Prussian blue nanoparticles for laser-induced photothermal therapy of tumors†
Hilary A. Hoffmana,
Lina Chakrabartiab,
Matthieu F. Dumonta,
Anthony D. Sandlerabcd and
Rohan Fernandes*ade
aThe Sheikh Zayed Institute for Pediatric Surgical Innovation, Children's National Medical Center, Washington, DC 20010, USA. E-mail: RFernand@childrensnational.org; Tel: +1-202-476-5290
bThe Joseph E. Robert Center for Surgical Care, Children's National Medical Center, Washington, DC 20010, USA
cDepartments of Surgery, George Washington University, Washington, DC 20037, USA
dPediatrics, George Washington University, Washington, DC 20037, USA
eRadiology, George Washington University, Washington, DC 20037, USA
First published on 26th June 2014
Abstract
We describe Prussian blue nanoparticles (PB NPs) for laser-induced photothermal therapy (PTT) of tumors. The PB NPs exhibit strong absorbance at near infrared (NIR) wavelengths, are stable, non-toxic, and have 20.5% photothermal conversion efficiencies. PTT with PB NPs in a mouse model of neuroblastoma resulted in marked tumor debulking, increased tumor-free days, and decreased tumor growth rates in tumor-bearing mice. These findings demonstrate the clinical potential of PB NPs for PTT of tumors.
Prussian blue is an ancient dye that was first serendipitously synthesized in the early 18th century. Prussian blue is comprised of colloidal nanoparticles that are made up of mixed valence iron hexacyanoferrate with the generalized formula AFeIII[FeII(CN)6]·yH2O (where y = 1–5 and A is a monovalent cation such as K+, Na+ or NH4+). Here, we describe the repurposing of Prussian blue nanoparticles (PB NPs) for laser-induced photothermal therapy (PTT) of tumors. The PTT capabilities of PB NPs are based on the fact that they exhibit defined absorbance at near infrared (NIR) wavelengths1–3 (650–900 nm; at which light exhibits maximum penetration of human tissue). Therefore, when PB NPs are injected into tumors and irradiated with a low-power NIR laser, the laser irradiation photothermally heats the nanoparticles ablating tumor cells leading to their destruction, and ultimately to tumor shrinkage. The photothermal heating effect is negligible in the absence of the nanoparticles (i.e. when using the low-power laser alone), thus having minimal bystander effect.
We demonstrate PB NPs for laser-induced PTT in a mouse model of neuroblastoma as proof of concept. Neuroblastoma is the most common type of extracranial solid tumor of childhood constituting roughly 7% of all newly diagnosed cases.4,5 About 60% of neuroblastoma patients present with advanced disease at diagnosis making it one of the most difficult tumors to treat.4,5 The various treatment options used to treat advanced neuroblastomas including surgery, chemotherapy, retinoid therapy, radiation therapy, high-dose radiation therapy/chemotherapy with stem cell transplant, and immunotherapy have made incremental but limited progress for this patient population.6,7 Therefore, a less invasive and more precise means for debulking advanced neuroblastomas would be a major step forward in the management of this disease.
