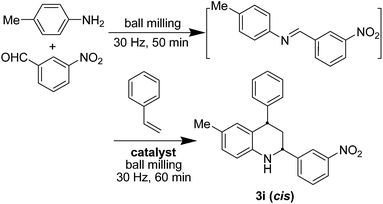
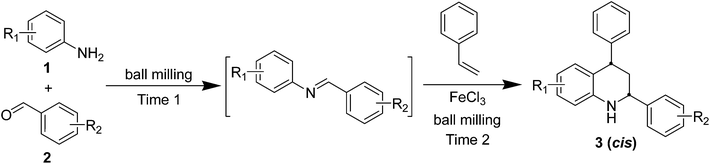
Mechanochemical milling promoted solvent-free imino Diels–Alder reaction catalyzed by FeCl3: diastereoselective synthesis of cis-2,4-diphenyl-1,2,3,4-tetrahydroquinolines†
Ya-Jun Tan,
Ze Zhang*,
Fang-Jian Wang,
Hao-Hao Wu and
Qing-Hai Li*
School of Biological and Chemical Engineering, Anhui Polytechnic University, Wuhu, 241000, China. E-mail: zhangze @ ustc. edu. cn; liqinghai@ahpu.edu.cn; Tel: +86 553 2871254
First published on 4th August 2014
Abstract
Under mechanochemical ball-milling at room temperature, FeCl3 promoted Diels–Alder cycloaddition of styrene with in situ generated N-aryl aldimines in the absence of any solvent afforded exclusively cis-2,4-diphenyltetrahydroquinolines in good to excellent yields within 90 minutes. The isolation work up just involves washing the resulting reaction mixture with water and recrystallization from EtOH–H2O. The advantages of high diastereoselectivity, short reaction time, free use of organic solvent, low cost, employment of cheap, easily available and nontoxic catalyst, and simple work-up procedure make this protocol a very efficient and green alternative to traditional methods for constructing these kinds of heterocyclic skeletons.
Natural and synthetic tetrahydroquinolines (THQs) are of great importance in medicinal chemistry, and this kind of compound has displayed various biological bioactivities.1 Therefore, the development of efficient synthesis of THQs has been actively investigated. Indeed, a large number of methods have been reported for the construction of polysubstituted THQs.2 Typically, the Povarov reaction, a [4 + 2] Diels–Alder reaction between N-arylimines and electron-rich dienophiles, is one of the most powerful and successful routes to rapidly construct this kind of privileged backbone.3 This imino Diels–Alder cycloaddition reactions can be promoted by many catalysts, such as various Lewis acids,3,4 transition metal carbonyls,5 lanthanide triflates,6 trifluoroacetic acid,7 2,3-dichloro-5,6-dicyano-pbenzoquinone (DDQ),8 triphenyl phosphonium perchlorate,9 etc. Frankly, most of these methods are efficient enough, but there are still some drawbacks such as rigorous or hazardous conditions, use of organic solvent, long reaction time, intractable side reactions or low selectivity, tedious isolation procedures and so on. To circumvent these problems, one of the best alternatives is to perform these reactions under solvent-free conditions. Among current solvent-free techniques, mechanochemical milling has recently been contributing more and more for efficient promotion of various organic reactions in a greener way.10,11 Keeping in mind the above facts and continuing our research on mechanochemical milling promoted reactions for synthesis of nitrogen-containing compounds,12 we herein wish to report a FeCl3-promoted solvent-free imino Diels–Alder reaction for diastereoselective synthesis of cis-2,4-diphenyl-1,2,3,4-tetrahydroquinolines.
Firstly, we chose the synthesis of cis-6-methyl-2-(3-nitrophenyl)-4-phenyl-1,2,3,4-tetrahydro-quinoline (3i) as the model reaction to screen an optimal catalyst (Table 1).
