Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reaction of α-amido sulfones with functionalized nitrocompounds: a new two-step synthesis of N-alkoxycarbonyl-2,5-disubstituted pyrroles†
Roberto Ballini,
Serena Gabrielli*,
Alessandro Palmieri and
Marino Petrini*
School of Science and Technology, Chemistry Division, Università di Camerino, via S. Agostino, 1, I-62032 Camerino, Italy. E-mail: serena.gabrielli@unicam.it; marino.petrini@unicam.it; Fax: +39 0737 402297
First published on 8th September 2014
Abstract
Reaction of α-amido sulfones with nitro ketals promoted by KF on alumina provides the corresponding adducts which, upon treatment with p-toluenesulfonic acid, generate the corresponding N-alkoxycarbonyl-2,5-disubstituted pyrroles. The latter transformation involves a cascade process including ketal cleavage, ring closure and final aromatization by nitrous acid elimination.
The pyrrole ring occurs frequently in many natural products of paramount importance for their notable bioactivity.1 Additionally, this nitrogenated heterocyclic system is present in several compounds of synthetic origin known for their antimycobacterial activity and DNA cross-linking properties.2,3 Pyrrole-containing macrocyclic derivatives are also involved in organic electronic materials and neutral anion receptors.4,5 The flourishing chemistry associated with pyrrole synthesis has evidenced a plethora of different procedures since the discovery of the Paal–Knorr and Hantzsch reactions.6 Modern variants of these old-fashioned methodologies involve multicomponent processes which are particularly attractive for their efficiency and eco-sustainability.7 Several of these new synthetic procedures no longer entail the use of dicarbonyl derivatives for the ring building but exploit other functionalized backbones such as 1,4-dihalodienes,8 enamides,9 aminoketones,10 aminoalcohols11 and isonitriles.12
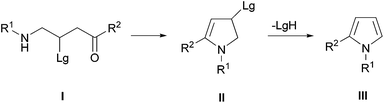
Intramolecular ring closure of amino derivatives I, bearing a carbonyl function in a suitable position of the alkyl framework, represents a viable process for the preparation of pyrrolines II (Scheme 1).13 This strategy can also be settled for the synthesis of pyrroles III but would require a tandem elimination process after the ring formation in order to provide the needed aromatic system. To this goal, some synthetic protocols aimed to the efficient preparation of precursors of type I have been devised.
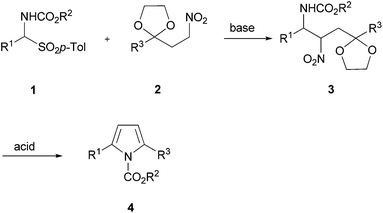
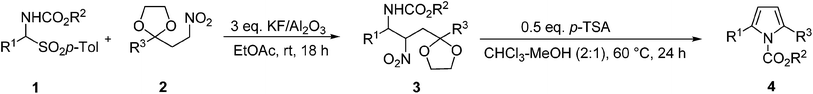
In a early work γ-amino ketones I (Lg![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OH) were obtained by reductive cleavage of isoxazolines which upon reaction with acetic acid were converted into pyrroles III.14 Later on, a procedure involving a Ti(IV) promoted Mukaiyama condensation between silyl enol ethers and azido acetals was employed to prepare γ-azido ketones that, under reductive conditions were converted into polysubstituted pyrroles.15 More recently, unsubstituted N-acylpyrroles have been prepared by acid promoted ring closure of 4-amido-3-methoxy aldehyde dimethyl acetals.16 Although quite efficient, the latter procedure is affected by poor versatility since only the acyl moiety can be changed in the target products. A common feature of the above cited methods is the utilization of a hydroxy or a methoxy group as leaving group used for the final aromatization step. Knowing the ability of the nitro system in acting as a good leaving group in elimination reactions, we devised a new procedure for the synthesis of N-alkoxycarbonyl-2,5-disubstituted pyrroles exploiting a two step strategy as depicted in Scheme 2.17 α-Amido sulfones 1 are well-known precursors of reactive N-acylimines which have been largely involved in nitro-Mannich reactions promoted or catalyzed under basic conditions.18 For our purpose, the N-acylimine is generated from 1 by base-promoted elimination of p-toluenesulfinc acid and then attacked by the nitronate anion corresponding to nitro ketal 2 leading to the nitrocarbamate adduct 3.