Our rationale for pursuing PTT (nanoparticle-based, laser-induced tissue ablation by exposure to temperatures >50 °C for at least 4–6 minutes) in tumor therapy is based on extensive literature demonstrating the reduced heat tolerance of tumors in comparison to normal cells.8–13 In PTT, ablation causes rapid loosening of cell membranes, denaturing of proteins ultimately irreversibly damaging tumor cells.14,15 The use of a laser to induce nanoparticle-based ablation allows for confined damage to tumor tissue, while limiting damage to surrounding healthy tissue. The use of NIR light is advantageous on account of its extensive safety profile in disease diagnostics and therapy.16 Other benefits of PTT include decreased cost and morbidity, the possibility of performing the procedure in an outpatient setting, and the ability to treat patients who would not be considered candidates for surgery due to age, comorbidity, or extent of disease progression.17 Mild hyperthermia (exposure of tissues to 40–45 °C) has also been shown to enhance the efficacy of radiation therapy and chemotherapy suggesting the utility of PTT in combination therapies.18,19
Alternate types of nanoparticle-based photothermal agents that are being concurrently studied include gold nanorods14,20 and nanoshells,21–23 copper sulfides,24,25 carbon nanotubes,8,26 semiconductor nanocrystals,27 palladium nanosheets,28 bismuth selenide nanoplates,29 polypyrrole nanoparticles,30 and hybrid nano-vesicles encapsulating NIR dyes such as indocyanine green.31 While Prussian blue has been explored in conjunction with other materials for PTT in vivo such as Prussian blue coated gold nanoparticles,32 this is the first study to examine unmodified PB NPs as a PTT agent in vivo. When compared to other nanoparticles, our PB NPs offer two important advantages: (1) they are easily synthesized in a single step at low cost with monodisperse, controlled size distributions without the need for specialized and costly chemicals that are required for the syntheses of the other nanoparticles and (2) PB NPs are already FDA approved for human use as their safety and low toxicity profiles in vivo have been rigorously established in clinical trials.33,34 In this study, we harness additional desirable features of PB NPs useful in the PTT of neuroblastomas including: (1) small sizes (∼60–90 nm diameters) that enable the PB NPs to easily penetrate tumors and circulate within the tumor interstitium35,36 and (2) defined absorption at NIR wavelengths allowing greater depth of tissue penetration of the NIR laser beneath the skin triggering photothermal heating of irradiated nanoparticles.
A previous study by Fu et al., presented citric acid-capped PB NPs as photothermal agents.1 Another recent study by Li et al. examined PB NPs functionalized with glypican-3 antibody for MR imaging and PTT.3 Here, we extend the previous studies by: (1) developing a citric acid-free scheme for synthesizing stable, monodisperse PB NPs, (2) measuring the photothermal conversion efficiencies of our PB NPs, and (3) conducting the first in vivo demonstration of PB NPs for laser-induced PTT in a mouse tumor model. The in vivo PTT capabilities of the PB NPs are studied in a mouse flank tumor model of neuroblastoma,37,38 which is a hard-to-treat model of the disease. We synthesized PB NPs using a modification of a scheme described by us previously2,39 by combining 2.5 × 10−5 mol of FeCl2·4H2O in 5 mL of Milli-Q H2O with 2.8 × 10−5 mol of K3Fe(CN)6 in 5 mL of Milli-Q H2O with vigorous stirring at room temperature. The synthesis yielded a deep blue suspension consisting of monodisperse PB NPs with a mean hydrodynamic size ∼65 nm as measured by dynamic light scattering (Fig. S1A†). The size and morphology of individual PB NPs were confirmed by TEM (Fig. S1B†).
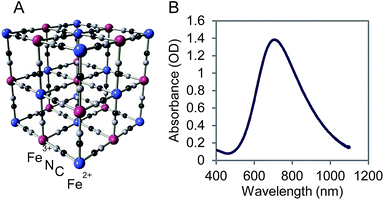
PB NPs have a lattice structure comprised of iron(III) hexacyanoferrate(II) (Fig. 1A) and the ability to bind metal ions for charge neutralization. On account of this metal binding ability, Prussian blue marketed as Radiogardase™, has been used by first responders in the event of a radioactive leak to sequester cesium and thallium ions, thereby reducing radioactive exposure in first responders.33,34 The visible-NIR spectrum of 1 mg mL−1 PB NPs showed an absorption band from 650–900 nm, corresponding to the energy of the metal-to-metal charge transfer between FeII and FeIII through the cyanide bridge (Fig. 1B). The first derivative of the absorbance spectrum yielded an accurate value of the position λmax for PB NPs of 705 nm.
