Substituent effect on the radical scavenging activity of 6-chromanol derivatives†
Keiko Inami*ab,
Mariko Suzukia,
Azusa Shimizua,
Miyuki Furukawab,
Mine Moritab and
Masataka Mochizukiab
aFaculty of Pharmaceutical Sciences, Tokyo University of Science, 2641 Yamazaki, Noda-shi, Chiba 278-8510, Japan. E-mail: inami@rs.noda.tus.ac.jp; Fax: +81-4-7121-3641; Tel: +81-4-7121-3641
bKyoritsu University of Pharmacy, Shibako-en 1-5-30, Minato-ku, Tokyo 105-8512, Japan
First published on 1st September 2014
Abstract
Several 6-chromanol derivatives with various substituents (one or two amino, acetylamino, chloro or nitro substituents at the 5-, 7-, 8- or 5,7-positions on the phenyl ring of 2,2-dimethyl-6-chromanol) were synthesized, and their second order rate constants (k) for a reaction that demonstrates radical scavenging activity (reaction with the galvinoxyl radical) were determined. Three monoacetylamino compounds, 8-nitro compound, and 5,7-diamino, 5,7-diacetylamino, and 5,7-dinitro compounds were newly synthesized. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k was plotted against the Hammett sigma (σm) or Taft sigma (σ*) constants for the compounds containing each of the four substituents to obtain their reaction constants (ρ) from the slopes. The σ plots representing radical scavenging activity showed a linear correlation with negative ρ values for all compounds with substituted positions. The results indicate that the electron-donating effect of the amino and acetylamino groups on the chroman ring enhanced radical scavenging activity, whereas the electron-withdrawing effect of the chloro and nitro groups decreased this activity. Furthermore, the magnitude of ρ for the substituted compounds increased in the following order with respect to the substitution position: meta-substituted (−3.71 for 6a–d), ortho-monosubstituted (−0.86 for 4a–d, −0.87 for 5a–d), and ortho-disubstituted (−0.47 for 7a–d). The greater ρ magnitudes for the meta-substituted compound indicated that the radical scavenging reactions were more sensitive to inductive substituent effects than for the ortho-substituent compounds. The ρ values for ortho-mono- and ortho-disubstituted compounds were smaller than that for the meta-substituted compound, despite the fact that the k values for the ortho-substituted compounds were higher than those for the meta-substituted compounds. Thus, electron-donating groups in ortho-substituted 6-chromanols accelerate the reaction rate through resonance stabilization in addition to the inductive substituent effect.
k was plotted against the Hammett sigma (σm) or Taft sigma (σ*) constants for the compounds containing each of the four substituents to obtain their reaction constants (ρ) from the slopes. The σ plots representing radical scavenging activity showed a linear correlation with negative ρ values for all compounds with substituted positions. The results indicate that the electron-donating effect of the amino and acetylamino groups on the chroman ring enhanced radical scavenging activity, whereas the electron-withdrawing effect of the chloro and nitro groups decreased this activity. Furthermore, the magnitude of ρ for the substituted compounds increased in the following order with respect to the substitution position: meta-substituted (−3.71 for 6a–d), ortho-monosubstituted (−0.86 for 4a–d, −0.87 for 5a–d), and ortho-disubstituted (−0.47 for 7a–d). The greater ρ magnitudes for the meta-substituted compound indicated that the radical scavenging reactions were more sensitive to inductive substituent effects than for the ortho-substituent compounds. The ρ values for ortho-mono- and ortho-disubstituted compounds were smaller than that for the meta-substituted compound, despite the fact that the k values for the ortho-substituted compounds were higher than those for the meta-substituted compounds. Thus, electron-donating groups in ortho-substituted 6-chromanols accelerate the reaction rate through resonance stabilization in addition to the inductive substituent effect.
Introduction
α-Tocopherol (1) is involved in immune function, cell signaling, gene expression regulation, and other metabolic processes.1,2 In addition to its physiological activities, α-tocopherol is an antioxidant and protects cells from damage caused by reactive oxygen species. Thus, α-tocopherol is thought to serve in preventing oxidative damage, such as that in cancer and ischemia/reperfusion tissue damage.3,4 The antioxidant activities of 1 are attributed to the radical scavenging function of their phenolic hydroxyl groups.5–7 A large number of tocopherol derivatives have been synthesized and assayed for their antioxidant and physiological activities.8–11The chemical properties of phenols have been widely studied and correlated with antioxidant activity that has radical scavenging activity,12–14 O–H bond dissociation enthalpy (BDE),14–17 and ionization enthalpy.18 Despite the large number of reports focused on the effect of substituents on the relationship between radical scavenging activity and calculated BDE in simple phenols,19–26 few reports have examined the effect of substituents in the chroman ring on radical scavenging activity.24–27 The aim of this study was to understand the structure–activity relationships of 6-chromanol derivatives in radical scavenging reactions. A series of 6-chromanol derivatives with substituents ortho or meta to the phenolic group was synthesized. The radical scavenging activities of these derivatives were then investigated based on the rate constants (k) of their reactions with the galvinoxyl radical (G˙), and then compared with substituent sensitivity for each positions, which obtained from the correlation of log k and Hammett sigma (σm) or Taft sigma (σ*) constants.
