electric field is the result of the change in the fibrous-like structures. The structural change is mainly dominated by the competition between the electric-field-induced electrostatic interaction and the shear-field-induced hydrodynamic force. The electrostatic interactions were responsible for the reorganization of ER structures and hinder the flow, while the hydrodynamic interactions tended to destroy ER structures and promote the flow.29 The large polarizability and sufficient polarization response of ER particles are important for producing strong and fast electrostatic interactions that can keep the structures and rheological properties stable under shear flow.30 When the strength of the electric field was enhanced, the shear stress of both TiOx–FA and TiOx–DMF suspensions increased due to an enhanced interaction force between particles. Relative to the TiOx–DMF-based ER fluids, the TiOx–FA-based ER fluid exhibited a higher shear stress at the same electric field strength, indicating that the electrostatic force between the TiOx–FA particles is larger than the interaction force between the TiOx–DMF particles.
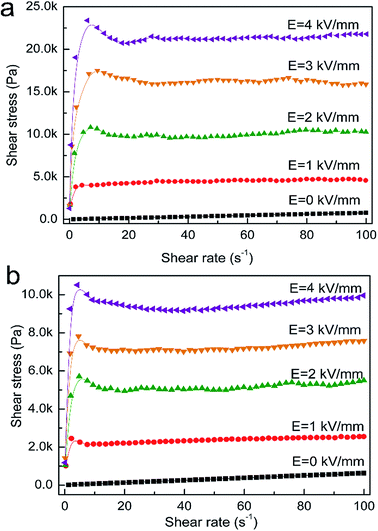 | ||
| Fig. 6 Shear stress of TiOx–FA (a) and TiOx–DMF (b) ER fluids (46 wt%) as a function of shear rate under various electric fields. | ||
It is known that electric-field-induced polarization in the contact region of neighboring particles causes their aggregation into fibers aligned along the electric field under an applied electric field. The high and stable shear stress over a wide shear rate region for the TiOx–FA suspension indicates that its polarizability is high and that the polarization response is proper. Thus, the TiOx–FA ER fluid can be greatly solidified by the electric field, and the particles aggregate into chains and columns fast enough to keep up with the destruction rate of the fibrous structure, maintaining stable rheological properties in the shear rate range of 1 to 100 s−1. A decrease in the shear stress is observed at low shear rates, and the flow behavior of the suspensions departs from Bingham fluid behavior. However, the Cho-Choi-Jhon (CCJ) model, a constitutive equation proposed for this special type of ER fluid, shows advantages in the analysis of a special type of ER phenomenon.31,32 This phenomenon indicated that as the shear rate increases, the destruction rate of the fibrils exceeds the reformation rate. Because of the higher volume fraction of GER suspensions and/or the high shear rate, the suspensions were actually expelled from the central region of the parallel-plate electrode, leading to a net decrease in the measured shear stress.
Dynamic tests using an oscillatory shear stress were performed to study the viscoelastic properties of the solidified ER fluid under an external electric field. The results of the stress sweep, which is sinusoidal in amplitude at a constant frequency, for the TiOx–FA and TiOx–DMF materials are shown in Fig. 7. It can be seen that both the storage modulus (G′) and the loss modulus (G′′) increase and that the linear viscoelastic region becomes wider as the electric field strength increases. The ER fluids exhibit solid-like behavior: G′ is substantially larger than G′′, and G′ remains nearly unchanged over a broad stress range. The plateau of the stress-dependent curves of the storage modulus is a characteristic of chain-like structures that are not destroyed under electric fields, which demonstrates that the elasticity is dominant in the linear viscoelastic region. When the applied stress exceeds a certain value, the storage modulus decreases sharply, revealing that liquid behavior is becoming dominant and the chain structure has been destroyed. The storage modulus and the loss modulus of the TiOx–FA suspension are larger than those of the TiOx–DMF suspension under the same electric field, reflecting its greater solidification (rigidity). This finding indicates that the stronger force between the TiOx–FA particles makes the structure more stable to shear stress, which is accordance with its higher yield stress.