OH) were obtained by reductive cleavage of isoxazolines which upon reaction with acetic acid were converted into pyrroles III.14 Later on, a procedure involving a Ti(IV) promoted Mukaiyama condensation between silyl enol ethers and azido acetals was employed to prepare γ-azido ketones that, under reductive conditions were converted into polysubstituted pyrroles.15 More recently, unsubstituted N-acylpyrroles have been prepared by acid promoted ring closure of 4-amido-3-methoxy aldehyde dimethyl acetals.16 Although quite efficient, the latter procedure is affected by poor versatility since only the acyl moiety can be changed in the target products. A common feature of the above cited methods is the utilization of a hydroxy or a methoxy group as leaving group used for the final aromatization step. Knowing the ability of the nitro system in acting as a good leaving group in elimination reactions, we devised a new procedure for the synthesis of N-alkoxycarbonyl-2,5-disubstituted pyrroles exploiting a two step strategy as depicted in Scheme 2.17 α-Amido sulfones 1 are well-known precursors of reactive N-acylimines which have been largely involved in nitro-Mannich reactions promoted or catalyzed under basic conditions.18 For our purpose, the N-acylimine is generated from 1 by base-promoted elimination of p-toluenesulfinc acid and then attacked by the nitronate anion corresponding to nitro ketal 2 leading to the nitrocarbamate adduct 3.
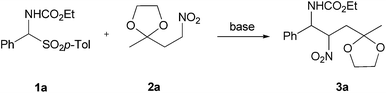
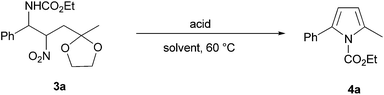
In order to test the feasibility of this approach, α-amido sulfone 1a was made to react with nitro ketal 2a under various reaction conditions as summarized in Table 1.
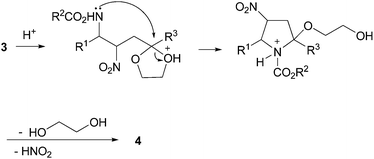
Sodium hydride was the first base used because of its known efficiency in promoting the addition reaction of nitromethane to α-amido sulfones.19 A preliminary trial using equimolar amount of reactants and 3 equivalents of NaH gave a modest result while a notable increase in the chemical yield was observed doubling the amount of the nitrocompound 2a (Table 1, entries 1–2). In order to circumvent the problems associated with the utilization of NaH as basic promoter (inflammability, dry conditions etc.), another couple of bases working under heterogeneous conditions were tested for our process. Potassium fluoride on alumina and Cs2CO3 were proved to be efficient promoters for this addition and after a careful evaluation we decided to employ the former base for all the next reactions.20 The second step of our protocol for the preparation of 2,5-disubstituted pyrroles 4 entails an acid-promoted cascade process involving a preliminary ketal protonation from compound 3, followed by ring closure to the intermediate pyrrolidine and a final aromatisation through nitrous acid and ethylene glycol elimination (Scheme 3). An alternative pathway involving direct formation of the carbonyl system, ring closure to the parent 1-pyrroline followed by nitrous acid elimination cannot obviously be ruled out.
Because of the superior stability toward cleavage of cyclic ketals over their open chain counterparts, the acidity level of the reaction mixture must be carefully tuned and accounting for the ability of macroreticular sulfonic resin Amberlyst 15 to carry out related processes, this solid acid was initially checked for this purpose (Table 2, entries 1–3).21
| Entry | Acid (g) | Solventb | 4a Yieldc [%] |
|---|---|---|---|
a Conditions: 1a (0.5 mmol), acid, 60 °C, 24 h.b CHCl3/MeOH, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.c Yields of isolated products.d Zeolite.e Carbon-sulfonic acid.22f Equivalents. 1.c Yields of isolated products.d Zeolite.e Carbon-sulfonic acid.22f Equivalents. |
|||
| 1 | Amb 15 (0.5) | MeOH | Trace |
| 2 | Amb 15 (0.5) | CHCl3/MeOH | 23 |
| 3 | Amb 15 (1.0) | CHCl3/MeOH | 19 |
| 4 | HSZ-320 (1.0) | CHCl3/MeOH | Traced |
| 5 | C-SO3H (1.0) | CHCl3/MeOH | 56e |
| 6 | p-TSA (1.0)f | CHCl3/MeOH | 72 |
| 7 | p-TSA (0.5)f | CHCl3/MeOH | 70 |
Reaction of compound 3a with Amberlyst 15 was proved ineffective in MeOH and only a modest yield of pyrrole 4a was obtained increasing the solubility of the substrate by adding CHCl3 to MeOH.