We further characterized PB NPs using Fourier transform infrared (FTIR; Fig. S2A†) spectroscopy and powder X-ray diffractomety XRD; Fig. S2B†). The FTIR spectrum of PB NPs exhibited a broad band at 2070 cm−1 corresponding to the characteristic FeII–CN–FeIII cyanide stretch energy of Prussian blue (Fig. S2A†).2,39 XRD of PB NPs exhibited two peaks corresponding to the 200 and 220 diffraction planes at 17.51 and 24.68 degrees (Fig. S2B†). These diffraction peaks were indexed to the Prussian blue lattice using the space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 225) and confirmed the presence of one phase constituted by Prussian blue. Using the (220) reflections fitted to a Gaussian function, the lattice parameters were calculated. We found the following lattice constant for Prussian blue, a = 10.17 Å.2,39 The measured values of the molar extinction coefficient of our PB NPs of 1.88 × 109 M−1 cm−1 (based on measurements conducted for Fig. 1B) was of the same order of magnitude as that measured for gold nanorods (5.24 × 109 M−1 cm−1)1 and at least an order of magnitude higher than those measured for carbon nanotubes and copper selenide nanocrystals indicating the suitability of the PB NPs as photothermal agents.
m (no. 225) and confirmed the presence of one phase constituted by Prussian blue. Using the (220) reflections fitted to a Gaussian function, the lattice parameters were calculated. We found the following lattice constant for Prussian blue, a = 10.17 Å.2,39 The measured values of the molar extinction coefficient of our PB NPs of 1.88 × 109 M−1 cm−1 (based on measurements conducted for Fig. 1B) was of the same order of magnitude as that measured for gold nanorods (5.24 × 109 M−1 cm−1)1 and at least an order of magnitude higher than those measured for carbon nanotubes and copper selenide nanocrystals indicating the suitability of the PB NPs as photothermal agents.
To understand the performance of our PB NPs as photothermal agents, we measured their photothermal conversion efficiencies. This was achieved by irradiating a known concentration of PB NPs (1 mg mL−1) in a 96 well plate using an 808 nm laser at 1 W until the system temperature stabilized at its maximum temperature (ESI† for details). At this point, the laser was turned off and we recorded the temporal decrease in temperature to calculate the rate of heat transfer from the system. To calculate the photothermal conversion efficiency ηT of the PB NPs, we used eqn (1) derived in the model described previously by Roper et al.3,40
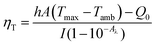 | (1) |
Using this method, we calculated (using Fig. S3 and S4†) the photothermal conversion efficiency (ηT) of the PB NPs to be 20.5% when irradiated with an 808 nm laser. Measuring the photothermal conversion efficiency is necessary as it allows us to fine tune the laser power, laser exposure time, and PB NP concentration to obtain optimal heating that is at a level just below the threshold where excessive heat starts to damage surrounding healthy cells.
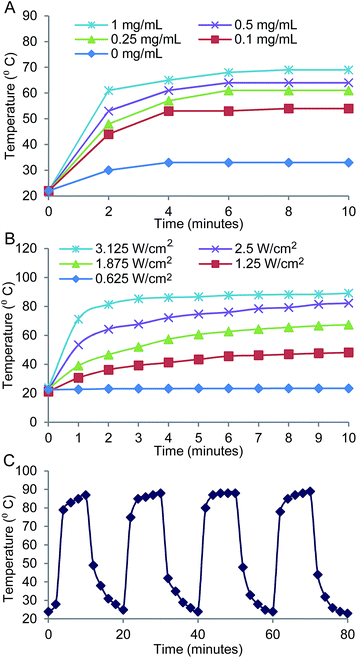
We investigated the photothermal heating characteristics of the PB NP-based PTT (Fig. 2). The photothermal heating effect was a function of PB NP concentration and was observed to increase with increasing PB NP concentrations (0–1 mg mL−1 PB NPs irradiated by an 808 nm NIR laser at 1.875 W cm−2 for 10 min; Fig. 2A). The photothermal heating effect was also dependent on the power of the incident laser and duration of the irradiation, and was observed to increase with increasing laser powers and durations (1 mg mL−1 PB NPs, 808 nm NIR laser at 0.625–3.125 W cm−2, 0–10 min; Fig. 2B). Furthermore, we demonstrated the stability of PB NPs as a PTT agent through a cyclic heating study. PB NPs showed consistent photothermal heating over four consecutive heating and cooling cycles indicating stability of the PB NPs as PTT agents (Fig. 2C).