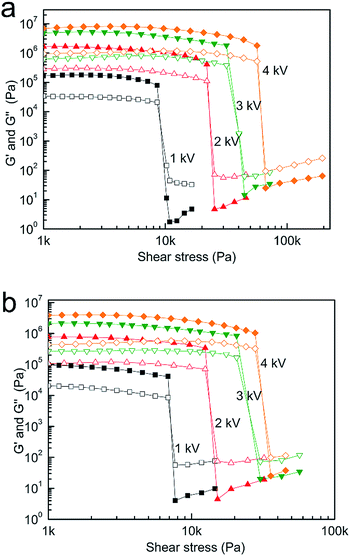 | ||
| Fig. 7 Stress dependence of storage modulus (G′, solid symbol) and loss modulus (G′′, open symbol) for the TiOx–FA (a) and TiOx–DMF (b) suspensions (56 wt%). | ||
Dielectric properties and ER structures
The interfacial polarization mechanism treats particle polarization using the complex dielectric constant ε = ε′ − iε′′, where ε′ (dielectric constant) is related to the polarizability and ε′′ (dielectric loss factor) is related to the polarization time and the stability of the interaction between particles.33 According to the polarization mechanism proposed by Block et al., the high Δε′ and the proper ε′′ peak in the frequency range of 102 to 105 Hz induce a strong interaction between the particles of ER fluids and maintain the stable chain structure formed by the particles under the applied electric and shear fields.Dielectric analysis of the pure-TiOx- and modified-TiOx-based ER fluids was conducted, and the dielectric spectra are shown in Fig. 8. In can be seen that the Δε′ values of pure TiOx ER fluids are very small, and no clear dielectric relaxation peak is observed between 100 Hz and 100 kHz. It generally believed that fast ionic or atomic polarization dominant in pure titanium oxide cannot supply optimal dielectric or polarization properties for high ER activity.34,35 After coating the surface of the TiOx particles with FA and DMF, the ε′ of the TiOx–FA and TiOx–DMF ER fluids exceeded that of the naked TiOx ER fluids, and a clear dielectric relaxation peak is observed. These findings clearly show the contributions of the FA and DMF shells. It can be concluded that the incorporation of polar molecules or polar groups on the surface of TiOx particles evidently enhanced the interfacial polarization. As a result, the larger Δε′ and proper polarization response induce stronger interactions between particles for high ER activity. Moreover, the presence of the dielectric loss peak is attributed to the orientation and the interaction of the polar molecules.36 Generally, the dipole is more difficult to reorient in the interior of solid particles than on the surface. The polar and dangling bonds on the particle surfaces and interfaces are less bounded by the interior lattice. In addition, the local electric field in the gap between the particles is much higher than the applied electric field. Thus, the orientation of the skin-deep polar molecules is more likely. The interaction between polar molecules begins at the substrate, with magnitude scaling within the interfacial area. Therefore, the interaction between the particles can be considered to be an interfacial phenomenon.37 Compared with other core/shell-structure ER materials, a thin layer of polar molecules could improve the dielectric properties effectively.38 Similarly, theoretical calculations have also shown that in polar-molecule-dominated ER fluids, particles with thinner shells yield larger yield stresses.39
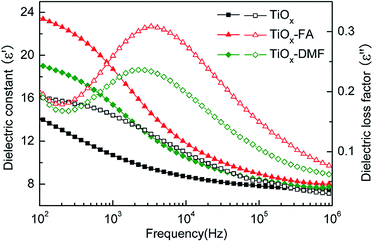 | ||
| Fig. 8 Dielectric spectra of different samples: dielectric constant (solid symbols) and dielectric loss factor (open symbols) (46 wt%). | ||
Conclusions
FA- and DMF-modified TiOx particles were successfully prepared via self-assembly. The TiOx–FA ER fluid delivered a very high yield stress (over 148 kPa at 5 kV mm−1) twice that of the TiOx–DMF ER fluid. The dielectric measurements showed that the TiOx–FA ER fluid features the larger dielectric constant enhancement Δε′ and a clear dielectric loss peak. The results demonstrate that the FA on the surface of the TiOx particles strengthened the polarizability and sequentially affected their ER activity. Our results not only constitute significant progress in the development of TiOx-based materials but also present a promising and flexible direction for the design and synthesis of more highly active ER materials.Acknowledgements
This research is supported by the National Natural Science Foundation of China (21003145, 21203225, 11374311), the Ningbo Natural Science Foundation (2013A610136, 2012A610128), the Ningbo Municipality (2009B21005), the Zhejiang Provincial National Science Foundation (LY14B070012), and the Major Project Technology Foundation of Zhejiang Province (2012C01034-3).Notes and references
- T. C. Halsey, Science, 1992, 258, 761–766 CAS.
- J. Yin, X. Zhao, L. Xiang, X. Xia and Z. Zhang, Soft Matter, 2009, 5, 4687–4697 RSC.
- H. J. Choi and M. S. Jhon, Soft Matter, 2009, 5, 1562–1567 RSC.
- K. P. S. Parmar, Y. Meheust, B. Schjelderupsen and J. O. Fossum, Langmuir, 2008, 24, 1814–1822 CrossRef CAS PubMed.
- W. Wen, X. Huang and P. Sheng, Soft Matter, 2008, 4, 200–210 RSC.
- H. J. Choi, M. S. Cho, K. K. Kang and W. S. Ahn, Microporous Mesoporous Mater., 2000, 39, 19–24 CrossRef CAS.
- J. Y. Hong, M. Choi, C. Kim and J. Jang, J. Colloid Interface Sci., 2010, 347, 177 CrossRef CAS PubMed.
- W. L. Zhang, Y. D. Liu and H. J. Choi, Carbon, 2012, 50, 290 CrossRef PubMed.
- J. B. Yin and X. P. Zhao, Chem. Mater., 2002, 14, 4633–4640 CrossRef CAS.