| Entry | Catalyst | Yieldb (%) |
|---|---|---|
| a Reactions were carried out with 4-methylaniline (2.0 mmol), 3-nitrobenaldehyde (2.0 mmol), styrene (2.5 mmol) and catalyst (0.5 mmol) under ball milling at room temperature.b Determined by HPLC analysis based on 4-methylaniline. | ||
| 1 | ZnCl2 | 76 |
| 2 | AlCl3 | 85 |
| 3 | CuCl2 | 15 |
| 4 | FeCl3 | 86 |
| 5 | Cu(OAc)2 | 10 |
| 6 | Co(OAc)2 | Not detected |
| 7 | Mn(OAc)3 | Trace |
| 8 | Pd(OAc)2 | Trace |
| 9 | BF3·OEt2 | 81 |
| 10 | Cu(OTf)2 | 56 |
| 11 | TFA | 48 |
| 12 | p-TsOH | 12 |
| 13 | KHSO4 | Trace |
| 14 | KH2PO4 | Not detected |
A variety of Lewis acids and Brønsted acids were attempted, which have been extensively studied for traditional synthesis of tetrahydroquinolines in solution. The results were outlined in Table 1, which demonstrated that strong Lewis acids such as ZnCl2, AlCl3, FeCl3 and BF3·OEt2 (entry 1, 2, 4, 9) promoted this Diels–Alder reaction to a great extent, while Brønsted acids and other relatively weak Lewis acids worked too inefficient or cannot work at all. In contrast, FeCl3 exhibits the best efficiency. Furthermore, considering its easy availability, low price, sustainability, non-toxicity, and environmentally friendly properties,13 we next selected FeCl3 to clarify the generality of substrate scope.
As shown in Table 2, a series of substituted anilines and benzaldehydes were examined. To our delight, the reactions worked well with substituted anilines and benzaldehydes bearing either electron-donating or electron-withdrawing groups on the benzene ring. In contrast, similar reactions with anilines bearing electron-donating substituents exhibited relatively lower reactivity when performed in organic solvents. Furthermore, all the desired products 3a–s with high structural diversity were obtained in good to excellent isolated yield through a simple work up, just including washing the resulting reaction mixture with water and recrystallization in EtOH–H2O. Under herein ball milling at room temperature, these cycloaddition reactions can be almost completely accomplished within 90 minutes, which were obviously faster than most conventional solution cases. The rapid conversion under this high speed vibration milling condition is probably caused by the high mechanical energy from local high pressure, friction, shear strain and so on.14
| Entry | R1 | R2 | Product 3b | Reaction time (min) | Yieldc (%) | |
|---|---|---|---|---|---|---|
| Time 1 | Time 2 | |||||
| a Reactions were carried out with aniline 1 (2.0 mmol), benzaldehyde 2 (2.0 mmol), and afterward added styrene (2.5 mmol) and FeCl3 (0.5 mmol) under ball milling (vibration frequency: 30 Hz) at room temperature.b Characterized by mp, IR, 1H NMR, 13CNMR and HRMS analysis.c Isolated yield combined from two parallel runs by washing the resulting reaction mixture with water and recrystallization in EtOH–H2O. | ||||||
| 1 | H | H | 3a | 90 | 90 | 71 |
| 2 | H | 4-Cl | 3b | 90 | 90 | 79 |
| 3 | H | 4-NO2 | 3c | 75 | 90 | 75 |
| 4 | H | 3-NO2 | 3d | 75 | 90 | 83 |
| 5 | 4-Me | H | 3e | 90 | 90 | 85 |
| 6 | 4-Me | 4-Me | 3f | 75 | 90 | 80 |
| 7 | 4-Me | 4-Cl | 3g | 60 | 90 | 82 |
| 8 | 4-Me | 4-NO2 | 3h | 50 | 90 | 83 |
| 9 | 4-Me | 3-NO2 | 3i | 50 | 90 | 74 |
| 10 | 4-OMe | H | 3j | 50 | 90 | 80 |
| 11 | 4-OMe | 4-Me | 3k | 40 | 90 | 81 |
| 12 | 4-OMe | 4-Cl | 3l | 30 | 90 | 77 |
| 13 | 4-OMe | 4-NO2 | 3m | 30 | 90 | 70 |
| 14 | 4-OMe | 3-NO2 | 3n | 30 | 90 | 73 |
| 15 | 4-Cl | 4-Cl | 3o | 60 | 90 | 91 |
| 16 | 4-Cl | 4-NO2 | 3p | 50 | 90 | 83 |
| 17 | 4-Cl | 3-NO2 | 3q | 50 | 90 | 82 |
| 18 | 3-Cl | 4-NO2 | 3r | 50 | 90 | 87 |
| 19 | 3-Cl | 3-NO2 | 3s | 50 | 90 | 86 |
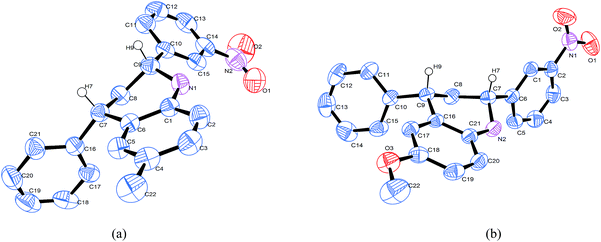