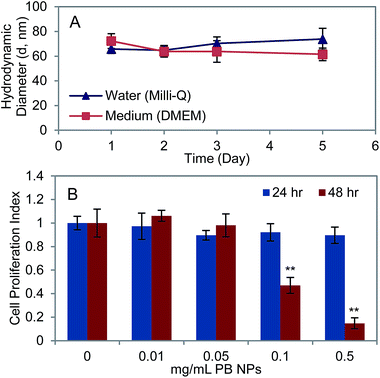
To determine the suitability of PB NPs as agents for PTT in vivo, we studied their stability and cytotoxicity in vitro. 1 mg mL−1 PB NPs were observed to be stable in both Milli-Q water and Dulbecco's Modified Eagle Media (DMEM) over a period of five days as measured by dynamic light scattering (Fig. 3A). The cytotoxicity of PB NPs on neuro2a cells, a mouse neuroblastoma cell line, was investigated by conducting an XTT assay to examine the viability of neuro2a after 24 and 48 hours of incubation with varying concentrations of PB NPs (0–0.5 mg mL−1 PB NPs co-incubated with 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 seeded cells of neuro2a; Fig. 3B). Since the PB NPs exhibited negligible absorbance at the XTT assay wavelength of 490 nm (Fig. 1B), we expected negligible optical artefacts introduced by the PB NPs in the XTT assay. Cells incubated for 24 hours with varying concentrations of PB NPs showed insignificant reduction in cell viability relative to the controls (no particles) at all concentrations of PB NPs.
000 seeded cells of neuro2a; Fig. 3B). Since the PB NPs exhibited negligible absorbance at the XTT assay wavelength of 490 nm (Fig. 1B), we expected negligible optical artefacts introduced by the PB NPs in the XTT assay. Cells incubated for 24 hours with varying concentrations of PB NPs showed insignificant reduction in cell viability relative to the controls (no particles) at all concentrations of PB NPs.
At 48 hours, PB NPs at concentrations ≥0.1 mg mL−1 exhibited cytotoxicity to neuro2a cells. These studies demonstrated the stability of our PB NPs and established the working concentrations of PB NPs for in vivo use. These results are in agreement with previous studies using PB NPs on a range of other cell lines including Eol-1 (eosinophilic), OE-21 (squamous epithelial), and BSG D10 (brainstem glioma) cell lines.2,39
We assessed the effect of PB NP-based, laser-induced PTT in mice with established flank, neuroblastoma tumors (i.e. tumor volumes ∼ 50 mm3). All mice studies were conducted in accordance with Children National's Institutional Animal Care and Use Committee approved protocols (Protocol# 198-12-06). To establish flank, neuroblastoma tumors, 6 wk-old female A/J mice were injected subcutaneously with 106 neuro2a cells. Established tumor-bearing mice were: (1) intratumorally injected with PB NPs (50 μL at 1 mg mL−1; well below the cytotoxic concentrations established in Fig. 3B) and irradiated with the NIR laser (10 min; 1.875 W cm−2); (2) irradiated with the NIR laser only without PB NPs (10 min; 1.875 W cm−2); or (3) left untreated. Since we used concentrations of PB NPs that were well below the established cytotoxic concentrations, we did not explicitly measure the effect of PB NPs by themselves on the tumor (so as to reduce the number of mice required for the study).
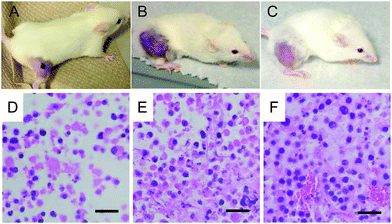
24 hours after treatment, tumors in mice that were intratumorally injected with PB NPs and irradiated with the NIR laser appeared necrotic (black mass; Fig. 4A). In contrast, the tumors in mice that were irradiated with the NIR laser (without PB NPs) or left untreated did not exhibit tumor necrosis (Fig. 4B and C). H&E stained tissue samples obtained from the tumors of the mice subject to the 3 different treatments described above demonstrated a marked decrease in the number of tumor cells (i.e. tumor debulking) in tumor-bearing mice that were intratumorally injected with PB NPs and irradiated with the NIR laser (Fig. 4D) relative to tumor-bearing mice that were irradiated with the NIR laser (without PB NPs; Fig. 4 E) or left untreated (Fig. 4F). The observed tumor debulking during PTT is consistent with an ablative mechanism of tumor debulking. These results indicate that both the PB NPs and the laser are simultaneously required for marked debulking of neuroblastomas.
