Formation of dispersed palladium–nickel bimetallic nanoparticles in microemulsions: synthesis, characterization, and their use as efficient heterogeneous recyclable catalysts for the amination reactions of aryl chlorides under mild conditions
Felora Heshmatpour* and
Reza Abazari
Department of Chemistry, Faculty of Science, K.N. Toosi University of Technology, P.O. Box 16315-1618, Tehran, 15418, Iran. E-mail: f.heshmatpour@yahoo.com; R.abazari@sina.kntu.ac.ir; Fax: +98 2122853650; Tel: +98 2123064230
First published on 21st October 2014
Abstract
A simple method for preparing spherical palladium–nickel bimetallic nanoparticles with a molar ratio of 1.0 (Pd0.5–Ni0.5 B-NPs) has been adopted using a water-in-oil microemulsion system of water/aerosol-OT/isooctane at room temperature. The morphology, size, structure, and elemental composition of these particles as the superior catalyst were characterized by field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), energy dispersive analysis using X-rays (EDAX), and X-ray fluorescence (XRF) analyses. The effects of the reaction parameters on the Buchwald–Hartwig amination reaction yield in the presence of these NPs as catalyst have been screened and the results are evaluated by using chlorobenzene and morpholine as a model reaction. Initially, differences in the catalytic properties of some synthesized NPs have been compared. In this context, Pd–Ni B-NPs with the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Pd/Ni = 1) showed the highest catalytic activity for this reaction. The Pd0.5–Ni0.5 B-NPs are stable and the leaching of catalyst in solution is very low. Therefore, they has been employed as efficient catalysts in the C–N coupling reaction. Finally, a mechanism for an amination reaction on the Pd/Ni catalyst surface was proposed.
1 (Pd/Ni = 1) showed the highest catalytic activity for this reaction. The Pd0.5–Ni0.5 B-NPs are stable and the leaching of catalyst in solution is very low. Therefore, they has been employed as efficient catalysts in the C–N coupling reaction. Finally, a mechanism for an amination reaction on the Pd/Ni catalyst surface was proposed.
Introduction
Arylamines and their derivatives are of significant importance in organic chemistry and play an important role as intermediates for pharmaceuticals, biologicals, agrochemicals, conducting polymers, and dyes in the chemical industry.1–7 Aromatic amines have also been demonstrated to be useful as ligands for transition metals.8 Employing aryl chlorides for this reaction has been a focus because aryl chlorides are cheaper and more available than their bromide and iodide counterparts.9–11 Also, it is well known that aromatic chlorides are much less reactive than aromatic bromides and iodides. Therefore, due to the much stronger C–Cl bond in the aromatic ring,9,11,13 the development of effective catalysts is still of great importance.Since the catalytic activity of transition metals is related to their (d) orbital properties, selecting a suitable metal is the first step for selectivity control and synthesis of the organic reactions.14 Aryl halides can be successfully transformed into the corresponding amines catalyzed by transition metals such as palladium,15–19 copper,20–23 nickel,24–26 and cobalt27 with various ligands through cross coupling reaction. Concerning the catalytic metals, palladium as an important tool in industrial and academic research (in different C–C, C–N, and C–O coupling reactions such as Negishi, Kumada, Suzuki Miyaura, Stille, aryl amination etc.) plays the most prominent part in the removal of halogen atom, especially chlorine, from Halogenated organic compounds.28–33 However, application of expensive transition metals, especially some sensitive ligands, in industry has been limited. Moreover, the ligands like phosphine ligands are typically unrecoverable, toxic, air-sensitive, expensive, and degradable at elevated temperatures. In addition, these ligands produce detrimental effects on biological systems,34–36 making the application of this catalytic system limited. A viable solution to limit the application of such expensive metal is to use other less expensive metals besides this metal and to allow for the removal of ligands from the catalytic system.
Another problem is that, most of these reactions are very often incomplete and require relatively high catalyst loadings, high heat, high pressure, strongly basic conditions, and poor recovery of catalyst, which negate the advantages associated with the use of aryl chlorides. In this context, a recent trend can be observed in the literature to evaluate progressively low catalyst loadings and short reaction times while taking the high recovery capability of the catalyst into consideration.37,38
As a result of the growing interest in the application of nano-catalysts in nano-chemistry and nano-technology, many researchers have recently realized the importance of these catalysts in organic reactions.39 Metal NPs are of the greatest importance due to their very unique chemical and physical properties, and they can also cause specific catalytic activities.40–42 According to the literature, several methods have been proposed for metal NPs preparation. Much interest has recently been attached to the synthesis of uniform NPs as new catalysts for organic reactions. Preparation of NPs via the microemulsion gives a proper control of size and composition.43–45 So, this technique is one of the most preferred candidates.
The yield of the catalysts can change by addition of another element to the metal, thus bimetallic NPs create new properties compared to the monometallic NPs.46 Also, since incorporation of a second catalytic metal like Ni and Cu onto the palladium surface produces enhanced coupling reaction rates, we have suggested the use of the Pd–Cu or Pd–Ni B-NPs stabilized by aerosol-OT, which can act as one of the best catalysts in this process. These additives modify the activity, functionality, and selectivity of the catalyst.47–49
In this paper, our initial investigations have focused on the development of an optimum set of reaction under green chemistry conditions. For example, water has clear advantages as a solvent in organic synthesis because it is readily available, cheap, and nontoxic. Also, we report that Pd–Ni B-NPs (at a mole ratio of Pd/Ni = 1), in combination with water as the solvent, is an extremely effective and reusable system for the C–N coupling reactions of aryl chlorides with amines for the Buchwald–Hartwig amination reaction.
Experimental
Materials
The following chemicals were used in the synthesis procedure: palladium(II) acetate (Pd(OAc)2 ≥ 99%, Merck), nickel(II) sulfate hexahydrate (NiSO4·6H2O 99%, Sigma-Aldrich), and copper(II) sulfate pentahydrate (CuSO4·5H2O ≥ 98%, sigma) as precursor salts; dioctyl sulfosuccinate sodium salt (aerosol-OT, AOT 98%, Aldrich) as a surfactant; hydrazine hydrate (N2H4·H2O ≥ 99%, Merck) as a reducing agent; isooctane (≥99%, Merck) as the oil phase; potassium carbonate (K2CO3 ≥99%, Merck) as a base, methanol (≥99.9%, Merck) as a washing agent and solvent; and water used in the experiments was deionized (DI) and doubly distilled prior to use as the aqueous phase in microemulsion system. Substrates were purchased from Merck Company, and were used without further purification or drying.Characterization
The shape (or morphology), size, crystal structure and elemental composition of the Pd–Ni B-NPs as the superior catalyst in this work (with Pd/Ni molar ratio of 1) were characterized by field emission scanning electron microscope (FE-SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), energy dispersive analysis of X-ray (EDAX), and X-ray fluorescence (XRF) analyses. FE-SEM images were obtained on a Hitachi S-1460 field emission scanning electron microscope using accelerating voltage of 15 kV. TEM measurement for Pd0.5–Ni0.5 B-NPs was obtained on a Zeiss EM10C transmission electron microscope using Acc voltage of 100 kV. Usually more than 100 particles from different parts of the grid were used to estimate the average size and size distribution of particles. XRD analysis of the Pd0.5–Ni0.5 B-NPs was carried out using a Philips diffractometer (Model TM-1800). Nickel filtered Cu-Kα radiation source was used to produce X-ray (λ = 1.542 Å), and scattered radiation was measured with a proportional counter detector at a scan rate of 4° per min. The scanning angle was from 25° to 90°, operating at a voltage of 40 kV applying potential current of 30 mA. EDAX spectrum was obtained on JEOL JSM-6380LV scanning electron microscope. The elemental composition of the Pd0.5–Ni0.5 B-NPs was determined by Philips model PW-1480 fluorescence spectrometer (XRF) operating at 4 kW with Rh Ka radiation source. Gas Chromatography (GC) data was recorded on a GC Shimadzu 14B with a SA13-5 capillary column (phenyl methyl siloxane, 30 m × 320 mm × 0.25 mm). The leaching of catalyst in the reaction products was checked using ICP-OES (inductively coupled plasma optical emission spectrometry) with an Agilent 720/730 Series apparatus.Preparation of the catalyst
| 2Pd2+ + N2H5+ + 5OH− → 2Pd0 + N2 + 5H2O | (1) |
| 2Ni2+ + N2H5+ + 5OH− → 2Ni0 + N2 + 5H2O | (2) |
The reaction was allowed to proceed with stirring for 1 h. Then, methanol was added to the beaker to make phase separation and to wash the solution. The final mixture was centrifuged to get samples. Finally, the synthesized NPs were characterized by FE-SEM analyses.