It is worthy to point out that herein solvent-free ball milling promoted reactions afforded the corresponding THQs 3 exclusively in cis-(2e, 4e) configuration based on HPLC analysis of the resulted reaction mixtures. The high diastereoselectivity may be ascribed to high local concentration of the reactants under this mechanochemical and solvent-free condition, which may result in an enhanced second-order reaction rate and thus prefer to selective formation of products via kinetic control.15 There is a fact that when the reaction for synthesis of tetrahydroquinolines 3i was performed by refluxing in organic solvents such as CH2Cl2 and THF, minor trans-diastereomer was detected. The cis-(2e, 4e) configuration is indicated by the diagnostic coupling constants of the relevant protons on the saturated THQ ring from 1H NMR analysis. That is, the large vicinal coupling constants J2a,3a and J3a,4a = 9.9–12.0 Hz for this form indicate an axial–axial relationship, and the aryl groups on C-2 and C-4 are both pseudoequatorial and thus located in cis-configuration.16 In addition, this stereochemistry was further unambiguously confirmed by single-crystal X-ray diffraction analysis of two selected examples 3i and 3n (Fig. 1), showing that the substituents in the saturated part of the tetrahydroquinoline occupy the equatorial positions, strongly confirming that the reaction was highly diastereoselective.17
In summary, under mechanochemical ball-milling at room temperature, the FeCl3 promoted Diels–Alder cycloaddition reactions of styrene with in situ generated N-aryl aldimines in absence of any solvent afforded exclusively cis-2,4-diphenyltetrahydroquinolines in good to excellent yields within 90 minutes. The isolation work up just involves washing the resulting reaction mixture with water and recrystallization in EtOH–H2O. This novel protocol exhibits the advantages of high diastereoselectivity, short reaction time, free use of organic solvent, low cost, employment of cheap, easily available and nontoxic catalyst, and simple work-up procedure. These merits make this protocol a very efficient and green alternative to traditional methods for synthesis of these kinds of compounds, and even can presumably be extended to the construction of other heterocyclic skeletons.
Acknowledgements
We are grateful to financial support from National Natural Science Foundation of China (21242013).Notes and references
- (a) R. Nagata, N. Tanno, T. Kodo, N. Ae, H. Yamaguchi, N. Tamiki, F. Antoku, T. Tatsuno, T. Kato, Y. Tanaka and M. Nakamura, J. Med. Chem., 1994, 37, 3956 CrossRef CAS; (b) F. Guo, B. H. Chang and C. J. Rizzo, Bioorg. Med. Chem. Lett., 2002, 12, 151 CrossRef CAS; (c) J. M. Singer, B. M. Barr, L. L. Coughenour and M. A. Walters, Bioorg. Med. Chem. Lett., 2005, 15, 4560 CrossRef CAS PubMed; (d) O. B. Wallace, K. S. Lauwers, S. A. Jones and A. Dodge, Bioorg. Med. Chem. Lett., 2003, 13, 1907 CrossRef CAS.
- (a) A. R. Katritsky, S. Rachwal and B. Rachwal, Tetrahedron, 1996, 52, 15031 CrossRef , and references cited therein; (b) V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 CrossRef CAS PubMed.
- L. S. Povarov, Russ. Chem. Rev., 1967, 36, 656 CrossRef PubMed.
- (a) D. L. Boger, Tetrahedron, 1983, 39, 2869 CrossRef CAS; (b) V. V. Kouznetsov, F. I. Zubkov, U. Mora, L. G. Voskressensky, L. Y. Vargas, L. Astudillo and E. E. Stashenko, Lett. Org. Chem., 2004, 1, 37 CrossRef CAS; (c) T. Kametani, H. Takeda, Y. Suzuki and T. Honda, Synth. Commun., 1985, 15, 499 CrossRef CAS; (d) C. Hertweck, J. Prakt. Chem., 2000, 342, 316 CrossRef CAS; (e) G. Savitha and P. T. Perumal, Tetrahedron Lett., 2006, 47, 3589 CrossRef CAS PubMed; (f) T. Kawabata, M. Kato, T. Mizugaki, K. Ebitani and K. Kaneda, Chem.–Eur. J., 2005, 11, 288 CrossRef PubMed; (g) A. Semwal and S. K. Nayak, Synth. Commun., 2006, 36, 227 CrossRef CAS.
- T. Jon and N. Hagihara, Jpn. J. Chem., 1970, 91, 373 Search PubMed.