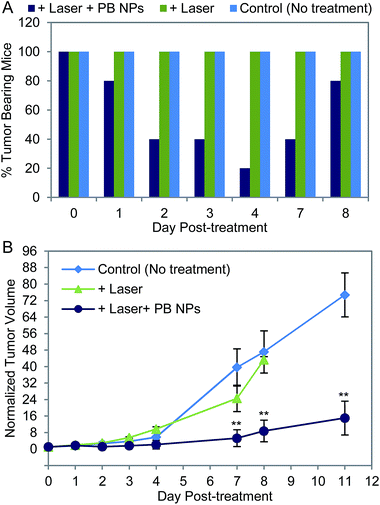
We evaluated the efficacy of the PB NP-based, laser-induced PTT in treating neuroblastomas. Tumor-bearing mice were: (1) intratumorally injected with PB NPs (50 μL, 1 mg mL−1) and irradiated for 10 min at 1.875 W cm−2 with the 808 nm NIR laser (“+Laser + PB NPs”, n = 5); (2) irradiated with the NIR laser without PB NPs (“+Laser”, n = 3); or (3) left untreated (“Control (No treatment)”, n = 10). The efficacy of the treatment was evaluated by temporally measuring the percentage of tumor-bearing mice in each group (tumor-bearing = measurable tumor; tumor-free = no measurable tumor) and by temporally monitoring the normalized tumor volumes. Normalized tumor volumes were obtained by dividing the tumor volume measured at every time-point by the tumor volume measured at day 0 for each individual mouse (Fig. 5B). Mice were removed from the study and humanely sacrificed when their absolute tumor volumes exceeded ∼2500 mm3. This resulted in mice being removed from the study at different times (i.e. different days post-treatment).
Mice in the “+Laser + PB NPs” group showed a marked decrease in tumor-bearing mice from 100% on day 0 to 20 % by day 4 post-treatment. 4 out of the 5 mice in this group showed complete tumor regression by day 4 and remained tumor-free until day 7 (Fig. 5A). In contrast, the “+Laser” and “Control (No treatment)” groups had tumor-bearing mice (100%) over the entire duration of the study (8 days). We noted that in mice that became tumor-free post-treatment in the “+Laser + PB NPs” group, the tumor temperatures measured using a hypodermic thermocouple reached 55–65 °C for at least 5 minutes during the laser PTT in 4 out of 5 mice. In the remaining mouse that did not show a response in this group, we observed a lower tumor temperature; <50 °C possibly caused by diffusion of the PB NPs outside the tumor and/or laser beam margins post-injection resulting in poorer response to treatment. In terms of normalized tumor volumes, mice in the “+Laser + PB NPs” group exhibited a significant decrease in normalized tumor volume relative to mice in the “+Laser” and “Control (No treatment)” groups from day 7 onward (Fig. 5B; ESI† for Statistical Analysis). Mice in the “+Laser + PB NPs” group also demonstrated a decreased tumor growth rate (slope of the normalized tumor volume plots) as compared to mice in the other two groups.
By day 7, we observed a reoccurrence of tumors in some mice (2 out of 5 mice) from the “+Laser + PB NPs” group (Fig. 5A). One mouse from this group remained tumor-free for over two weeks (when the study was terminated). Importantly, tumor regrowth was not detected in the tumor areas that were injected with PB NPs and irradiated by the laser (defined as “overlapping areas”). Tumor growth was observed around the margins of these irradiation (overlapping) areas–areas that were not irradiated by the laser and consequently insufficiently heated to yield effective ablation. These findings indicate the effect of tumor morphology on the outcome of PB NP-based laser PTT. Tumors with greater surface area (for the same tumor volume) are more difficult to effectively ablate and remain tumor-free as the laser PTT effects are inaccessible to the entire tumor volume. Such tumors likely require multiple rounds of laser PTT, larger beam-width lasers for efficient laser PTT, or laser PTT in combination with other therapies (e.g. chemotherapy and radiotherapy) which is the focus of ongoing studies in our group. Clearly, our current findings (Fig. 5) demonstrate the potential of our PB NPs as agents for laser-induced PTT of tumors. Future studies will elucidate the effects of thermal diffusion during PTT, the systemic effects of PTT, and investigate the integration of imaging modalities into PB NPs such as optical imaging and MRI,2,39 which confer theranostic (simultaneous therapy and diagnostic) capabilities to the PB NPs.