Experimental methods
Material and methods
2-Methyl-3-buten-2-ol was obtained from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan). The galvinoxyl radical was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Acetonitrile, which was used for spectral measurements, was obtained from Dojindo Laboratories (Kumamoto, Japan). Other reagents were purchased from Wako Pure Chemical Industries (Osaka, Japan). All of the reagents and solvents used were of the best commercially available quality and were not further purified unless otherwise noted.Reaction progression was monitored using thin-layer chromatography (TLC) on silica gel 60 F254 (0.25 mm, Merck, Darmstadt, Germany). Column chromatography was performed using silica gel 60 (230–400 mesh, Merck). Melting points were determined using a Yanaco micro-melting point apparatus without correction. NMR spectra were recorded with a JEOL JNM-LA400 spectrometer (Tokyo, Japan). Chemical shifts were expressed in terms of ppm downfield shifted from TMS. High-resolution mass spectra were collected using a JEOL JMS-SX102A mass spectrometer. FT-IR spectra were recorded with a Perkin Elmer Spectrum 100 (Waltham, USA). UV-Vis spectrophotometry was obtained using a Hewlett-Packard 8453 photodiode array spectrophotometer (Hanover, USA) equipped with an Applied Photophysics RX 2000 stopped-flow device (Leatherhead, UK).
2,2-Dimethyl-6-chromanol (3) was synthesized using the method described by Nilsson et al. (mp: 75.3–76.0 °C).28 Amino (4a, 5a, 6a), chloro (4c, 5c, 6c, 7c), and nitro (4d, 5d) 6-chromanols and 6-acetoxy-2,2-dimethyl-8-nitrochroman (mp: 56.5–57.0 °C) and 6-acetoxy-8-amino-2,2-dimethylchroman (mp: 86.5–87.5 °C) were synthesized as reported previously.29,30
Syntheses of 6-acetoxyacetylaminochromans (4b-OAc, 5b-OAc)
4d or 5d was dissolved in methanol, followed by the addition of an excess of acetic anhydride and palladium/carbon (5%). The reaction mixture was stirred overnight at room temperature under a hydrogen atmosphere to produce the following two distinct spots via TLC: acetylamino (4b, 5b) and acetoxylated acetylamino compounds (4b-OAc, 5b-OAc). The reaction mixture was filtered through celite, and the filter cake was washed with CH2Cl2. Water was added to the filtrate, and the organic layer was extracted twice with CH2Cl2. The combined organic layer was washed with saturated NaHCO3 and water and dried over anhydrous sodium sulfate, filtered, and then the solvent was evaporated under reduced pressure. The crude product was dissolved in pyridine, followed by the addition of an excess of acetic anhydride. The reaction mixture was stirred at room temperature for 2 h to produce a single spot by TLC. The reaction mixture was poured onto crushed ice and extracted twice with CH2Cl2. The combined organic layer was washed with saturated NaHCO3, water, 1 M HCl, and water, then dried over anhydrous sodium sulfate, filtered, and the solvent evaporated under reduced pressure. The crude product was purified on a silica gel column to afford the single desired product.4b-OAc; white needle-like crystals (recrystallized from ether and hexane); yield 40%; mp 121.0–123.0 °C; 1H NMR (400 MHz, CDCl3) δ 6.85 (d, J = 9.0 Hz, 1H), 6.75 (d, J = 9.0 Hz, 1H), 6.66 (br, 1H), 2.64 (t, J = 6.8 Hz, 2H), 2.29 (s, 3H), 2.15 (s, 3H), 1.76 (t, J = 6.8 Hz, 2H), 1.33 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 169.8, 168.2, 152.4, 139.1, 127.3, 120.6, 120.3, 116.9, 74.2, 32.0, 26.7, 23.3, 20.9, 19.5; IR (cm−1, neat) 3266, 2932, 1766, 1660; HRMS (FAB) 278.1394 (calcd for C15H19NO4 277.1314).
5b-OAc; white needle-like crystals (recrystallized from ether and hexane); yield 27%; mp 147.5–150.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.51 (s, 1H), 6.99 (br, 1H), 6.80 (s, 1H), 2.71 (t, J = 6.6 Hz, 2H), 2.32 (s, 3H), 2.14 (s, 3H), 1.77 (t, J = 6.6 Hz, 2H), 1.31 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 169.3, 167.8, 151.9, 133.9, 128.3, 121.9, 117.4, 111.7, 74.4, 32.5, 26.9, 24.5, 22.2, 21.0; IR (cm−1, neat) 3315, 2976, 1752, 1669; HRMS (FAB) 278.1392 (calcd for C15H19NO4 277.1314).
Synthesis of 6-acetoxy-8-acetylaminochroman (6b-OAc)
6-Acetoxy-8-amino-2,2-dimethylchroman (39.7 mg, 0.17 mmol) was dissolved in pyridine (0.5 mL), followed by the addition of an excess of acetic anhydride (1 mL). The reaction mixture was stirred at room temperature for 3 h. The reaction mixture was then poured onto crushed ice, and the precipitate was filtered via suction to afford the desired product.6b-OAc; white needle-like crystals (recrystallized from ethanol and H2O); yield 40%; mp 186.0–187.5 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 2.7 Hz, 1H) 7.72 (br, 1H), 6.54 (d, J = 2.9 Hz, 1H), 2.75 (t, J = 6.8 Hz, 2H), 2.26 (s, 3H), 2.19 (s, 3H), 1.81 (t, J = 6.8 Hz, 2H), 1.36 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 170.2, 168.0, 143.0, 139.6, 127.8, 120.5, 116.2, 111.0, 75.7, 32.6, 27.0, 25.0, 22.4, 21.1; IR (cm−1, neat) 3339, 1744, 1675; HRMS (FAB) 278.1394 (calcd for C15H19NO4 277.1314).