Other solid acids were tested for this conversion such as Zeolite HSZ-320 and carbon-sulfonic acid,22 but only the latter reagent provided encouraging results (Table 2, entries 4–5). Finally, the utilization of p-toluenesulfonic acid gave a rather satisfactory yield of pyrrole 4a when employed in equimolar amount (Table 2, entry 6). Since a reduction of half the amount of acid used resulted only in a negligible decrease in the yield of the obtained pyrrole 4a, these conditions were selected for the method development. N-Alkoxycarbonyl groups in α-amido sulfones 1 other than N-ethoxy and N-methoxycarbonyl were tested with the aim of providing a possible easier cleavage of the carbamoyl moiety in the final pyrrole 4. N-Benzyloxycarbonyl sulfones 1 (R2 = Bn) were less reactive than their methyl and ethyl counterparts in the nitro-Mannich reaction, while nitrocarbamates 3 bearing the N-t-butoxycarbamoyl moiety (R2 = t-Bu) gave disappointing results in the final step probably because of the acidic conditions affecting the t-butyl group.
The optimised conditions found for the two-step transformation were applied to the reaction of different α-amido sulfones 1 with nitro ketals 2 (Table 3). The addition reaction generating the nitro carbamate 3 was quite efficient for most of the combinations tested. The use of the solid base is instrumental in order to simplify the work up operations which involve filtration of the solid base and evaporation of the solvent. The excess of nitro ketal 2 employed, can be almost completely recovered after column chromatography together with the wanted adduct 3. The subsequent cascade process leading to the pyrrole derivative 4 was rather satisfactory for most of the adducts 3 obtained. For unknown reasons compound 3i obtained from α-amido sulfone 1h bearing an alkyl framework gave disappointing results when converted into the corresponding pyrrole (Table 3, entry 9). Conversely, the nature of substituent R3 in adduct 3 did not affect the outcome of the process so that alkyl or aryl groups can be inserted as substituents in the pyrrole ring. Although isolation of nitrocarbamates 3 is instrumental for the recovery of unreacted nitro ketals 2, an attempt to use crude nitrocarbamates for the next cyclization step has been pursued in order to evidence possible advantages in the overall process. Crude compound 3a, obtained by filtration of the basic promoter and evaporation of the solvent, has been used for the next step leading to the formation of pyrrole 4a in 53% yield based on substrate 1a. This value is close to that recorded (60%) for the whole process carried out on purified 3a thus demonstrating that isolation of nitrocarbamates can be avoided with a minimum loss in the overall yield of the target pyrroles 4.