Conclusions
We have described the synthesis of Prussian blue nanoparticles (PB NPs) as agents for laser-induced photothermal therapy (PTT) in a mouse model of neuroblastoma – a common cancer of childhood. When irradiated by an 808 nm near infrared (NIR) laser, the NIR-absorbing PB NPs are photothermally heated with 20.5% photothermal conversion efficiencies. The photothermal heating effect was observed to be a function of PB NP concentration, incident laser power and duration. We established the stability and safe concentrations of the PB NPs in vitro. Finally, in vivo studies in a hard-to-treat mouse model of neuroblastoma demonstrated that PB NP-based, laser-induced PTT resulted in significant tumor debulking, increased tumor-free days, and decreased tumor growth rates. These findings indicate the therapeutic potential of local tumor ablation with PB NP-based, laser-induced PTT.Acknowledgements
This work was supported by the Sheikh Zayed Institute for Pediatric Surgical Innovation (RAC Awards #30000174 and 30001489).Notes and references
- G. Fu, W. Liu, S. Feng and X. Yue, Chem. Commun., 2012, 48, 11567–11569 RSC
.
- M. F. Dumont, H. A. Hoffman, P. R. S. Yoon, L. S. Conklin, S. R. Saha, J. Paglione, R. W. Sze and R. Fernandes, Bioconjugate Chem., 2014, 25, 129–137 CrossRef CAS PubMed
.
- Z. Li, Y. Zeng, D. Zhang, M. Wu, L. Wu, A. Huang, H. Yang, X. Liu and J. Liu, J. Mater. Chem. B, 2014, 2, 3686–3696 RSC
.
- R. Siegel, D. Naishadham and A. Jemal, Ca-Cancer J. Clin., 2013, 63, 11–30 CrossRef PubMed
.
- E. Ward, C. DeSantis, A. Robbins, B. Kohler and A. Jemal, Ca-Cancer J. Clin., 2014, 64, 83–103 CrossRef PubMed
.
- G. M. Brodeur, M. D. Hogarty, Y. P. Mosse and J. M. Maris, Principles and Practice of Pediatric Oncology, ed. P. A. Pizzo and D.G. Poplack, Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, 6th edn, 2010, pp. 886–922 Search PubMed
.
- N. Esiashvili, C. Anderson and H. M. Katzenstein, Curr. Probl. Cancer, 2009, 33, 333–360 CrossRef PubMed
.
- A. R. Burke, R. N. Singh, D. L. Carroll, J. C. S. Wood, R. B. D'Agostino Jr, P. M. Ajayan, F. M. Torti and S. V. Torti, Biomaterials, 2012, 33, 2961–2970 CrossRef CAS PubMed
.
- J. Overgaard, Cancer, 1977, 39, 2637–2646 CrossRef CAS
.
- J. L. Roti Roti, Int. J. Hyperthermia, 2008, 24, 3–15 CrossRef PubMed
.
- G. Crile Jr, Cleve. Clin. J. Med., 1961, 28, 75–89 CrossRef CAS
.
- C. F. Babbs and D. P. DeWitt, Med. Instrum., 1981, 15, 367–373 CAS
.
- F. Luchetti, B. Canonico, M. Della Felice, S. Burattini, M. Battistelli, S. Papa and E. Falcieri, Histol. Histopathol., 2003, 18, 1041–1052 CAS
.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Lasers Med. Sci., 2008, 23, 217–228 CrossRef PubMed
.
- K. F. Chu and D. E. Dupuy, Nat. Rev. Cancer, 2014, 14, 199–208 CrossRef CAS PubMed
.
- M. Chu, Y. Shao, J. Peng, X. Dai, H. Li, Q. Wu and D. Shi, Biomaterials, 2013, 34, 4078–4088 CrossRef CAS PubMed
.