Syntheses of acetylamino-6-chromanols via acetylated compound hydrolyses
The purified acetylated compounds were dissolved in methanol and treated with 1 M sodium methoxide. After the disappearance of the starting material observed by TLC, the reaction mixture was neutralized with 0.5 M HCl and extracted twice with CH2Cl2. The combined organic layer was washed with water, dried over anhydrous sodium sulfate and filtered. Following concentration under reduced pressure, the product was purified on a short silica gel column, even when a single compound was obtained.4b; tan yellow solid; yield 96%; mp 184.0–186.0 °C (decomposition); 1H NMR (400 MHz, CDCl3) δ 7.51 (s, 1H), 7.12 (br, 1H), 6.85 (d, J = 9.0 Hz, 1H), 6.67 (d, J = 8.8 Hz, 1H), 2.59 (t, J = 6.8 Hz, 2H), 2.28 (s, 3H), 1.81 (t, J = 6.8 Hz, 2H), 1.30 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 170.3, 147.8, 143.2, 123.5, 119.1, 116.8, 113.3, 73.1, 32.1, 26.4, 23.6, 19.4; IR (cm−1, neat) 3267, 2974, 2929, 1639; HRMS (FAB) 236.1209 (calcd for C13H17NO3 235.1208).
5b; tan yellow solid; yield 97%; mp 205.0–206.5 °C (decomposition); 1 H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H), 7.48 (br, 1H), 6.72 (s, 1H), 6.41 (s, 1H), 2.71 (t, J = 6.3 Hz, 2H), 2.23 (s, 3H), 1.76 (t, J = 6.8 Hz, 2H), 1.29 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 170.3, 147.5, 141.8, 124.4, 120.2, 119.8, 110.0, 74.1, 32.7, 26.7, 23.7, 22.1; IR (cm−1, neat) 3415, 2970, 2929, 1655, 1608; HRMS (FAB) 235.1287 (calcd for C13H17NO3 235.1208).
6b; tan yellow solid; yield 99%; mp 193.0–194.5 °C (decomposition); 1H NMR (400 MHz, CDCl3) δ 8.62 (s, 1H), 8.24 (d, J = 2.9 Hz, 1H), 7.87 (br, 1H), 6.37 (d, J = 2.9 Hz, 1H), 2.72 (t, J = 6.7 Hz, 2H), 2.24 (s, 3H), 1.78 (t, J = 6.7 Hz, 2H), 1.33 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 169.2, 150.0, 135.2, 126.8, 121.0, 110.1, 105.5, 74.9, 32.9, 26.8, 24.9, 22.4; IR (cm−1, neat) 3560, 2978, 2933, 1637; HRMS (FAB) 235.1203 (calcd for C13H17NO3 235.1208).
Synthesis of 2,2-dimethyl-8-nitro-6-chromanol (6d)
6-Acetoxy-2,2-dimethyl-8-nitrochroman (27 mg, 0.1 mmol) was dissolved in methanol (1 mL). Sodium methoxide (0.2 mL) was then added to the solution, and this mixture was stirred for 0.5 h at room temperature. The reaction mixture was acidified with 1 M HCl and extracted three times with CH2Cl2. The combined organic phase was washed with 5% NaHCO3 and H2O, dried over Na2SO4, filtered, and evaporated to give an orange oil. The crude product was purified using silica gel column chromatography (silica gel 60, 4% methanol–chloroform) to afford a yellow solid.6d; yellow solid; yield 101%; mp 88.0–91.0 °C; 1H NMR (400 MHz, CDCl3) δ 7.17 (d, J = 3.2 Hz, 1H), 6.83 (d, J = 3.2 Hz, 1H), 2.80 (t, J = 6.8 Hz, 2H), 1.85 (t, J = 6.8 Hz, 2H), 1.36 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 147.1, 142.5, 139.0, 125.5, 121.3, 110.1, 76.1, 32.0, 26.6, 22.7; IR (cm−1, neat) 3539, 3413, 2975, 1524, 1358; HRMS (EI) 223.0842 (calcd for C11H13NO4 223.0845).
Synthesis of 2,2-dimethyl-5,7-dinitro-6-chromanol (7d)
2,2-Dimethyl-6-chromanol (592 mg, 3.33 mmol) was dissolved in acetic acid (40 mL) and cooled to below 15 °C while stirring. Nitric acid (422 μL, 2 eq.) was then added to the solution, and the mixture was stirred below 15 °C for 10 min. The reaction mixture was poured onto crushed ice and extracted three times with CH2Cl2. The combined organic layer was extracted three times with 10% Na2CO3. The aqueous layer was acidified to pH 3 upon concentrated HCl addition, at which time, a precipitate appeared. The solid was filtered via suction to afford a yellow solid.7d; orange prism crystals (recrystallized from carbon tetrachloride); yield 46%; mp 168.0–168.1 °C; 1H NMR (400 MHz, CDCl3) δ 10.3 (s, 1H), 7.69 (s, 1H), 2.80 (t, J = 6.8 Hz, 2H), 1.86 (t, J = 6.8 Hz, 2H), 1.36 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 146.6, 141.0, 140.3, 132.9, 125.2, 114.0, 75.7, 30.8, 26.5, 19.3; IR (cm−1, neat) 3123, 2980, 1530, 1369; HRMS (EI) 268.0695 (calcd for C11H12N2O6 268.0695).
Synthesis of 5,7-diamino-2,2-dimethyl-6-chromanol (7a)
7d (20 mg, 0.07 mmol) was first dissolved in methanol (5 mL), and 5% palladium/carbon (19 mg) was then added to the solution. The reaction mixture was stirred under hydrogen gas for 30 min at room temperature. The reaction mixture was filtered through celite, and the filter cake was washed with deaerated CH2Cl2 under nitrogen gas. The filtrate was blown dry with argon to afford a red solid.7a; red solid; yield 97%; 1H NMR (400 MHz, CDCl3) δ 7.36 (s), 2.55 (br), 2.32 (br), 2.19 (br), 1.81 (br), 1.37 (br), 1.30 (s); HRMS (FAB) 209.1288 (calcd for C11H16N2O2 208.1212). 13C NMR data could not be obtained owing to the compound's high instability in air.