General procedure of the Buchwald–Hartwig amination reaction of chlorobenzene with morpholine
In a 25 mL two-necked flask, the mixture of chlorobenzene (2 mmol), morpholine (2.2 mmol), potassium carbonate (3 mmol) and the nano-catalyst (4.5 × 10−4 mmol) were added in methanol (5 mL). The flask was sealed and the mixture was allowed to stir in a preheated oil bath at 60 °C for the appropriate time. The reactions were monitored by thin layer chromatography (TLC). After the reaction was completed, the reaction mixture was then cooled at room temperature. The solid nano-catalyst was separated by centrifuge and washed with water to remove base and salt. The remaining solution was then washed with acetone to remove adsorbed organic substrate. Finally, the prepared samples were analyzed by GC.Results and discussion
Analysis of Pd0.5–Ni0.5 B-NPs
The property which is very evident for metal NPs after the addition of the reducing agent is their color change which indicates that the metal NPs are reduced. For instance, the color of Pd0.5–Ni0.5 B-NPs was changed immediately from brown into black after the addition of hydrazine hydrate. This change in the color before and after reduction process is shown in Fig. 1. | ||
| Fig. 1 Digital photograph of Pd0.5–Ni0.5 B-NPs before (right-hand) and after (left-hand) reduction with hydrazine hydrate. | ||
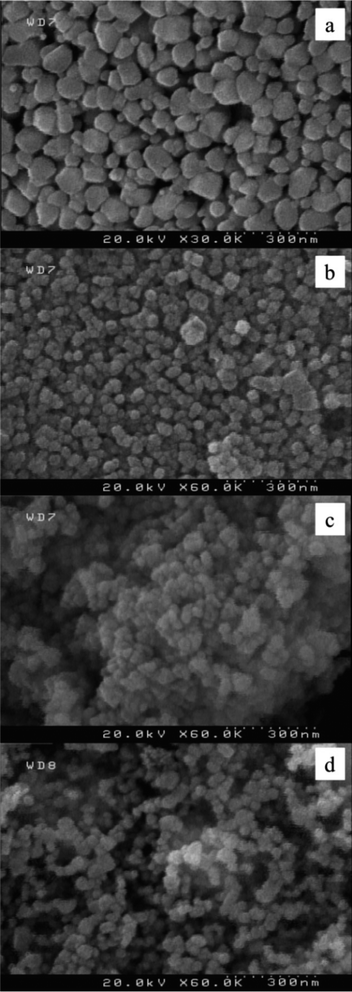
The first question relating to the nano-materials is to investigate the aggregation state, size, shape, and morphology of the particles. The preparation conditions affect the structure, shape (or morphology), and size distribution of the nanostructures. As previously mentioned (in the introduction), if the nanostructures are synthesized using the reverse microemulsion, then the size and composition of the structures can properly be controlled. In view of this, first the surface morphology of the synthesized NPs (Ni, Pd, Pd–Cu, and Pd–Ni) has been investigated using FE-SEM analysis – as shown in Fig. 2. Morphology of NPs is assumed to be a function of the reaction parameters (i.e. the surfactant ratio, water concentration, time, temperature, and precursor). In preparation of the NPs, stabilizers such as surfactants are applied to prevent the aggregation. Moreover, these surfactants act as surface ligands and have the ability to control the shape and size of the growing particles. FE-SEM image indicates that the synthesized NPs are approximately uniform and spherical in shape and with some agglomeration. However, a few larger loose aggregates were also observed which may be due to aggregation during the washing steps and to the quite high calcination temperature.
For further investigation of the Pd0.5–Ni0.5 B-NPs (as the superior catalyst in this study), the structure, size and elemental composition of the considered NPs were characterized using TEM, XRD, XRF, and EDAX analyses. In the first step, for a better understanding of the structural and morphological characteristics of Pd0.5–Ni0.5 B-NPs, these particles have also been investigated through the TEM analysis (in addition to the FE-SEM analysis). They give comparable information for morphology and size investigations. Among the most frequently used techniques, TEM analysis is indispensable for the nanostructures study.
Fig. 3a and b shows a typical TEM image and the particle size distribution histogram for the Pd0.5–Ni0.5 B-NPs. As can be seen, the particles have spherical shape, highly monodispersed, and all the particles exhibit uniform size and morphology, which is in good agreement with the FE-SEM result. Also, the edges of the NPs are clearly seen without any agglomeration. Recent literature has shown that synthesizing uniform NPs for organic reactions has attracted much attention. The mean diameter and standard deviation were calculated by counting over 100 particles from a TEM image at ×450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 magnification.
000 magnification.
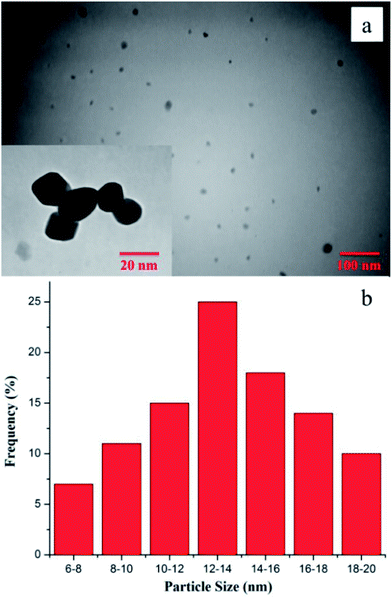 | ||
| Fig. 3 TEM image (a) and the particle size distribution histogram (b) of spherical Pd0.5–Ni0.5 B-NPs. | ||
It can be observed from TEM micrograph that these Pd0.5–Ni0.5 B-NPs are with a nearly narrow size distribution of average diameter in the range of 6–20 nm. To be precise, TEM is a more suitable and powerful tool for determining the morphology and size of the NPs. Consequently, due to the fact that the particle morphology may change in terms of the synthesis conditions and since the particles in the nano-scale include size-dependent properties, it is essential to adopt a reliable method for development of uniform and de-agglomerated particles.