- B. Wang and X. Zhao, J. Mater. Chem., 2002, 12, 2869–2871 RSC.
- Y. G. Ko and U. S. Choi, Soft Matter, 2012, 8, 253–259 RSC.
- J.-Y. Hong and J. Jang, Soft Matter, 2012, 8, 7348–7350 RSC.
- Y. Tian, Y. G. Meng and S. Z. Wen, J. Appl. Phys., 2001, 90, 493–496 CrossRef CAS PubMed.
- C. Li, J. Y. Huang, Q. G. Tang, J. P. Huang, J. W. Zhang and L. W. Zhou, Soft Matter, 2012, 8, 5250–5255 RSC.
- C. S. Orellana, J. He and H. M. Jaeger, Soft Matter, 2011, 7, 8023–8029 RSC.
- F. F. Fang, J. H. Kim, H. J. Choi and Y. Seo, J. Appl. Polym. Sci., 2007, 105, 1853–1860 CrossRef CAS.
- W. J. Wen, X. X. Huang, S. H. Yang, K. Q. Lu and P. Sheng, Nat. Mater., 2003, 2, 727–730 CrossRef CAS PubMed.
- K. Q. Lu, R. Shen, X. Z. Wang, G. Sun, W. J. Wen and J. X. Liu, Chin. Phys., 2006, 15, 2476–2480 CrossRef CAS.
- K. Di, Y. Zhu, X. Yang and C. Li, Colloids Surf., A, 2006, 280, 71–75 CrossRef CAS PubMed.
- C. S. Orellana, J. He and H. M. Jaeger, Soft Matter, 2011, 7, 8023–8029 RSC.
- W. Wen, H. Ma, W. Y. Tam and P. Sheng, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1997, 55, R1294–R1297 CrossRef CAS.
- X. Gong, J. Wu, X. X. Huang, W. Wen and P. Sheng, Nanotechnology, 2008, 19, 165602 CrossRef PubMed.
- E. Y. Chu, E. M. Pearce, T. K. Kwei, T. F. Yeh and Y. Okamoto, Macromol. Rapid Commun., 1991, 12, 1–4 CrossRef CAS.
- Y. G. Ko, U. S. Choi and B. H. Sung, J. Appl. Polym. Sci., 2004, 93, 1559–1566 CrossRef CAS.
- J. B. Jun, S. Y. Uhm and K. D. Suh, Macromol. Chem. Phys., 2003, 204, 451–459 CrossRef CAS.
- P. Tan, J. P. Huang, D. K. Liu, W. J. Tian and Z. W. Zhou, Soft Matter, 2010, 6, 4800–4806 RSC.
- E. C. McIntyre, H. J. Oh and P. F. Green, ACS Appl. Mater. Interfaces, 2010, 2, 965–968 CAS.
- X. Huang, W. Wen, S. Yang and P. Sheng, Solid State Commun., 2006, 139, 581–588 CrossRef CAS PubMed.
- J. B. Yin, X. Xia, L. Xiang and X. Zhao, Carbon, 2010, 48, 2958–2967 CrossRef CAS PubMed.
- B. Wang, Y. Yin, C. Liu, S. Yu and K. Chen, Dalton Trans., 2013, 42, 10042–10055 RSC.
- S. G. Kim, J. Y. Lim, J. H. Sung, H. J. Choi and Y. Seo, Polymer, 2007, 48, 6622–6631 CrossRef CAS PubMed.
- Y. D. Liu, F. F. Fang and H. J. Choi, Langmuir, 2010, 26, 12849–12854 CrossRef CAS PubMed.
- B. X. Wang and X. P. Zhao, Adv. Funct. Mater., 2005, 15, 1815–1820 CrossRef CAS.
- J. P. Huang, J. Phys. Chem. B, 2005, 109, 4824–4828 CrossRef CAS PubMed.
- J. B. Yin and X. P. Zhao, Chem. Mater., 2004, 16, 321–328 CrossRef CAS.
- J. Wu, F. Liu, J. Guo, P. Cui, G. Xu and Y. Cheng, Colloids Surf., A, 2012, 410, 136–143 CrossRef CAS PubMed.
- J. Wu, G. Xu, Y. Cheng, F. Liu, J. Guo and P. J. Cui, J. Colloid Interface Sci., 2012, 378, 36–43 CrossRef CAS PubMed.
- P. Tan, W. J. Tian, X. F. Wu, J. Y. Huang, L. W. Zhou and J. P. Huang, J. Phys. Chem. B, 2009, 113, 9092–9097 CrossRef CAS PubMed.
- S. Chen, X. Huang, N. F. A. van der Vegt, W. Wen and P. Sheng, Phys. Rev. Lett., 2010, 105, 046001 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: XRD of the TiOx and modified TiOx, dielectric constant and dipole moment of the water, FA and DMF. See DOI: 10.1039/c4ra04469j |
| This journal is © The Royal Society of Chemistry 2014 |