Synthesis of 5,7-diacetylamino-2,2-dimethyl-6-chromanol (7b)
7d (55 mg, 0.21 mmol) was dissolved in methanol (10 mL), followed by the addition of an excess of acetic anhydride (4.2 mL) and 5% palladium/carbon (26 mg). The reaction mixture was stirred under a hydrogen atmosphere at room temperature for 9.5 h. The reaction mixture was filtered through celite, and the filter cake was washed with CH2Cl2. The filtrate was acidified to pH 3 upon 1 M HCl addition and extracted three times with CH2Cl2. The combined organic layer was washed with water, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The residues were termed 7b and acetoxylated 7b (7b-OAc). The mixture was then dissolved in methanol (2 mL), and 0.5 M NaOMe (0.2 mL) was added. The mixture was stirred for 10 min at room temperature under an argon atmosphere. The mixture was neutralized by the addition of 1 M HCl and then extracted three times with CH2Cl2. The combined organic layer was washed with water, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The crude product was purified on a silica gel column (ethyl acetate–CHCl3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desired single product.
1) to afford the desired single product.
7b-OAc; 1H-NMR (400 MHz, CDCl3) δ 6.79 (s, 1H), 2.56 (t, 2H, J = 6.1 Hz), 2.30 (s, 3H), 2.14 (s, 6H), 1.75 (t, 2H, J = 6.7 Hz), 1.31 (s, 6H).
7b; white prism crystals (recrystallized from ethanol); yield 64%; mp 224.0–224.2 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 8.03 (br, 1H), 7.48 (s, 1H), 2.55 (t, J = 6.7 Hz, 2H), 2.29 (s, 3H), 2.19 (s, 3H), 1.77 (t, J = 6.7 Hz, 2H), 1.27 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 170.6, 168.8, 147.5, 133.2, 127.9, 123.3, 109.2, 107.9, 73.3, 32.1, 26.4, 24.6, 23.5, 19.0; IR (cm−1, neat) 3259, 2974, 2929, 1639; HRMS (FAB) 293.1501 (calcd for C15H20N2O4 292.1423).
Kinetics measurements
The kinetics were determined by measuring the disappearance of absorbance at 428 nm under pseudo-first-order conditions at 25 °C.31 Substituted 6-chromanols (with the following final concentrations: 0–100 μM for compounds 4a, 5a, and 7a; 0–300 μM for compounds 4b, 4c, 5b, 6a, 6b, 7b, and 7c; 0–600 μM for compounds 4d, 5d, 6d, and 7d; and 0–1000 μM for compounds 5c and 6c) and G˙ (final concentration of 5.0 μM) were dissolved in argon deaerated with acetonitrile. The two syringes were loaded with 2 mL of each compound and G˙ solution. The pneumatic drive accessory initiated mixing after the initiation of data acquisition by the spectrophotometer at 300–600 nm and 0.5 s intervals. Half of the starting concentration of G˙ solution and antioxidant was used after mixing.The radical scavenging rates were determined by monitoring the changes in absorbance due to the galvinoxyl radical at 428 nm. Pseudo-first-order rate plots of ln(A − A∞) versus time, where A and A∞ refer to the absorbance at a given time and the final absorbance, respectively, were linear until three or more half-lives. The A∞ used was the absorbance 10 min after each reaction started. To avoid the influence of minor absorption from the G˙ reduction products at this wavelength, only the first G˙ absorption decay values were used in kinetics analyses. Pseudo-first-order rate constants were determined using the least-squares method. The observed pseudo-first-order rate constant (kobs) was compound concentration-dependent. The second-order rate constants (k) for the reactions between the compounds and G˙ were obtained from the slopes of the linear functions of kobs versus various compound concentrations under pseudo-first order reaction conditions. The data were collected in at least three different experimental sessions.
Results
Chemistry
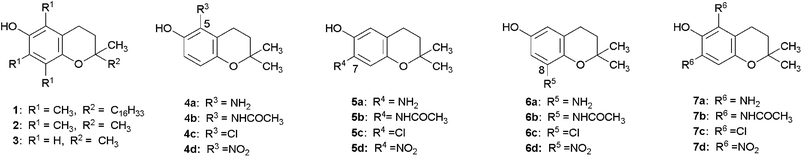
The lead compound optimized in this manuscript is 2,2-dimethyl-6-chromanol (3). Compounds 1 and 2 are considered to be derivatives of 3. The substituents were chosen based on their ability to donate electrons (NH2 and NHCOCH3) or accept electrons (Cl and NO2). Compounds 4a–d and 5a–d possess a substituent at position 5 or 7, respectively, which are ortho to the phenolic OH group. Compounds 6a–d have a substituent at position 8, which is meta to the phenolic OH group. Compounds 7a–d have two substituted groups, both in ortho positions (Fig. 1).Three monoacetylamino compounds (4b, 5b, 6b), a nitro compound (6d), and 5,7-diamino, 5,7-diacetylamino, and 5,7-dinitro compounds (7a, 7b, 7d) were newly synthesized.
The nitro compounds were prepared by nitration, reduced by hydrogen gas in the presence of palladium/carbon to the corresponding amino compound. The acetylamino compounds were prepared using simple catalytic reduction of the corresponding nitro compounds (4d, 5d, 6d, 7d) in the presence of acetic anhydride to obtain the acetoxy acetylaminochromans for storage. The acetoxy compounds were hydrolyzed with alkaline reagents immediately before use (Schemes 1–3).