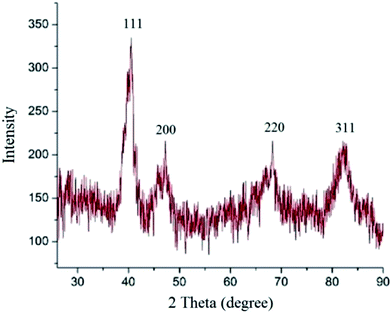
X-Ray diffraction is an effective method for investigation of the solid structure of nanostructures and to confirm the phase purity. Because crystal structure is closely related to the properties of the particles, it is important to investigate their crystallinity. Considerable effort has been devoted to nanostructure studies, i.e. core–shell and alloy particles, because of their valuable properties which make them useful for composition-dependent catalysis applications etc. Therefore, crystallinity, crystallite phase and mean size of the particles were further characterized by the XRD analysis. Fig. 4 shows the phase composition of Pd0.5–Ni0.5 catalyst by the XRD analysis. The pattern can be well indexed without any trances of impurity phase, conforming that the sample is single phase. The phase was identified by comparison with the Joint Committee on Powder Diffraction Standards (JCPDSs). Because of the different peak positions of Pd, Ni and Pd–Ni bimetallic in the XRD pattern, it is very effective to employ the XRD analysis for the determination of the Pd0.5–Ni0.5 B-NPs structure. The characteristic peaks for Pd (JCPDS number of card, 05-0681; 2θ = 40°, 46.7°, 68°, and 82°) and those for Ni (JCPDS number of card, 04-0850; 2θ = 44.5°, 51.8°, and 76.4°), marked by their indices ((111), (200), (220), and (311)), demonstrate the presence of the face-centered cubic (fcc) Pd and Ni crystallite planes. The XRD pattern of the Pd0.5–Ni0.5 catalyst in Fig. 4 appears at 2θ values of 40.36°, 47.20°, 68.28°, and 82.46° corresponding to the planes (111), (200), (220), and (311) with fcc structure. This issue indicates that a portion of Ni has entered the Pd lattice, forming the Pd–Ni alloy phase.
Using the Debye–Scherrer equation (i.e. D = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), the Pd–Ni NPs mean particles size (D) are calculated to be 17 nm. As a result, the average crystallite size calculated from the peaks broadening in the XRD pattern is consistent with particle size obtained from the TEM micrograph. This partial difference may be accounted for by the fact that XRD represents a global picture while TEM depicts a local feature. In this equation, D is the mean particle size, k is the so-called shape factor, which usually takes a value of about 0.9, λ is the wavelength of X-ray source used in XRD, β is the full width in radians at half-maximum intensity (FWHM), and θ is the angle at maximum diffraction curve intensity.
θ), the Pd–Ni NPs mean particles size (D) are calculated to be 17 nm. As a result, the average crystallite size calculated from the peaks broadening in the XRD pattern is consistent with particle size obtained from the TEM micrograph. This partial difference may be accounted for by the fact that XRD represents a global picture while TEM depicts a local feature. In this equation, D is the mean particle size, k is the so-called shape factor, which usually takes a value of about 0.9, λ is the wavelength of X-ray source used in XRD, β is the full width in radians at half-maximum intensity (FWHM), and θ is the angle at maximum diffraction curve intensity.
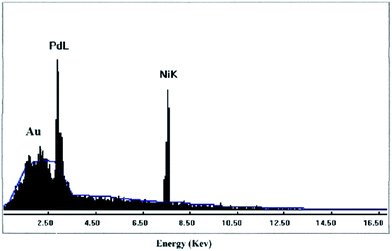
XRF and EDAX studies were done to fully analyze the elemental composition of the Pd0.5–Ni0.5 B-NPs. XRF analysis is one of the most important techniques for the analysis of metals and trace elements, which is independent of the chemical form of the element. For this reason, to confirm the existence of Pd and Ni species, Pd0.5–Ni0.5 B-NPs is examined by XRF analysis as the superior catalyst for the Buchwald–Hartwig amination reaction. The element analysis shows the molar ratio of Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni is 53.13
Ni is 53.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46.87, this is very close to the designed molar ratio (theoretical value), suggesting that almost all elements have been involved into the final products.
46.87, this is very close to the designed molar ratio (theoretical value), suggesting that almost all elements have been involved into the final products.
Fig. 5 shows the chemical composition of the Pd0.5–Ni0.5 B-NPs determined by EDAX analysis, which reveals only palladium and nickel elements existed in the products with the molar ratio of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Pd
1 (Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 55.68
Ni = 55.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44.32), which agrees well with the XRF result (the presence of signal of Au can be ascribed to the Au grid). In other words, no elements other than Pd and Ni are detected which confirm the purity of the Pd0.5–Ni0.5 B-NPs synthesized in the present work. In addition, the absence of sulfur and sodium atoms indicated that aerosol-OT and sodium salts were no more present. Therefore, the prepared Pd0.5–Ni0.5 B-NPs were free of impurities under the current synthetic route. Also, it coincides with the above conclusion of the formation of alloy between Pd and Ni from XRD results.
44.32), which agrees well with the XRF result (the presence of signal of Au can be ascribed to the Au grid). In other words, no elements other than Pd and Ni are detected which confirm the purity of the Pd0.5–Ni0.5 B-NPs synthesized in the present work. In addition, the absence of sulfur and sodium atoms indicated that aerosol-OT and sodium salts were no more present. Therefore, the prepared Pd0.5–Ni0.5 B-NPs were free of impurities under the current synthetic route. Also, it coincides with the above conclusion of the formation of alloy between Pd and Ni from XRD results.
Optimization of the reaction conditions
First, for optimization of the reaction conditions, a series of catalyst sources with different molar ratios and loading of the catalysts were investigated in the Buchwald–Hartwig amination reaction of chlorobenzene with morpholine as a model reaction. Since most papers in the literature indicate that the use of the metals such as Pd, Cu, and Ni in different catalytic systems can activate the C–X bond (X = Cl, Br, and I) in the coupling reactions, we have investigated these metals as bimetallic NPs in our system. For practical application in the Buchwald–Hartwig amination reaction, the lifetime, thermal stability and reusability of the employed catalysts are very important factors. Furthermore, the ability to use relatively low catalyst loadings is most significant on process development and manufacturing scales in order to reduce the cost of the catalyst and to ease the obtainment of catalyst separated from the product for catalyst reusability. When metal salts were used, results were not acceptable (entries 1 and 2). However, comparing the catalytic activity of Pd–Cu and Pd–Ni (as the B-NPs) with Pd and Ni (as the monometallic NPs) at 60 °C for 8 h (entries 3–6), it was observed that using B-NPs and especially the Pd0.5–Ni0.5 B-NPs, the rate and activity of the reaction increased. In other words, the incorporation of Ni and Cu to Pd enhances catalytic activity, compared to the Pd and Ni monometallic NPs. This high catalytic activity can be due to the charge transfer effect between different metals of these NPs, which can cause to minimize the activation energy of the C–N bond in the coupling reactions. Since, when a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of the bimetallic NPs was applied (entries 5 and 6) and Pd–Ni NPs showed the highest product yield, different molar ratios of this catalyst were investigated (entries 7 and 8). When this molar ratio changed to 1
1 of the bimetallic NPs was applied (entries 5 and 6) and Pd–Ni NPs showed the highest product yield, different molar ratios of this catalyst were investigated (entries 7 and 8). When this molar ratio changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Pd–Ni), the reaction yield decreased (entry 7). To continue our investigations, in spite of the fact that a molar ratio of Pd to Ni as 2
2 (Pd–Ni), the reaction yield decreased (entry 7). To continue our investigations, in spite of the fact that a molar ratio of Pd to Ni as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 slightly increases the product yield compared to the time when this ratio is as 1
1 slightly increases the product yield compared to the time when this ratio is as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 6 and 8), we chose the same ratio of 1
1 (entries 6 and 8), we chose the same ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the catalyst as the optimal catalyst since we sought to exploit the more cost-efficient conditions for the catalyst involved (Table 1).