| Entry | α-Amido Sulfone 1 | R1 | R2 | Nitro ketal 2 | R3 | 3 Yielda (%) | Pyrrole 4 | Yieldb (%) | |
|---|---|---|---|---|---|---|---|---|---|
a Reaction conditions: α-amido sulfone (2.0 mmol), nitro ketal (4.0 mmol), KF/Al2O3 (6.0 mmol) in EtOAc (20 mL), at rt, 18 h. Yield of pure isolated product.b Reaction conditions: nitrocarbamate (1.0 mmol), p-TSA (0.5 mmol), in CHCl3–MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9 mL) at 60 °C, 24 h. Yield of pure isolated product.c Yield in parenthesis refers to the reaction carried out directly on crude 3a obtained after filtration and evaporation of the solvent calculated on compound 1a.d Reaction time 48 h. 1, 9 mL) at 60 °C, 24 h. Yield of pure isolated product.c Yield in parenthesis refers to the reaction carried out directly on crude 3a obtained after filtration and evaporation of the solvent calculated on compound 1a.d Reaction time 48 h. |
|||||||||
| 1 | 1a | Ph | Et | 2a | Me | 3a | 86 | 4a | 70 (53)c |
| 2 | 1b | 4-NO2Ph | Et | 2b | 4-MeOPh | 3b | 65 | 4b | 55 |
| 3 | 1b | 4-NO2Ph | Et | 2a | Me | 3c | 63 | 4c | 84 |
| 4 | 1c | 4-MeOPh | Et | 2c | 4-t-BuPh | 3d | 80 | 4d | 55 |
| 5 | 1d | Ph | Me | 2c | 4-t-BuPh | 3e | 99 | 4e | 75 |
| 6 | 1e | 4-CNPh | Et | 2d | Ph(CH2)2 | 3f | 97 | 4f | 79 |
| 7 | 1f | 2-FPh | Et | 2e | 1-Hexyl | 3g | 99 | 4g | 53 |
| 8 | 1g | 1-Naphthyl | Et | 2a | Me | 3h | 67 | 4h | 74 |
| 9 | 1h | Et | Et | 2f | Ph | 3i | 90 | 4i | 28 |
| 10 | 1i | 4-FPh | Et | 2g | Ph(Me)CH | 3j | 95 | 4j | 57d |
| 11 | 1b | 4-NO2Ph | Et | 2h | Et | 3k | 88 | 4k | 73 |
| 12 | 1d | Ph | Me | 2i | 4-MePh | 3l | 72 | 4l | 54 |
Conclusions
In conclusion, a novel procedure for the preparation of 2,5-disubstituted pyrrole derivatives has been devised starting from α-amido sulfones 1 which are made to react with nitro ketals 2 in a base promoted addition reaction. The obtained adducts 3 upon treatment with p-toluenesulfonic acid undergo to a cascade reaction involving ketal cleavage, cyclization and final aromatization to the target substituted pyrrole. The overall process is featured by a ready availability of the starting materials employed, inexpensive reactants and solvents used, mild reaction conditions and molecular diversity of the substituted pyrroles prepared. Further studies aimed at enlarging the synthetic significance of this procedure to other polysubstituted pyrrole systems are currently underway in our laboratory.Acknowledgements
We acknowledge financial support from University of Camerino, and FIRB National Project ‘Metodologie di nuova generazione nella formazione di legami carbonio-carbonio e carbonio-eteroatomo in condizioni eco-sostenibili’.Notes and references
- (a) D. L. J. Clive and P. Cheng, Tetrahedron, 2013, 69, 5067–5078 CrossRef CAS PubMed; (b) I. S. Young, P. D. Thornton and A. Thompson, Nat. Prod. Rep., 2010, 27, 1801–1839 RSC; (c) H. Fan, J. Peng, M. T. Hamann and J.-F. Hu, Chem. Rev., 2008, 108, 264–287 CrossRef CAS PubMed; (d) C. T. Walsh, S. Garneau-Tsodikova and A. R. Howard-Jones, Nat. Prod. Rep., 2006, 23, 517–531 RSC.
- M. Biava, G. C. Porretta, G. Poce, C. Battilocchio, S. Alfonso, A. de Logu, F. Manetti and M. Botta, ChemMedChem, 2011, 6, 593–599 CrossRef CAS PubMed.
- (a) T.-L. Su, T.-C. Lee and R. Kakadiya, Eur. J. Med. Chem., 2013, 69, 609–621 CrossRef CAS PubMed; (b) T. Vaijayanthi, T. Bando, G. N. Pandian and H. Sugiyama, ChemBioChem, 2012, 13, 2170–2185 CrossRef CAS PubMed.
- S. C. Rasmussen and S. J. Everson, Prog. Polym. Sci., 2013, 38, 1773–1804 CrossRef CAS PubMed.
- P. Dydio, D. Lichosyt and J. Jurczak, Chem. Soc. Rev., 2011, 40, 2971–2985 RSC.
- G. W. H. Cheeseman and C. W. Bird, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, Oxford, 1984, vol. 4, pp. 89–147 Search PubMed.