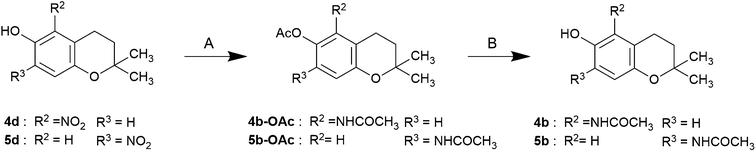 | ||
| Scheme 1 Synthesis of 5- or 7-subsituted 6-chromanols reagents and conditions: (A) (i) 5% Pd–C, H2 gas, Ac2O, rt, (ii) Ac2O, pyridine, rt; (B) 1 M NaOMe, MeOH, rt. | ||
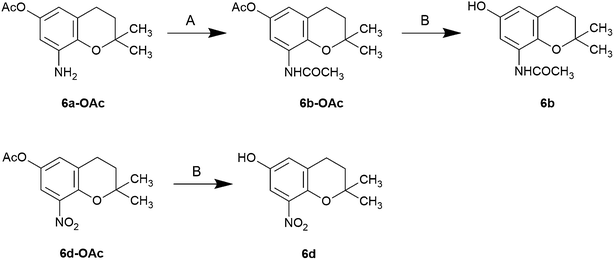 | ||
| Scheme 2 Synthesis of 8-subsituted 6-chromanols Reagents and conditions: (A) Ac2O, pyridine, rt; (B) 1 M NaOMe, MeOH, rt. | ||
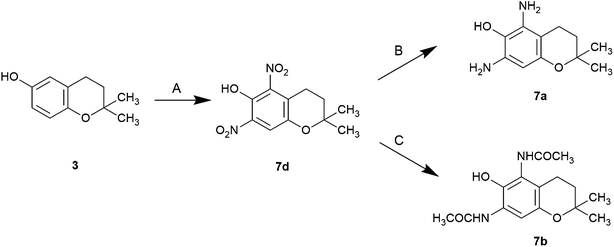 | ||
| Scheme 3 Synthesis of 5- and 7-disubsituted 6-chromanols Reagents and conditions: (A) HNO3, AcOH, 15 °C; (B) 5% Pd–C, H2 gas, rt; (C) (i) 5% Pd–C, H2 gas, Ac2O, rt, (ii) 0.5 M NaOMe, MeOH, rt. | ||
Galvinoxyl radical scavenging activity of the substituted 6-chromanols
The ability of a series of 6-chromanols to scavenge G˙ was evaluated in acetonitrile. G˙ is a reactive oxygen species with a strong absorption band at 428 nm.31 Upon addition of a compound to deaerated G˙ solutions in acetonitrile, the absorption band at 428 nm rapidly decreased. The absorbance decay at 428 nm obeyed pseudo-first-order reaction kinetics when the compound concentration was at more than a 10-fold excess compared to the G˙ concentration. The observed pseudo-first-order rate constant (kobs) was compound concentration-dependent in a linear manner. Second-order rate constants (k) for the reactions between 6-chromanols and G˙ were obtained from the slopes of the linear functions of kobs versus compound concentration (Table 1).| Compound | ka (M−1 s−1) | Compound | ka (M−1 s−1) |
|---|---|---|---|
| a Standard error of the regression line for each concentration. | |||
| 4a | 50840 ± 0.09 | 6a | 23970 ± 0.25 |
| 4b | 831.0 ± 0.003 | 6b | 396.8 ± 0.002 |
| 4c | 100.3 ± 0.001 | 6c | 45.2 ± 0.003 |
| 4d | 31.2 ± 0.001 | 6d | 23.3 ± 0.001 |
| 5a | 48610 ± 0.02 | 7a | 61300 ± 0.26 |
| 5b | 914.1 ± 0.009 | 7b | 859.4 ± 0.004 |
| 5c | 100.5 ± 0.001 | 7c | 181.7 ± 0.001 |
| 5d | 33.2 ± 0.001 | 7d | 30.1 ± 0.000 |
The aromatic ring substituent effects on G˙-scavenging activity showed that the amino 6-chromanols exhibited the highest radical scavenging activity, followed by acetylamino, chloro, and finally nitro 6-chromanols at any of the substituent positions. In terms of substituent position, the ortho-disubstituted or ortho-monosubstituted compounds demonstrated higher radical scavenging activities, whereas meta-substituted compounds were less efficient.
The data also showed that the electron-donating substituents (NH2 and NHCOCH3) led to an accelerated G˙-scavenging reaction rate, whereas the electron-withdrawing substituents (Cl and NO2) led to a decreased reaction rate.
Correlation of radical scavenging activity with Hammett and Taft substituent constants for 6-chromanol derivatives
The Hammett sigma constant is one of the most widely used means for studying and interpreting organic reactions and their mechanisms. The Hammett sigma constant (σm) for the position meta to the phenolic OH group, which has been shown to interact with radicals, was used.32–34 The Taft sigma constant (σ*) describes the electronic effect, which includes a substituent's steric effect. In this case, the σ* for substituents in positions ortho to the phenolic OH group were applied.34To identify the electronic effects of all of the substituents in a given compound in each reaction with the series of 6-chromanols and G˙, the sum of the sigma constants was plotted against log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k for the radical scavenging activity at each substituent position. The reaction constants (ρ) were then obtained from the slope values.35 For meta-substituted 6-chromanols, a linear relationship between log
k for the radical scavenging activity at each substituent position. The reaction constants (ρ) were then obtained from the slope values.35 For meta-substituted 6-chromanols, a linear relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k and σ was observed (ρmeta = −3.71, r2 = 0.91). The ortho-monosubstituted 6-chromanols (ρ5-ortho = −0.86, r2 = 0.89; ρ7-ortho = −0.87, r2 = 0.89) and the 5,7-disubstituted 6-chromanols (ρ5,7-diortho = −0.47, r2 = 0.90) also exhibited linear relationships between their σ and G˙ scavenging activities. In all cases, σ versus G˙ scavenging activity showed negative linear slopes with good correlation.
k and σ was observed (ρmeta = −3.71, r2 = 0.91). The ortho-monosubstituted 6-chromanols (ρ5-ortho = −0.86, r2 = 0.89; ρ7-ortho = −0.87, r2 = 0.89) and the 5,7-disubstituted 6-chromanols (ρ5,7-diortho = −0.47, r2 = 0.90) also exhibited linear relationships between their σ and G˙ scavenging activities. In all cases, σ versus G˙ scavenging activity showed negative linear slopes with good correlation.