1 for the catalyst as the optimal catalyst since we sought to exploit the more cost-efficient conditions for the catalyst involved (Table 1).
| Entry | Catalyst | mmol × 10−4 | Yieldb (%) |
|---|---|---|---|
| a Reaction condition: chlorobenzene (2 mmol), morpholine (2.2 mmol), Cs2CO3 (3 mmol), MeOH (5 mL), 60 °C, 8 h.b Yields was determined by GC, which was then compared to an internal standard.c Yield is an average of two runs. | |||
| 1 | Pd(OAc)2 | 6.8 | 13 |
| 2 | Pd(OAc)2 + Ni(OAc)2·4H2O | 6.8 | 19 |
| 3 | Pd | 6.8 | 60 |
| 4 | Ni | 6.8 | 34 |
| 5 | Pd0.5–Cu0.5 | 6.8 | 67 |
| 6 | Pd0.5–Ni0.5 | 6.8 | 80 |
| 7 | Pd0.5–Ni1 | 6.8 | 51 |
| 8 | Pd1–Ni0.5 | 6.8 | 83 |
| 9 | Pd0.5–Ni0.5 | 9 | 82 |
| 10c | Pd0.5–Ni0.5 | 4.5 | 78 |
| 11 | Pd0.5–Ni0.5 | 3.2 | 55 |
| 12 | None | — | Trace |
Recent studies have shown that spherical shape and monodispersity of the particles can exhibit excellent catalytic performances when used as catalyst in the organic reactions due to their high volume specific capacity. As shown in Fig. 2, the spherical shape of the particles in (a–c) is less than those in (d). Moreover, there seems to be a better uniformity among the size and shape of the particles in (d) compared with (a–c). For example, in Fig. 2c which is related to the sample Pd0.5–Cu0.5 B-NPs, there are quasi-spherical particles with less monodispersity. Also, in the Ni NPs (a), the particles became more disordered and no more spherical, and also agglomeration increased among the particles. As a result, among the different samples, Pd0.5–Ni0.5 B-NPs had good homogeneity and appropriate separation with more spherical geometries. Consequently, in combination with the relationship between catalytic activity and particles morphology, it can be foreseen that sample with the highest spherical shape results in the highest catalytic activity. Also, it means that there is a direct relation between the catalytically activity and the uniformity among the shape of the particles for the samples.
In an effort to evolve the minimum acceptable loading of a catalyst for C–N bond in the coupling reaction using the preferred Pd0.5–Ni0.5 B-NPs, the reactions with different amounts of the loading of this catalyst was carried out at 60 °C for 8 h (entries 9–11). An increase (from 6.8 × 10−4 to 9 × 10−4 mmol) in the concentration of the catalyst did not result in the increase in the yield (entries 6 and 9) and indeed, further increased amount of the catalyst lead to slight increase in the percentage of yield (from 80% to 82%). When the catalytic amount decreased to 4.5 × 10−4 mmol, the product yield was 78% (entry 10), which is a negligible decrease in relation to 4.5 × 10−4 mmol reduction in the amount of the catalyst from 82% to 78% (entries 9 and 10). In other words, by reducing this amount to 4.5 × 10−4 mmol, the result was still satisfactory. But, the yield of reaction was observed to decrease with a decrease in catalyst loading from 4.5 × 10−4 mmol to 3.2 × 10−4 mmol (entries 11). Therefore, the minimum acceptable amount of the catalyst to be used in our system is 4.5 × 10−4 mmol. To illustrate the importance and role of this catalyst in this reaction, an experiment was conducted in the absence of the said catalyst. According to the observations, only a slight conversion was obtained after 8 h (entry 12).
The practical application of catalysts to the organic reactions is always accompanied by the problem of the deactivation of the catalyst and few effective general methods have been reported in the literature. Therefore, in practical use, developing catalysts that keep their catalytic activity for a prolonged time is a major problem.50–52 Great importance is attached to these aspects, and so a number of examples exist in the literature that report recycling experiments.53 In the present work, we have studied the potential recycling process of Pd0.5–Ni0.5 B-NPs stabilized by aerosol-OT as the superior catalyst. In order to evaluate the catalytic life of the said catalysts, the C–N coupling reaction was repeated several times with the catalyst recovered after each reaction and the reuse experiments were repeated until lower yield was observed. One of the major advantages of the heterogeneous catalyst is the convenient separation of the catalyst from the reaction mixture. This approach is interesting from an industrial point of view, because this gives an opportunity for lowering of the production costs. Recycle efficiency and catalytic stability of this catalyst up to seven runs at 60 °C (in the Buchwald–Hartwig amination reaction of chlorobenzene with morpholine) is shown in Fig. 6.
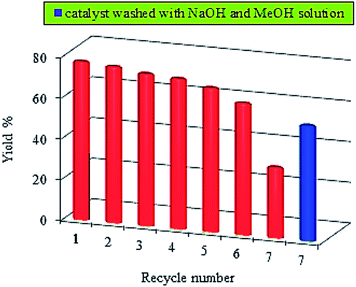 | ||
| Fig. 6 Yield of the recycle experiments by using Pd0.5–Ni0.5 B-NPs as catalyst in the amination reaction with chlorobenzene and morpholine as a model reaction at 60 °C for 8 h. | ||
Before the next run, the catalyst system was easily separable-by centrifugation-from the reaction mixture by decantation of the liquid phase, and then the catalyst was washed with diethyl ether, dried at 50 °C, and was used for a new run. Each reaction was run for 8 h, which was the necessary time for completing the reaction. When this treatment was performed, almost no significant loss of catalytic activity was observed after six runs. However, in the seventh run, a decrease in the yield was observed, i.e., a 43% decrease compared with the first run. These results can be attributed to the decreasing amount of Pd–Ni catalyst versus the number of cycles, which shows that the contribution of Pd–Ni catalyst in the Buchwald–Hartwig amination reaction is important.
Also, this can be explained by FE-SEM analysis which shows that the Pd0.5–Ni0.5 B-NPs agglomerated during the reaction (after seven recycling) which would lead to lower availability of catalytic sites (Fig. 7a). Moreover, according to the TEM image (Fig. 7b), the particles became larger, their shape became more disordered, and their separation decreased more. However, as shown in Fig. 7c, the particles structure of the Pd0.5–Ni0.5 B-NPs was slightly changed. X-ray diffraction analysis was further used to identify the crystal structure of the Pd0.5–Ni0.5 B-NPs after the catalytic reaction.