- (a) Y. Makioka, T. Shindo, Y. Taniguchi, K. Takaki and Y. Fujiwara, Synthesis, 1995, 801 CrossRef CAS PubMed; (b) M. Yamanaka, A. Nishida and M. Nakagana, Org. Lett., 2000, 2, 159 CrossRef CAS PubMed; (c) K. Hattori and H. Yamamoto, Tetrahedron, 1993, 49, 1749 CrossRef CAS.
- H. Ishitani and S. Kobayashi, Tetrahedron Lett., 1996, 37, 7357 CrossRef CAS.
- P. A. Grieco and A. Bahsas, Tetrahedron Lett., 1988, 29, 5855 CrossRef CAS.
- R. Nagarajan, S. Chitra and P. T. Perumal, Tetrahedron, 2001, 57, 3419 CrossRef CAS.
- For some reviews, see: (a) E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719 RSC; (b) L. Takacs, Chem. Soc. Rev., 2013, 42, 7649 RSC; (c) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC; (d) S.-E. Zhu, F. Li and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535 RSC; (e) J. Huot, D. B. Ravnsbæk, J. Zhang, F. Cuevas, M. Latroche and T. R. Jensen, Prog. Mater. Sci., 2013, 58, 30 CrossRef CAS PubMed; (f) R. B. N. Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559 RSC; (g) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC; (h) A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317 RSC; (i) B. Rodríguez, A. Bruckmann, T. Rantanen and C. Bolm, Adv. Synth. Catal., 2007, 349, 2213 CrossRef PubMed.
- For some recent examples, see: (a) D. Tan, V. Štrukil, C. Mottillo and T. Friščić, Chem. Commun., 2014, 50, 5248 RSC; (b) M. Hestericová and R. Šebesta, Tetrahedron, 2014, 70, 901 CrossRef PubMed; (c) J. G. Hernández, N. A. J. Macdonald, C. Mottillo, I. S. Butler and T. Friščić, Green Chem., 2014, 16, 1087 RSC; (d) L. R. Chen, B. E. Lemma, J. S. Rich and J. Mack, Green Chem., 2014, 16, 1101 RSC; (e) M. Ferguson, N. Giri, X. Huang, D. Apperley and S. L. James, Green Chem., 2014, 16, 1374 RSC; (f) A. Bose and P. Mal, Tetrahedron Lett., 2014, 55, 2154 CrossRef CAS PubMed; (g) M. Zille, A. Stolle, A. Wild and U. S. Schubert, RSC Adv., 2014, 4, 13126 RSC; (h) G.-P. Fan, Z. Liu and G.-W. Wang, Green Chem., 2013, 15, 1659 RSC.
- (a) Z. Zhang, Y.-J. Tan, C.-S. Wang and H.-H. Wu, Heterocycles, 2014, 89, 103 CrossRef CAS; (b) Z. Zhang, H.-H. Wu and Y.-J. Tan, RSC Adv., 2013, 3, 16940 RSC; (c) Z. Zhang, Z.-W. Peng, M.-F. Hao and J.-G. Gao, Synlett, 2010, 2895 CrossRef CAS PubMed; (d) Z. Zhang, J. Gao, J.-J. Xia and G.-W. Wang, Org. Biomol. Chem., 2005, 3, 1617 RSC; (e) Z. Zhang, G.-W. Wang, C.-B. Miao, Y.-W. Dong and Y.-B. Shen, Chem. Commun., 2004, 40, 1832 RSC.
- (a) Y.-T. Su and G.-W. Wang, Org. Lett., 2013, 15, 3408 CrossRef CAS PubMed; (b) A. Correa, O. G. Mancheno and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (c) B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef CAS PubMed; (d) J. Fan, L. Gao and Z. Wang, Chem. Commun., 2009, 45, 5021 RSC; (e) J. Bonnamour and C. Bolm, Org. Lett., 2008, 10, 2665 CrossRef CAS PubMed.
- G.-W. Wang, T.-H. Zhang, E.-H. Hao, L.-J. Jiao, Y. Murata and K. Komatsu, Tetrahedron, 2003, 59, 55 CrossRef CAS.
- M. A. Fox and J. K. Whitesell, in Organic Chemistry, Jones & Bartlett, Boston, 2nd edn, 1997, ch. 6, p. 298 Search PubMed.
- V. V. Kouznetsov, J. S. Bello and D. F. Amado-Torres, Tetrahedron Lett., 2008, 49, 5855 CrossRef CAS PubMed.
- ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, characterization details, NMR spectra for all products. CCDC 993488 and 993489. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra05252h |
| This journal is © The Royal Society of Chemistry 2014 |