The magnitude of ρ was compared among substituent positions.32,33 The ρ of meta-substituted 6-chromanols (3.71) was the greatest, indicating that the G˙ scavenging reaction was more sensitive to the inductive substituents. The ρ5-ortho (0.86) and ρ7-ortho (0.87) values were similar in magnitude, suggesting that a substituent at either the 5- or the 7-ortho position has the same effect on the radical scavenging reaction. The ρ5,7-diortho (0.47) showed a smaller value (Fig. 2).
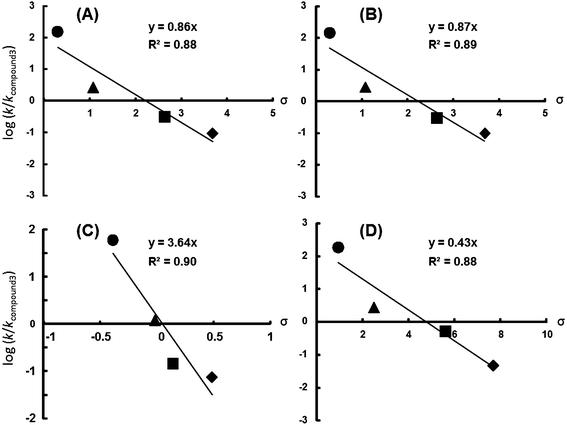 | ||
Fig. 2 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k/k3 versus calculated σ value plots. (A) 5-Substituted 6-chromanols: 4a (●), 4b (▲), 4c (■), 4d (◆); (B) 7-substituted 6-chromanols: 5a (●), 5b (▲), 5c (■), 5d (◆); (C) 8-substituted 6-chromanols: 6a (●), 6b (▲), 6c (■), 6d (◆); (D) 5,7-disubstituted 6-chromanols: 7a (●), 7b (▲), 7c (■), 7d (◆). k3 is presented as k (340.3 M−1 s−1) for compound 3. The σm and the σ* value are quoted from ref. 34. k/k3 versus calculated σ value plots. (A) 5-Substituted 6-chromanols: 4a (●), 4b (▲), 4c (■), 4d (◆); (B) 7-substituted 6-chromanols: 5a (●), 5b (▲), 5c (■), 5d (◆); (C) 8-substituted 6-chromanols: 6a (●), 6b (▲), 6c (■), 6d (◆); (D) 5,7-disubstituted 6-chromanols: 7a (●), 7b (▲), 7c (■), 7d (◆). k3 is presented as k (340.3 M−1 s−1) for compound 3. The σm and the σ* value are quoted from ref. 34. | ||
Discussion
A series of 6-chromanol derivatives containing amino, acetylamino, chloro, or nitro groups were synthesized, and the effects of the substituents and their position on G˙ scavenging activity were investigated.Phenolic compounds scavenge oxyl radicals via the following two primary mechanisms: one-step hydrogen atom transfer (HAT), and electron transfer followed by proton transfer (ET-PT).36 One-step HAT involves the abstraction of a hydrogen atom from the phenolic hydroxyl group to form a phenoxyl radical. By this mechanism, the O–H bond dissociation is homolytic, and the reaction is driven by BDE. In contrast to HAT, ET-PT involves the loss of an electron followed by the exothermic loss of a proton and formation of a radical cation. ET-PT is driven by IP.36
We report that BDE of chlorinated 6-chromanols (4c, 5c, 6c, 7c) correlate with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k in G˙ scavenging reactions, indicating that the hydrogen atom transfer mechanism is the rate-determining step in these reactions.37,38 Moreover, high radical scavenging activity of aminochromanols (4a, 5a, 6a) related with the high stability of the corresponding phenoxyl radicals.30
k in G˙ scavenging reactions, indicating that the hydrogen atom transfer mechanism is the rate-determining step in these reactions.37,38 Moreover, high radical scavenging activity of aminochromanols (4a, 5a, 6a) related with the high stability of the corresponding phenoxyl radicals.30
At any substituent position, all of the 6-chromanols showed G˙ scavenging activity, with increasing k values for substituents in the following order: amino, acetylamino, chloro, and then nitro groups. The data indicated that 6-chromanols with electron-donating substituents significantly increased G˙ scavenging activity. Furthermore, the introduction of an electron-donating substituent at a position ortho to the phenolic OH group in the chroman ring has been reported to significantly enhance G˙ scavenging activity. These data agree well with our previous report.30
Many studies have reported that the hydrogen atom abstraction rates for a series of meta- or para-substituted phenols linearly correlate with the BDE or Hammett sigma constant (σ).22,23,25,27,39 In this study, the sums of σm and σ* were used to investigate the relationship between the effects of substituents in 6-chromanols and the radical scavenging activity of the compounds. σ was plotted against log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k. For many reactions, a series of log
k. For many reactions, a series of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k values is linearly related to the respective σ values. By contrast, a number of reactions have been shown to deviate from this linearity.40,41 Nonlinear Hammett plots are due to a variation in transition state or reaction mechanism for a series of compounds with different substituents. In this study, nonlinear relationships between the σ value and log
k values is linearly related to the respective σ values. By contrast, a number of reactions have been shown to deviate from this linearity.40,41 Nonlinear Hammett plots are due to a variation in transition state or reaction mechanism for a series of compounds with different substituents. In this study, nonlinear relationships between the σ value and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k are possible. However, calculated BDEs in phenols are linearly related to σ values.22,23,25,27,39
k are possible. However, calculated BDEs in phenols are linearly related to σ values.22,23,25,27,39
The linear slope of σ value versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k for each substituent position was obtained. The reaction constants for the G˙ scavenging reactions were extrapolated from the slopes (ρ).32,33 The ρ value magnitudes reveal the susceptibility of a reaction to substituent effects. The ρmeta value was the greatest, indicating that the radical scavenging reaction was highly sensitive to the inductive substituent effect at the meta position. Even though the ortho-substituted 6-chromanols demonstrated high radical scavenging activity, the ρ value magnitudes were low. The magnitude of ρ depends on several factors, including temperature, solvent, and transition state stability.41–44 The 5- and 7-aminochromanoxyl radicals have been detected by ESR,30 indicating that their intermediates are highly stable at room temperature (half-life of 43 min). These data agreed with our results and explained that the low magnitude of ρ was caused by the stability of the intermediate radical. A relatively small ρ value reflects high 6-chromanol derivative reactivity. Therefore, the radical reactions of the ortho-monosubstituted and disubstituted 6-chromanols are largely dependent on the stabilities of the intermediates to increase radical scavenging activity.