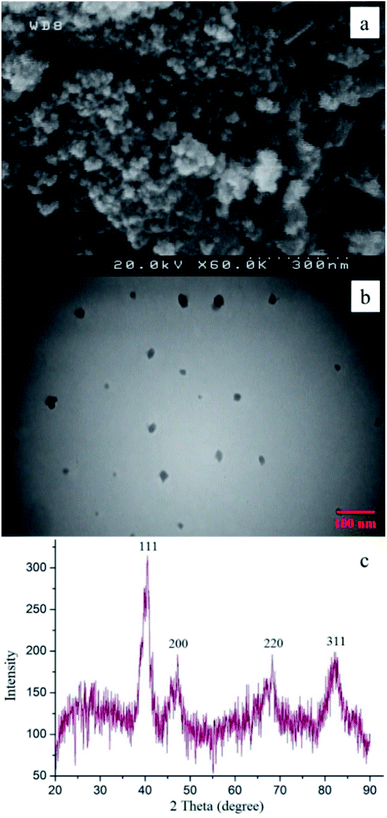 | ||
| Fig. 7 FE-SEM image (a), TEM image (b), and X-ray diffraction spectrum (c) of Pd0.5–Ni0.5 B-NPs after the seventh run in the C–N coupling reaction between chlorobenzene and morpholine. | ||
Another reason for such a decrease in the catalytic activity is probably due to the accumulation of produced salts on the catalyst surface and/or surface deactivation with the produced HCl. The accumulated salts on the catalytic surface can be removed by washing with diethyl ether or water. The addition of bases such as NaOH and NH3 is effective in suppressing the deactivation and causes the catalysts to restore the activity, since the halogen ions responsible for the poisoning are removed from the catalyst surface.54–56 In view of this, in our experiment, after the Pd0.5–Ni0.5 catalyst was used in the sixth run, it was washed with a solution of NaOH (at a NaOH-to-catalyst molar ratio of 2.0) in MeOH (5 mL) at room temperature for 10 min. In the seventh run, the yield after this treatment increased from 35% to 57% which is probably because the catalyst surface became cleaner and/or reduced-as shown in Fig. 6 in a blue bar. In brief, this reveals the excellent stability and recyclability of the catalyst Pd0.5–Ni0.5 stabilized by aerosol-OT in the absence of organic and inorganic supports while no ligands exist.
In the next step, we investigated the effect of various bases, solvents, reaction temperatures, and reaction time on the yields of the C–N coupling reaction between chlorobenzene and morpholine. The detailed results are listed in Table 2. Bases influenced the progress of the reaction greatly. As shown in Table 2, entries 1–6, the yield of the C–N coupling reaction by Pd0.5–Ni0.5 B-NPs as catalyst is highly dependent on the base. Using MeOH as solvent at 60 °C for 8 h, NEt3 gives the lowest yield while NaOtBu and K3PO4 also give low yields. Cs2CO3 gives a fairly good yield and among the bases explored, K2CO3 gave the best result (entry 3). Consequently, bases perform an essential role in the Buchwald–Hartwig amination reaction due they can accelerate and regenerate catalysts. Also, in order to regenerate catalyst for coupling reactions, bases are required to neutralize and remove HX from H-Cat-X intermediate. In this context, the reaction with no base yielded only a small amount of product (entry 6).
| Entry | Base | Solvent | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: chlorobenzene (2 mmol), morpholine (2.2 mmol), base (3 mmol), solvent (5 mL), Pd0.5–Ni0.5 B-NPs (4.5 × 10−4 mmol).b Yields was determined by GC, which was then compared to an internal standard. | |||||
| 1 | Cs2CO3 | MeOH | 60 | 8 | 78 |
| 2 | K3PO4 | MeOH | 60 | 8 | 55 |
| 3 | K2CO3 | MeOH | 60 | 8 | 85 |
| 4 | NEt3 | MeOH | 60 | 8 | 27 |
| 5 | NaOtBu | MeOH | 60 | 8 | 34 |
| 6 | None | MeOH | 60 | 8 | Trace |
| 7 | K2CO3 | 1,4-Dioxane | 60 | 8 | 37 |
| 8 | K2CO3 | DMSO | 60 | 8 | 53 |
| 9 | K2CO3 | THF | 60 | 8 | 49 |
| 10 | K2CO3 | H2O | 60 | 8 | 83 |
| 11 | K2CO3 | DMF | 60 | 8 | 87 |
| 12 | K2CO3 | CH3CN | 60 | 8 | 56 |
| 13 | K2CO3 | n-Hexane | 60 | 8 | Trace |
| 14 | K2CO3 | H2O | 50 | 8 | 73 |
| 15 | K2CO3 | H2O | 80 | 8 | 95 |
| 16 | K2CO3 | H2O | 90 | 8 | 96 |
| 17 | K2CO3 | H2O | 80 | 6 | 84 |
| 18 | K2CO3 | H2O | 80 | 10 | 95 |
In line with our examinations to obtain the optimal reaction conditions, several organic solvents, such as MeOH, 1,4-dioxane, DMSO, THF, H2O, DMF, CH3CN, and n-hexane were screened. The effects of these polar as well as non-polar solvents (with the catalyst molar ratio of Pd–Ni as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and in the presence of K2CO3 as base at 60 °C for 8 h) were compared and listed in Table 2, entries 7–13. In our system, DMF was found to be more effective than other solvents. Solvents like CH3CN and DMSO did not produce a good yield possibly due to their high coordination ability. Using 1,4-dioxane and THF was not much successful and only 37% and 49% yield was observed, respectively. A change in the solvent from one with less polarity to a nonpolar n-hexane solvent resulted in steep drop in the yield which can be due to the poor solubility of starting materials in the latter solvent. Among the other solvent investigated, water was found to be the solvent of choice. Consequently, the choice of solvents is particularly important for the catalytic reaction. Use of DMF as solvent gives a higher yield, but the high boiling point of DMF is disadvantageous. Moreover, it has already been reported that H2O molecule is sometimes required to activate the catalyst or to improve the solubility of inorganic bases. So, H2O has been chosen as the select solvent for the amination reaction.
1 and in the presence of K2CO3 as base at 60 °C for 8 h) were compared and listed in Table 2, entries 7–13. In our system, DMF was found to be more effective than other solvents. Solvents like CH3CN and DMSO did not produce a good yield possibly due to their high coordination ability. Using 1,4-dioxane and THF was not much successful and only 37% and 49% yield was observed, respectively. A change in the solvent from one with less polarity to a nonpolar n-hexane solvent resulted in steep drop in the yield which can be due to the poor solubility of starting materials in the latter solvent. Among the other solvent investigated, water was found to be the solvent of choice. Consequently, the choice of solvents is particularly important for the catalytic reaction. Use of DMF as solvent gives a higher yield, but the high boiling point of DMF is disadvantageous. Moreover, it has already been reported that H2O molecule is sometimes required to activate the catalyst or to improve the solubility of inorganic bases. So, H2O has been chosen as the select solvent for the amination reaction.
In the next step, we studied the effect of the reaction temperature on the reaction outcome, while performing the reaction at different reaction temperatures ranging from 50 °C to 90 °C by keeping other reaction parameters constant (Table 2, entries 10, 14–16). The yield of the reaction was observed to decrease with a decrease in the reaction temperature from 60 °C to 50 °C (entry 14). However, it was observed that the reaction proceeded smoothly at 80 °C within 8 h and gave excellent yield (entry 15). Further increase in the reaction temperature from 80 °C to 90 °C resulted in a slight increase in the yield percentage (entry 16). Therefore, the minimum acceptable amount of the reaction temperature to be used in our system is 80 °C. As a result, the temperature was also a crucial factor for the C–N coupling reaction. Then, we performed the reaction at 80 °C and checked the yield at different time intervals (6–10 h), Table 2, entries 15, 17, and 18. It was previously observed that the reaction completed within 8 h and gave excellent yield (entry 15). As the reaction time decreased to 6 h, the reaction yield approximately decreased. Also, with the aim of increasing the reaction yield, we expanded the reaction time to 10 h. however, no change in the reaction yield was observed, and the maximum time for reaction completion was 8 h. In other words, the yield could not increase obviously in more than 8 h and this time was the enough time for completing the reaction. As a result, the reaction was sensitive to temperature reaction. From the above discussions, it can be seen that the best reaction condition was obtained by using K2CO3 as base in H2O solvent at 80 °C for 8 h in the open air.