- Reviews: (a) V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2014, 43, 4633–4657 RSC; (b) V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2010, 39, 4402–4421 RSC for some recent examples see: ; (c) M. Zhang, X. Fang, H. Neumann and M. Beller, J. Am. Chem. Soc., 2013, 135, 11384–11388 CrossRef CAS PubMed; (d) C. C. Silveira, S. R. Mendes, G. M. Martins, S. C. Schlösser and T. S. Kaufman, Tetrahedron, 2013, 69, 9076–9085 CrossRef CAS PubMed; (e) B.-L. Li, P.-H. Li, X.-N. Fang, C.-X. Li, J.-L. Sun, L.-P. Mo and Z.-H. Zhang, Tetrahedron, 2013, 69, 7011–7018 CrossRef CAS PubMed; (f) X. Wang, X.-P. Xu, S.-Y. Wang, W. Zhou and S. J. Ji, Org. Lett., 2013, 15, 4246–4249 CrossRef CAS PubMed.
- (a) M. Zhan, S. Zhang, W.-X. Zhang and Z. Xi, Org. Lett., 2013, 15, 4182–4185 CrossRef CAS PubMed; (b) W. Geng, W.-X. Zhang, W. Hao and Z. Xi, J. Am. Chem. Soc., 2012, 134, 20230–20233 CrossRef CAS PubMed; (c) S. Li, Q. Liao, F. Wang, C. Xi and L. Zhang, Eur. J. Org. Chem., 2010, 5426–5431 CrossRef PubMed.
- (a) Y.-H. Xu, T. He, Q.-C. Zhang and T.-P. Loh, Chem. Commun., 2014, 50, 2784–2786 RSC; (b) M.-N. Zhao, Z.-H. Ren, Y.-Y. Wang and Z. H. Guan, Chem.–Eur. J., 2014, 20, 1839–1842 CrossRef CAS PubMed; (c) B. Li, N. Wang, Y. Liang, S. Xu and B. Wang, Org. Lett., 2013, 15, 136–139 CrossRef CAS PubMed; (d) M. Yamagishi, K. Nishigai, T. Hata and H. Urabe, Org. Lett., 2011, 13, 4873–4875 CrossRef CAS PubMed; (e) A. Saito, O. Konishi and Y. Hanzawa, Org. Lett., 2010, 12, 372–374 CrossRef CAS PubMed.
- (a) R. Yan, X. Kang, X. Zhou, X. Li, X. Liu, L. Xiang, Y. Li and G. Huang, J. Org. Chem., 2014, 79, 465–470 CrossRef CAS PubMed; (b) M. Egorov, B. Delpech, G. Aubert, T. Cresteil, M. C. Garcia-Alvarez, P. Collin and C. Maranzano, Org. Biomol. Chem., 2014, 12, 1518–1524 RSC; (c) K. Ohta, F. Taguchi and Y. Endo, Heterocycles, 2012, 86, 165–170 CrossRef CAS PubMed; (d) A. Palmieri, S. Gabrielli, C. Cimarelli and R. Ballini, Green Chem., 2011, 13, 3333–3336 RSC; (e) Q. Li, A. Fan, Z. Lu, Y. Cui, W. Lin and X. Jia, Org. Lett., 2010, 12, 4066–4069 CrossRef CAS PubMed.
- (a) S. Qu, Y. Dang, C. Song, M. Wen, K.-W. Huang and Z.-X. Wang, J. Am. Chem. Soc., 2014, 136, 4974–4991 CrossRef CAS PubMed; (b) S. Michlik and R. Kempe, Nat. Chem., 2013, 5, 140–144 CrossRef CAS PubMed; (c) D. Srimani, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2013, 52, 4012–4015 CrossRef CAS PubMed.
- (a) N. Ono and T. Okujima, Synthesis of Pyrroles and Their Derivatives from Isocyanides, in Isocyanide Chemistry: Applications in Synthesis and Material Science, ed. V. G. Nenajdenko, Wiley-VCH, Weinheim, 2012, ch. 11, pp. 385–430 Search PubMed; (b) T. Wu, L. Pan, X. Xu and Q. Liu, Chem. Commun., 2014, 50, 1797–1800 RSC; (c) I. Yavari and M. Nematpour, Helv. Chim. Acta, 2013, 96, 2098–2102 CrossRef CAS PubMed; (d) R. Sharma, K. Kumar, M. Chouhan, V. Grover and V. A. Nair, RSC Adv., 2013, 3, 14521–14527 RSC; (e) M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 6958–6961 CrossRef CAS PubMed; (f) S. Kamijo, C. Kanazawa and Y. Yamamoto, J. Am. Chem. Soc., 2005, 127, 9260–9266 CrossRef CAS PubMed.