k for each substituent position was obtained. The reaction constants for the G˙ scavenging reactions were extrapolated from the slopes (ρ).32,33 The ρ value magnitudes reveal the susceptibility of a reaction to substituent effects. The ρmeta value was the greatest, indicating that the radical scavenging reaction was highly sensitive to the inductive substituent effect at the meta position. Even though the ortho-substituted 6-chromanols demonstrated high radical scavenging activity, the ρ value magnitudes were low. The magnitude of ρ depends on several factors, including temperature, solvent, and transition state stability.41–44 The 5- and 7-aminochromanoxyl radicals have been detected by ESR,30 indicating that their intermediates are highly stable at room temperature (half-life of 43 min). These data agreed with our results and explained that the low magnitude of ρ was caused by the stability of the intermediate radical. A relatively small ρ value reflects high 6-chromanol derivative reactivity. Therefore, the radical reactions of the ortho-monosubstituted and disubstituted 6-chromanols are largely dependent on the stabilities of the intermediates to increase radical scavenging activity.
There is a possibility that radical scavengers can be developed as anti-inflammatory agents and disease chemopreventive agents.45 As our 6-chromanols showed positive radical scavenging activities, a protecting group will be introduced into a phenolic hydroxyl group and/or amino groups, and then their cytotoxicity will be evaluated.
Conclusion
For a series of substituted 6-chromanols, amino-6-chromanols substituted at ortho positions demonstrated the highest radical scavenging activities. Additionally, Hammett or Taft sigma constants can be used to predict the radical scavenging activities of substituted 6-chromanols to design new antioxidants based on chromanols.References
- J. M. Zingg and A. Azzi, Curr. Med. Chem., 2004, 11(9), 1113–1133 CrossRef CAS.
- R. Brigelius-Flohé, Nutr. Res. Rev., 2006, 19(2), 174–186 CrossRef PubMed.
- E. Niki and M. G. Traber, Ann. Nutr. Metab., 2012, 61(3), 207–212 CrossRef CAS PubMed.
- V. D. Kancheva and O. T. Kasaikina, Curr. Med. Chem., 2013, 20(37), 4784–4805 CrossRef CAS.
- G. W. Burton and K. U. Ingold, Acc. Chem. Res., 1986, 19, 194–201 CrossRef CAS.
- E. Niki and N. Noguchi, Acc. Chem. Res., 2004, 37, 45–51 CrossRef CAS PubMed.
- J. Tsuchiya, E. Niki and Y. Kamiya, Bull. Chem. Soc. Jpn., 1983, 56, 229–232 CrossRef CAS.
- (a) L. R. C. Barclay, M. R. Vinqvist, K. Mukai, S. Itoh and H. Motimoto, J. Org. Chem., 1993, 58, 7416–7420 CrossRef CAS; (b) K. Mukai, Y. Kageyama, T. Ishida and K. Fukuda, J. Org. Chem., 1989, 54, 552–556 CrossRef CAS; (c) W. Gregor, G. Grabner, C. Adelwohrer, T. Rosenau and L. Gille, J. Org. Chem., 2005, 70, 3472–3483 CrossRef CAS PubMed; (d) G. W. Burton, T. Doba, E. J. Gabe, L. Hughes, F. L. Lee, L. Prasad and K. U. Ingold, J. Am. Chem. Soc., 1985, 107, 7053–7065 CrossRef CAS.
- (a) D. Shanks, R. Amorai, M. Fumo, F. Pedulli, L. Valgimigli and L. Engman, J. Org. Chem., 2006, 71, 1033–1038 CrossRef CAS PubMed; (b) N. Al-Maharik, L. Engman, J. Malmstrom and C. H. Schiesser, J. Org. Chem., 2001, 66, 6286–6290 CrossRef CAS PubMed.
- (a) V. N. Povalishev, G. I. Polozov and O. I. Shadyro, Bioorg. Med. Chem. Lett., 2006, 16, 1236–1239 CrossRef CAS PubMed; (b) R. Amorati, A. Cavalli, M. Fumo, M. Masetti, S. Menichetti, C. Pagliuca, G. F. Pedulli and C. Viglianisi, Chem.–Eur. J., 2007, 13(29), 8223–8230 CrossRef CAS PubMed.
- L. Bamonti, T. Hosoya, K. F. Pirker, S. Böhmdorfer, F. Mazzini, F. Galli, T. Netscher, T. Rosenau and L. Gille, Bioorg. Med. Chem., 2013, 21(17), 5039–5046 CrossRef CAS PubMed.
- R. Apak, S. Gorinstein, V. Böhm, K. M. Schaich, M. Özyürek and K. Güçlü, Pure Appl. Chem., 2013, 85(5), 957–998 CrossRef CAS , (IUPAC Technical Report).
- W. Brand-Williams, M. E. Cuvelier and C. Berset, LWT–Food Sci. Technol., 1994, 28(1), 25–30 CrossRef.
- M. Lucarini and G. F. Pedulli, Chem. Soc. Rev., 2010, 39(6), 2106–2119 RSC.