- G. S. Gazelle, S. N. Goldberg, L. Solbiati and T. Livraghi, Radiology, 2009, 217, 633–646 CrossRef PubMed
.
- P. Wust, B. Hildebrandt, G. Sreenivasa, B. Rau, J. Gellermann, H. Riess, R. Felix and P. M. Schlag, Lancet Oncol., 2012, 3, 487–497 CrossRef
.
- B. Hildebrandt, P. Wust, O. Athers, A. Dieing, G. Sreenivasa, T. Kerner, R. Felix and H. Riess, Crit. Rev. Oncol. Hematol., 2012, 43, 33–56 CrossRef
.
- E. B. Dickerson, E. B. Dreaden, X. Huang, I. H. El-Sayed, H. Chu, S. Pushpanketh, J. F. McDonald and M. A. Elsayed, Cancer Lett., 2008, 269, 57–66 CrossRef CAS PubMed
.
- L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef CAS PubMed
.
- C. Loo, A. Lowery, N. Halas, J. West and R. Drezek, Nano Lett., 2005, 5, 709–711 CrossRef CAS PubMed
.
- D. P. O'Neal, L. R. Hirsch, N. J. Halas, J. D. Payne and J. L. West, Cancer Lett., 2004, 209, 171–176 CrossRef PubMed
.
- Q. Tian, F. Jiang, R. Zou, Q. Liu, Z. Chen, M. Zhu, S. Yang, J. Wang, J. Wang and J. Hu, ACS Nano, 2011, 5, 9761–9771 CrossRef CAS PubMed
.
- B. Li, Q. Wang, R. Zou, X. Liu, K. Xu, W. Li and J. Hu, Nanoscale, 2014, 6, 3274–3282 RSC
.
- H. K. Moon, S. H. Lee and H. C. Choi, ACS Nano, 2009, 3, 3707–3713 CrossRef CAS PubMed
.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560–2566 CrossRef CAS PubMed
.
- S. Tang, H. Xiaoqing and N. Zheng, Chem. Commun., 2011, 47, 3948 RSC
.
- J. Li, F. Jiang, B. Yang, X. Song, Y. Liu, H. Yang, D. Cao, W. Shi and G. Chen, Sci. Rep., 2013, 3, 1–7 Search PubMed
.
- K. Yang, H. Xu, L. Cheng, C. Sun, J. Wang and Z. Liu, Adv. Mater., 2012, 24, 5586–5592 CrossRef CAS PubMed
.
- B. Bahmani, D. Bacon and B. Anvari, Sci. Rep., 2013, 3, 1–7 Search PubMed
.
- L. Jing, X. Liang, Z. Deng, S. Feng, X. Li, M. Huang, C. Li and Z. Dai, Biomaterials, 2014, 35, 5814–5821 CrossRef CAS PubMed
.
- Heyltex, http://www.heyltex.com/radiogardase.php, accessed April 2014.
- FDA, Radiogardase: ferric hexacyanoferrate(II) capsule, http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021626s007lbl.pdf, accessed 12 May 2014.
- F. M. Kievit and M. Zhang, Adv. Mater., 2011, 23, H217–H247 CrossRef CAS PubMed
.
- Y. Matsumura and H. Maeda, Cancer Res., 1986, 46, 6387–6392 CAS
.
- L. Chakrabarti, B. D. Wang, N. H. Lee and A. D. Sandler, PLoS One, 2013, 8, e83521 Search PubMed
.
- L. Chakrabarti, T. Abou-Antoun, S. Vukmanovic and A. D. Sandler, Front. Oncol., 2012, 2, 82 CrossRef PubMed
.
- M. F. Dumont, S. Yadavilli, R. W. Sze, J. Nazarian and R. Fernandes, Int. J. Nanomed., 2014, 9, 2581–2595 Search PubMed
.
- D. K. Roper, W. Ahn and M. Hoepfner, J. Phys. Chem. C, 2007, 111, 3636–3641 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: (1) Size and morphology of PB NPs; (2) characterization of PB NPs; (3) calculation of the photothermal conversion efficiency of PB NPs; (4) statistical analysis; (5) references. See DOI: 10.1039/c4ra05209a |
| This journal is © The Royal Society of Chemistry 2014 |