Buchwald–Hartwig amination reaction of aryl chlorides with amines through the optimized reaction conditions
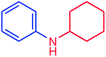
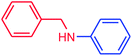
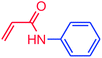
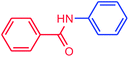
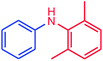
The use of aryl chlorides would be more attractive for industrial applications because of their low cost and wide availability. However, deactivated aryl chlorides are reluctant to undergo catalytic reactions due to their strong C–Cl bonds. In other words, although the aryl iodides and aryl bromides are interesting substrates, we focused our attention on N-arylation with aryl chlorides, which are less reactive electrophiles but of much greater interest for industrial applications. In the first step (after optimizing the reaction conditions), we explored the scope of the C–N coupling reactions between chlorobenzene (as one of the substrates) and cyclohexylamine, aminomethylbenzene, acrylamide, benzamide, 2,6-dimethylaniline, morpholine, piperidine, ethylaniline, diphenylamine, indole, and imidazole (as a nitrogen-fragment donor), respectively. All the substrates are converted to the corresponding N-aryl amines in excellent yields. The results are depicted in Table 3, entries 1–11. Also, by keeping the other reaction parameters constant, the rate and activity of the prepared catalysts in the Buchwald–Hartwig amination reaction are compared and listed in Table 3. It should be noted the turnover numbers (TONs) and the turnover frequencies (TOFs) which are defined as mmol product/mmol catalyst and mmol product/mmol catalyst per hour, were calculated from the isolated yield.| Entry | substrate | Product | Yieldb (%) | TONc | TOFc (h−1) |
|---|---|---|---|---|---|
| a Reaction condition: substrate (aryl chlorides or aryl bromides (2 mmol); amines (2.2 mmol)), K2CO3 (3 mmol), Pd0.5–Ni0.5 B-NPs (4.5 × 10−4 mmol), H2O (5 mL), 80 °C, 8 h.b All compounds are characterized by comparison of GC analysis.c Calculated from the isolated yields.d At 50 °C for 5 h.e Morpholine (3 mmol). | |||||
| 1 | 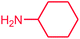 |
 |
94 | 3954 | 482 |
| 2 | 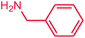 |
 |
100 | 4327 | 541 |
| 3 | 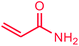 |
 |
93 | 4107 | 513 |
| 4 | 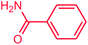 |
 |
96 | 4253 | 532 |
| 5 | 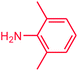 |
 |
97 | 4350 | 544 |
| 6 | 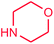 |
 |
95 | 4277 | 535 |
| 7 | 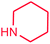 |
 |
93 | 4131 | 516 |
| 8 |  |
 |
86 | 3804 | 475.5 |
| 9 |  |
 |
95 | 4103 | 513 |
| 10 | 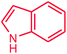 |
 |
93 | 4065 | 508 |
| 11 |  |
 |
98 | 4346 | 543 |
| 12 | 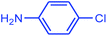 |
 |
96 | 4199 | 525 |
| 13 |  |
 |
100 | 4404 | 550.5 |
| 14 | 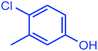 |
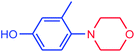 |
91 | 4022 | 503 |
| 15 | 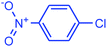 |
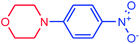 |
95 | 3873 | 484 |
| 16 | 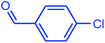 |
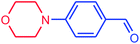 |
99 | 4320 | 540 |
| 17 | 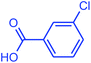 |
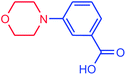 |
90 | 3879 | 485 |
| 18 |  |
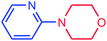 |
92 | 3938 | 492 |
| 19d | 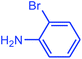 |
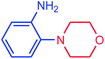 |
95 | 4229 | 846 |
| 20d |  |
 |
100 | 4406 | 881 |
| 21e | 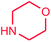 |
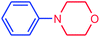 |
69 | 3151 | 394 |
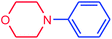
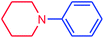
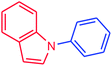
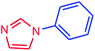
It is clear that the reaction of chlorobenzene with primary and secondary amines took about 8 h for completion. Primary amines reacted with chlorobenzene without difficulty. Initially, aliphatic amines including cyclohexylamine and aminomethylbenzene were evaluated using this protocol with 94% and 100% yields, respectively (entries 1 and 2). Also, it is found that the alkyl and aryl amides give high yields (entries 3 and 4). According to the literature, compared with less hindered substrates, the more hindered substrates cause the reaction rate to decrease due to a slower oxidative–addition step. However, in our experiment, sterically hindered 2,6-dimethyl substituted aniline can react with chlorobenzene to give excellent result in the N-aryl coupling reaction by using Pd0.5–Ni0.5 B-NPs (entry 5). In addition, some other kinds of amines were also tested in this study. Various secondary amines can be coupled with chlorobenzene to give the desired products in high yields, entries 6–11. Arylaminations using cyclic secondary amines were first examined under similar experimental conditions and desired couplings were obtained by 95% and 93% yields using morpholine and piperidine as amines, respectively (entries 6 and 7). A slight decrease in the yield was observed for substituted secondary amine containing aryl group (entry 8). However, sterically hindered diphenylamine was converted to the tertiary amine with a very good result (entry 9). We were delighted to find that the N-arylation of indole with chlorobenzene proceeded successfully and the yield was satisfactory (entry 10). Subsequently, other similar N-hetereocycle such as imidazole was also examined as the substrate. The C–N coupling reactions showed high yields (entry 11). In general, reactions of chlorobenzene with primary and secondary amines (including acyclic, cyclic, and aromatic amines) occurred in yields of more than 86%.
In the next step, a wide range of aryl chlorides (including aniline, anisole, phenol, methyl, nitro, aldehyde, and benzoic acid groups) with morpholine were examined under the optimized reaction conditions, Table 3, entries 12–17. These substituent groups at either ortho, meta, or para positions can be coupled with morpholine to examine the effect of these groups in the amination reaction. As recorded in the literature, it is usually difficult to activate aryl chlorides because the C–Cl bond has relatively high bond energy.9,12,13 However, in our catalytic system, chlorinated aryl compounds were successfully activated. As shown in Table 3, morpholine is N-coupled with both electron-donating (entries 12–14) and -withdrawing (entries 15–17) chloroarenes in excellent yields. In other words, in our experiment using the catalytic system involved, we could not observe a strong dependence of the product yield on donor or acceptor substitution of the chloroarenes. It can be accounted for by the fact that use of two metals as catalyst in the reaction has successfully facilitated the completion of oxidation–addition reaction throughout the catalytic cycle for both electron-donating and electron-withdrawing chloroarenes. Moreover, the amination cross-coupling reaction of hetrocyclic chloroarene such as 2-chloropyridine was resulted with 92% yield (entry 18). According to our results, the reaction conditions optimized for the amination of aryl chlorides were also effective for the amination of aryl bromides. Generally, the rates for amination of aryl bromides were faster than those for amination of aryl chlorides. In our protocol aryl bromides were found to react smoothly giving excellent yield under mild reaction conditions. For instance, reactions of 2-bromoaniline and 4-bromoanisole with morpholine gave 95% and 100% yields, respectively (entries 19 and 20). It is clear that the reaction of aryl bromides with morpholine took about 5 h for completion. In other words, as indicated in the Table 3, the coupling reactions of aryl chlorides with amines required higher temperatures and extended reaction times because the oxidative–addition of C–Cl bond to catalyst species is usually difficult.