- Y. Kadoma, T. Atsumi, N. Okada, M. Ishihara, I. Yokoe and S. Fujisawa, Molecules, 2007, 12(2), 130–138 CrossRef CAS.
- M. C. Foti, C. Daquino, I. D. Mackie, G. A. DiLabio and K. U. Ingold, J. Org. Chem., 2008, 73(23), 9270–9282 CrossRef CAS PubMed.
- N. Nenadis, L. F. Wang, M. Z. Tsimidou and H. Y. Zhang, J. Agric. Food Chem., 2005, 53(2), 295–299 CrossRef CAS PubMed.
- S. Fujisawa, M. Ishihara, Y. Murakami, T. Atsumi, Y. Kadoma and I. Yokoe, In Vivo, 2007, 21(2), 181–188 CAS.
- P. Mulder, H. G. Korth, D. A. Pratt, G. A. DiLabio, L. Valgimigli, G. F. Pedulli and K. U. Ingold, J. Phys. Chem. A, 2005, 109(11), 2647–2655 CrossRef CAS PubMed.
- R. Bosque and J. Sales, J. Chem. Inf. Comput. Sci., 2003, 43(2), 637–642 CrossRef CAS PubMed.
- C. X. Xue, R. S. Zhang, H. X. Liu, X. J. Yao, M. C. Liu, Z. D.Hu and B. T. Fan, J. Chem. Inf. Comput. Sci., 2004, 44(2), 669–677 CrossRef CAS PubMed.
- M. Lucarini, P. Pedrielli, G. F. Pedulli, S. Cabiddu and C. Fattuoni, J. Org. Chem., 1996, 61(26), 9259–9263 CrossRef CAS.
- D. J. V. A. dos Santos, A. S. Newton, R. Bernardino and R. C. Guedes, Int. J. Quantum Chem., 2008, 108(4), 754–761 CrossRef CAS PubMed.
- J. S. Wright, E. R. Johnson and G. A. DiLabio, J. Am. Chem. Soc., 2001, 123(6), 1173–1183 CrossRef CAS PubMed.
- Y. Luo, Int. J. Chem. Kinet., 2002, 34(8), 453–466 CrossRef CAS PubMed.
- J. S. Wright, D. J. Carpenter, D. J. McKay and K. U. Ingold, J. Am. Chem. Soc., 1997, 119(18), 4245–4252 CrossRef CAS.
- M. Najafi, K. H. Mood, M. Zahedi and E. Klein, Comput. Theor. Chem., 2011, 969(1–3), 1–12 CrossRef CAS PubMed.
- J. L. G. Nilsson, H. Sievertsson and H. Selander, Acta Chem. Scand., 1968, 22, 3160–3170 CrossRef CAS PubMed.
- K. Inami, Y. Iizuka, M. Furukawa, I. Nakanishi, K. Ohkubo, K. Fukuhara, S. Fukuzumi and M. Mochizuki, Bioorg. Med. Chem., 2012, 20(13), 4049–4055 CrossRef CAS PubMed.
- K. Inami, I. Nakanishi, M. Morita, M. Furukawa, K. Ohkubo, S. Fukuzumi and M. Mochizuki, RSC Adv., 2012, 2(33), 12714–12717 RSC.
- N. Gotoh, K. Shimizu, E. Komuro, J. Tsuchiya, N. Noguchi and E. Niki, Biochim. Biophys. Acta, 1992, 1128(2–3), 147–154 CAS.
- P. S. Marrs, J. Chem. Educ., 2001, 78(4), 527–529 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91(2), 165–195 CrossRef CAS.
- G. Gokel, Deans Handbook of Organic Chemistry, 2nd edn, McGraw-Hill, New York, 2004, Section 7 Search PubMed.
- B. A. Hathaway and B. Olesen, J. Chem. Educ., 1993, 70(11), 953–955 CrossRef CAS.
- E. Anouar, C. A. Calliste, P. Košinová, F. D. Meo, J. L. Duroux, Y. Champavier, K. Marakchi and P. Trouillas, J. Phys. Chem. A, 2009, 113(50), 13881–13891 CrossRef CAS PubMed.
- I. Nakanishi, T. Kawashima, K. Ohkubo, H. Kanazawa, K. Inami, M. Mochizuki, K. Fukuhara, H. Okuda, T. Ozawa, S. Itoh, S. Fukuzumi and N. Ikota, Org. Biomol. Chem., 2005, 3(4), 549–704 Search PubMed.
- I. Nakanishi, K. Fukuhara, T. Shimada, K. Ohkubo, Y. Iizuka, K. Inami, M. Mochizuki, S. Urano, S. Itoh, N. Miyata and S. Fukuzumi, J. Chem. Soc., Perkin Trans. 2, 2002, 1520–1524 RSC.
- T. Yoshida, K. Hirozumi, M. Harada, S. Hitaoka and H. Chuman, J. Org. Chem., 2011, 76(11), 4564–4570 CrossRef CAS PubMed.
- J. O. Schreck, J. Chem. Educ., 1971, 48(2), 103–107 CrossRef CAS.
- I. Um, L. Im, E. Kim and J. Shin, Org. Biomol. Chem., 2010, 8(16), 3801–3806 CAS.
- J. E. Hodgkins and E. D. Megarity, J. Am. Chem. Soc., 1965, 87(23), 5322–5326 CrossRef CAS.
- P. Mulder, O. W. Saastad and D. Griller, J. Am. Chem. Soc., 1988, 110(12), 4090–4092 CrossRef CAS.
- L. Valgimigli, K. U. Ingold and J. Lusztyk, J. Am. Chem. Soc., 1996, 118(15), 3545–3549 CrossRef CAS.
- Q. Jiang, Free Radical Biol. Med., 2014, 72, 76–90 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra05782a |
| This journal is © The Royal Society of Chemistry 2014 |