- (a) M. L. Wong, I. A. Guzei and L. L. Kiessling, Org. Lett., 2012, 14, 1378–1381 CrossRef CAS PubMed; (b) T. J. Harrison and G. R. Dake, J. Org. Chem., 2005, 70, 10872–10874 CrossRef CAS PubMed; (c) J. R. Lennox, S. C. Turner and H. Rappoport, J. Org. Chem., 2001, 66, 7078–7083 CrossRef CAS PubMed.
- (a) S. K. Mukerji, K. K. Sharma and K. G. B. Torssell, Tetrahedron, 1983, 39, 2231–2235 CrossRef CAS; (b) S. S. Ghabrial, I. Thomsen and K. G. B. Torssell, Acta Chem. Scand., Ser. B, 1987, 41, 426–434 CrossRef PubMed.
- (a) H. Bertschy, A. Meunier and R. Neier, Angew. Chem., Int. Ed. Engl., 1990, 29, 777–778 CrossRef PubMed; (b) E. Bellur, H. Gorls and P. Langer, J. Org. Chem., 2005, 70, 4751–4761 CrossRef CAS PubMed.
- (a) T. Maehara, R. Kanno, S. Yokoshima and T. Fukuyama, Org. Lett., 2012, 14, 1946–1948 CrossRef CAS PubMed; (b) R. A. W. Neves Filho, S. Stark, M. C. Morejon, B. Westermann and L. A. Wessjohann, Tetrahedron Lett., 2012, 53, 5360–5363 CrossRef CAS PubMed.
- (a) N. Ono, The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001 CrossRef PubMed; (b) R. Ballini, A. Palmieri and L. Barboni, Chem. Commun., 2008, 2975–2985 RSC.
- (a) M. Petrini, Chem. Rev., 2005, 105, 3949–3977 CrossRef CAS PubMed; (b) A. Noble and J. C. Anderson, Chem. Rev., 2013, 113, 2887–2939 CrossRef CAS PubMed For some very recent examples see: ; (c) D. M. Barber, A. Ďuriš, A. L. Thompson, H. J. Sanganee and D. J. Dixon, ACS Catal., 2014, 4, 634–638 CrossRef CAS PubMed; (d) T. A. Davis, A. E. Vilgelm, A. Richmond and J. N. Johnston, J. Org. Chem., 2013, 78, 10605–10616 CrossRef CAS PubMed; (e) D. Cao, Z. Chai, J. Zhang, Z. Ye, H. Xiao, H. Wang, J. Chen, X. Wu and G. Zhao, Chem. Commun., 2013, 49, 5972–5974 RSC.
- (a) R. Ballini and M. Petrini, Tetrahedron Lett., 1999, 40, 4449–4452 CrossRef CAS; (b) E. Foresti, G. Palmieri, M. Petrini and R. Profeta, Org. Biomol. Chem., 2003, 1, 4275–4281 RSC.
- Reviews: (a) B. Basu and B. Mandal, Curr. Org. Chem., 2011, 15, 3870–3893 CrossRef CAS; (b) B. E. Blass, Tetrahedron, 2002, 58, 9301–9320 CrossRef CAS.
- (a) R. Ballini, S. Gabrielli, A. Palmieri and M. Petrini, Adv. Synth. Catal., 2010, 352, 2459–2462 CrossRef CAS PubMed; (b) A. Palmieri, S. Gabrielli and R. Ballini, Chem. Commun., 2010, 46, 6165–6167 RSC.
- B. M. Rao, G. N. Reddy, T. V. Reddy, B. L. A. P. Devi, R. B. N. Prasad, J. S. Yadav and B. V. S. Reddy, Tetrahedron Lett., 2013, 54, 2466–2471 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures for the preparation of compounds 3 and 4. Copies of the 1H NMR and 13C NMR spectra for new compounds prepared. See DOI: 10.1039/c4ra08112a |
| This journal is © The Royal Society of Chemistry 2014 |