Surprisingly, in a separate experiment, when the amount of amine increased from 2.2 to 3 mmol in 4.5 × 10−4 mmol of Pd0.5–Ni0.5 at 80 °C for 8 h, the amination reaction of chlorobenzene and morpholine decreased in the yield percentage from 95% to 69% (entries 6 and 21). This result may be attributed to the chelating properties of the starting amine (i.e. morpholine) towards the Pd–Ni catalyst.
As a result, it is observed that the yields of the Buchwald–Hartwig amination reactions in the C–N coupling process were in the range of 86–100%, corresponding to the TONs from 3804 to 4406. For this catalyst system, these obtained amounts of TONs and TOFs were relatively higher than some of the other catalytic systems. Also, it is well-known that catalyst TONs of around 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 are required to consider large scale application.57–59 As a good advantage of the presented catalyst by this method, product was obtained with excellent yield and complete reaction (100%).
000 are required to consider large scale application.57–59 As a good advantage of the presented catalyst by this method, product was obtained with excellent yield and complete reaction (100%).
According to the recent results, the addition of the second metal to the catalytic system can improve the activity, selectivity, and stability of catalysts in the organic reactions. Fig. 8 suggests a mechanism for the C–N coupling reaction on the Pd–Ni catalyst surface using chlorobenzene and diphenylamine as a model reaction. Many studies indicate that the breaking of the C–X bond is involved in the rate determining step.60,61 The resulting electron-rich Pd center is able to active the C–X bond in an oxidative–addition process. Therefore, in the first step of our proposed mechanism, Pd can cause the C–Cl bond to weaken in an oxidative–addition step, followed by base-assisted displacement of the chlorine atom by diphenylamine. This is then followed by a reductive elimination step which regenerates the Pd0 species.
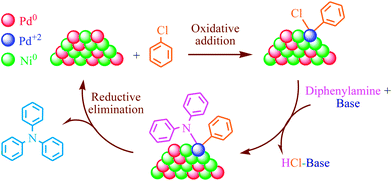 | ||
| Fig. 8 Proposed mechanism for catalytic cycle of amination reaction on the Pd0.5–Ni0.5 B-NPs surface. | ||
We suggest that the presence of Ni in Pd0.5–Ni0.5 B-NPs results in the facilitation of electron transfer in the catalytic cycle. Ni is prone to release electrons to form Ni2+ since the standard electrode potential of Ni2+/Ni (−0.257 V vs. NHE) is markedly lower than that of Pd/Pd2+ (0.915 V vs. NHE). In other words, the additional amount of Ni can make Pd metal regenerate once again in an electron transfer process as follows,62,63 (eqn (3)).
| Pd2+ + Ni0 → Pd0 + Ni2+ | (3) |
Determination of catalyst leaching (Pd0.5–Ni0.5 B-NPs)
Catalyst leaching is one of the major obstacles in heterogenizing homogeneous catalytic reactions, in which case the reaction is actually catalyzed by the leached active species presented in solution. To investigate the catalyst leaching in these reactions, the filtrate of the amination reaction between chlorobenzene and morpholine after completion was analyzed by ICP-OES technique. Due to the observation of low catalyst concentration in the filtrate (2.7%), it can be concluded that the catalyst has high stability for recycling. In other words, these results show that the amount of leached metals from the catalyst is very low and their contribution in the total activity of the catalyst in the reaction is probably negligible.Conclusions
In conclusion, the purpose of the present study is to design an effective and proper catalytic system for the C–N coupling reaction using Pd0.5–Ni0.5 B-NPs. These NPs have been prepared based on the microemulsion route. In this method, nearly spherical shape, uniform, and well-dispersed with a relatively narrow particle size distribution (in the range of 6–20 nm) was successfully synthesized. Using water as solvent and the Pd0.5–Ni0.5 catalyst in combination with K2CO3 as base and at 80 °C for 8 h, optimum conditions for this reaction has been obtained. The best molar ratios of these two metals as Pd and Ni are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Pd/Ni = 1). Finally, it has been shown that a broad range of primary and secondary amines can be coupled with functionalized aryl chlorides including electron-withdrawing and electron-donating groups in the presence of Pd0.5–Ni0.5 B-NPs in water solvent. This catalytic system could be recycled and simply recovered six times without loss of catalytic activity. In general, this catalytic system is nontoxic, thermally stable, reusable, and also makes it possible for the reactions to occur in air. Moreover, after the reaction, the leaching of Pd0.5–Ni0.5 B-NPs into the solution is very low by ICP-OES. To the best of our knowledge, this is the first report on the use of Pd0.5–Ni0.5 B-NPs, stabilized by aerosol-OT, used in the C–N coupling reaction of aryl chlorides with amines.
1 (Pd/Ni = 1). Finally, it has been shown that a broad range of primary and secondary amines can be coupled with functionalized aryl chlorides including electron-withdrawing and electron-donating groups in the presence of Pd0.5–Ni0.5 B-NPs in water solvent. This catalytic system could be recycled and simply recovered six times without loss of catalytic activity. In general, this catalytic system is nontoxic, thermally stable, reusable, and also makes it possible for the reactions to occur in air. Moreover, after the reaction, the leaching of Pd0.5–Ni0.5 B-NPs into the solution is very low by ICP-OES. To the best of our knowledge, this is the first report on the use of Pd0.5–Ni0.5 B-NPs, stabilized by aerosol-OT, used in the C–N coupling reaction of aryl chlorides with amines.
Acknowledgements
The authors are grateful to K.N. Toosi University of Technology for the help to undertake this work.Notes and references
- L. Jiang and S. L. Buchwald in Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed.
- F. A. Beland and F. F. Kadlubar, Handbook of Experimental Pharmacology, Carcinogenesis and Mutagenesis; Grove, Springer Verlag, Heidelberg, Germany, 1990 Search PubMed.
- D. R. Waring and G. Hallas, The Chemistry and Application of Dyes, Plenum, New York, 1990 Search PubMed.
- Y. Liu, M. Prashad and W.-C. Shieh, Org. Process Res. Dev., 2014, 18, 239–245 CrossRef CAS.
- A. Bouhlel, C. Curti, A. Dumètre, M. Laget, M. D. Crozet, N. Azas and P. Vanelle, Bioorg. Med. Chem., 2010, 18, 7310–7320 CrossRef CAS PubMed.
- S. A. Lawrence in Amines: Synthesis Properties, and Application, Cambridge University, Cambridge, 2004 Search PubMed.
- A. Gangjee, O. A. Namjoshi, S. Raghavan, S. F. Queener, R. L. Kisliuk and V. Cody, J. Med. Chem., 2013, 56, 4422–4441 CrossRef CAS PubMed.
- I. Westmoreland, I. J. Munslow, P. N. O'Shaughnessy and P. Scott, Organometallics, 2003, 22, 2972–2976 CrossRef CAS.
- A. F. Littke and G. G. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef CAS.
- V. V. Grushin and H. Alper in Activation of Unreactive Bonds and Organic Synthesis, ed. S. Murai, Springer, Berlin, 1999 Search PubMed.
- V. V. Grushin and H. Alper, Chem. Rev., 1994, 94, 1047–1062 CrossRef CAS.
- Y.-R. Luo in Handbook of Bond Dissociation Energies in Organic Compounds, CRC Press, New York, 2003 Search PubMed.
- W. A. Herrmann, K. Öfele, D. V. Preysing and S. K. Schneider, J. Organomet. Chem., 2003, 687, 229–248 CrossRef CAS PubMed.
- N. Yan, C. Xiao and Y. Kou, Coord. Chem. Rev., 2010, 254, 1179–1278 CrossRef CAS PubMed.
- C. Doebelin, P. Wagner, I. Bertin, F. Simonin, M. Schmitt, F. Bihel and J. –J. Bourguignon, RSC Adv., 2013, 3, 10296–10300 RSC.
- N. Marion, O. Navarro, J. Mei, E. D. Stevens, N. M. Scott and S. P. Nolan, J. Am. Chem. Soc., 2006, 128, 4101–4111 CrossRef CAS PubMed.
- S. M. Raders, J. N. Moore, J. K. Parks, A. D. Miller, T. M. Leißing, S. P. Kelley, R. D. Rogers and K. H. Shaughnessy, J. Org. Chem., 2013, 78, 4649–4664 CrossRef CAS PubMed.
- J. Dutta, S. Datta, D. K. Seth and S. Bhattacharya, RSC Adv., 2012, 2, 11751–11763 RSC.
- G. L. Duc, S. Meiries and S. P. Nolan, Organometallics, 2013, 32, 7547–7551 CrossRef.
- S. k. Rasheed, D. N. Rao, K. R. Reddy, S. Aravinda, R. A. Vishwakarma and P. Das, RSC Adv., 2014, 4, 4960–4969 RSC.
- X. Zhu, Y. Ma, L. Su, H. Song, G. Chen, D. Liang and Y. Wan, Synthesis, 2006, 23, 3955–3962 Search PubMed.
- M. A. Bhosale and B. M. Bhanage, RSC Adv., 2014, 4, 15122–15130 RSC.
- S. M. Islam, S. Mondal, P. Mondal, A. Singha Roy, K. Tuhina, N. Salam and M. Mobarak, J. Organomet. Chem., 2012, 696, 4264–4274 CrossRef CAS PubMed.
- R. Omar-Amrani, A. Thomas, E. Brenner, R. Schneider and Y. Fort, Org. Lett., 2003, 5, 2311–2314 CrossRef CAS PubMed.
- L. Ackermann, W. Song and R. Sandmann, J. Organomet. Chem., 2011, 696, 195–201 CrossRef CAS PubMed.
- X.-H. Fan, G. Li and L.-M. Yang, J. Organomet. Chem., 2011, 696, 2482–2484 CrossRef CAS PubMed.
- G. Cahiez and A. Moyeux, Chem. Rev., 2010, 110, 1435–1462 CrossRef CAS PubMed.
- I. P. Beletskaya and A. V. Cheprakv, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed.
- G. Hervé, G. Sartori, G. Enderlin, G. Mackenzie and C. Len, RSC Adv., 2014, 4, 18558–18594 RSC.
- J. Dutta and S. Bhattacharya, RSC Adv., 2013, 3, 10707–10721 RSC.
- B. M. Choudary, S. Madhi, N. S. Chowdari, M. L. Kantam and B. Sreedhar, J. Am. Chem. Soc., 2002, 124, 14127–14136 CrossRef CAS PubMed.
- M. Nasrollahzadeh, M. Maham, A. Ehsani and M. Khalaj, RSC Adv., 2014, 4, 19731–19736 RSC.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685–4696 CrossRef CAS PubMed.
- L. Yin and J. Liebscher, Chem. Rev., 2007, 107, 133–173 CrossRef CAS PubMed.
- H. V. Huynn and J. Wu, J. Organomet. Chem., 2009, 694, 323–331 CrossRef PubMed.
- E. Mieczynska, A. Gniewek, I. Pryjomska-Ray, A. M. Trzeciak, H. Grabowska and M. Zawadzki, Appl. Catal., A, 2011, 393, 195–205 CrossRef CAS PubMed.
- A. Molnar, Chem. Rev., 2011, 111, 2251–2320 CrossRef CAS PubMed.
- V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957–11986 RSC.
- N. Yan, C. Xiao and Y. Kou, Coord. Chem. Rev., 2010, 254, 1179–1278 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- H. Liu, D. Wang, S. Shang and Z. Song, Carbohydr. Polym., 2011, 83, 38–43 CrossRef CAS PubMed.
- D. H. Chen, C. C. Wang and T. C. Huang, J. Colloid Interface Sci., 1999, 210, 123–129 CrossRef CAS PubMed.
- B. L. Cushing, V. L. Kolesnichenko and C. Ơconnor, J. Chem. Rev., 2004, 104, 3893–3946 CrossRef CAS PubMed.
- B. Kar, S. Bardhan, K. Kundu, S. K. Saha, B. K. Paul and S. Das, RSC Adv., 2014, 4, 21000–21009 RSC.
- R. Abazari, S. Sanati and L. A. Saghatforoush, Chem. Eng. J., 2014, 236, 82–90 CrossRef CAS PubMed.
- L. Guczi, Catal. Today, 2005, 101, 53–64 CrossRef CAS PubMed.
- L. S. Carvalho, C. L. Pieck, M. C. Rangel, N. S. Fígoli, J. M. Grau, P. Reyes and J. M. Parera, Appl. Catal., A, 2004, 269, 91–103 CrossRef CAS PubMed.
- R. Abazari, F. Heshmatpour and S. Balalaie, ACS Catal., 2013, 3, 139–149 CrossRef CAS.
- L. S. Carvalho, C. L. Pieck, M. C. Rangel, N. S. Fígoli, C. R. Vera and J. M. Parera, Appl. Catal., A, 2004, 269, 105–116 CrossRef CAS PubMed.
- R. A. W. Johnstone, A. H. Wilby and I. D. Entwistle, Chem. Rev., 1985, 85, 129–170 CrossRef CAS.
- K. A. Frankel, B. W.-L. Jang, G. W. Roberts and J. J. Spivey, Appl. Catal., A, 2001, 209, 401–413 CrossRef CAS.
- S. Ordonez, F. W. Diez and H. Sastre, Appl. Catal., B, 2001, 31, 113–122 CrossRef CAS.
- E. Mieczynska, T. Borkowski, M. Cypryk, P. Pospiech and A. M. Trzeciak, Appl. Catal., A, 2014, 470, 24–30 CrossRef CAS PubMed.
- P. Forni, L. Prati and M. Rossi, Appl. Catal., B, 1997, 14, 49–53 CrossRef CAS.
- F. J. Urbano and J. M. Marinas, J. Mol. Catal. A: Chem., 2001, 173, 329–345 CrossRef CAS.
- M. A. Aramendía, V. Boráu, I. M. García, C. Jiménez, J. M. Marias and F. J. Urbano, Appl. Catal., B, 1999, 20, 101–110 CrossRef.
- A. Zapf and M. Beller, Top. Catal., 2002, 19, 101–109 CrossRef CAS.
- J. G. de Vries, Can. J. Chem., 2001, 79, 1086–1092 CrossRef CAS.
- H.-U. Blaser and M. Studer, Appl. Catal., A, 1999, 189, 191–204 CrossRef CAS.
- B. Coq, J. –M. Cognion, F. Figueras and D. Tournigant, J. Catal., 1993, 141, 21–33 CrossRef CAS.
- C. D. Thompson, R. M. Rioux, N. Chen and F. H. Ribeiro, J. Phys. Chem. B, 2000, 104, 3067–3077 CrossRef CAS.
- S. Wang, B. Yang, T. Zhang, G. Yu, S. Deng and J. Huang, Ind. Eng. Chem. Res., 2010, 49, 4561–4565 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical methods fundamentals and applications, John Wiley & Sons, Inc., 2nd edn, 2001 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |