Fabrication of porous TiO2–SiO2 multifunctional anti-reflection coatings by sol–gel spin coating method†
Qiangqiang Maoa,
Dawen Zeng*ab,
Keng Xua and
Changsheng Xieb
aState Key Laboratory of Materials Processing and Die & Mould Technology, Huazhong University of Science and Technology (HUST), No. 1037, Luoyu Road, Wuhan 430074, P. R. China. E-mail: dwzeng@mail.hust.edu.cn
bNanomaterials and Smart Sensors Research Lab (NSSRL), Department of Materials Science and Engineering, HUST, No. 1037, Luoyu Road, Wuhan 430074, P. R. China
First published on 30th October 2014
Abstract
In this study, we report that an antireflective coating with multifunctional properties, including high transparency, self-cleaning and abrasion resistance, was prepared by a facile sol–gel spin-coating method. The average transmittance of single coated glass substrates was greater than 93% in the wavelength range of 300–1000 nm compared to 90.1% for bare glass substrate. The photocatalytic activity of the coatings showed a close dependence on the content of TiO2 nanoparticles. The coatings showed the ability to decompose air borne organic contaminants (gaseous benzene) and applied contaminants (fingerprints) under UV irradiation when the content of TiO2 was greater than 10%. After illumination by UV light, the TiO2–SiO2 coatings showed hydrophilic properties with a water contact angle of about 1°, which favored greatly the self-cleaning function of the coatings. When the content of TiO2 was 10%, the critical load of the coating increased up to 305 μN as compared to that of the base-catalyzed SiO2 coating (198 μN), which indicated the abrasion resistance property of this coating. This approach suggested novel opportunities to a variety of practical applications, including windows of high rise buildings, solar thermal collectors and solar cell cover glasses, etc.
Introduction
Anti-reflection coatings (ARCs) have been widely used in energy-related applications including solar thermal collectors, windows of high rise buildings and covers of solar cells.1–5 Up to now, many research works have been conducted to improve the transmittance property of the ARCs.6–8 But, a coating with only anti-reflection capacity will not satisfy the increasing demands of more competitive technologies and practical applications.9 More and more recent research works consolidate the interest and feasibility of elaboration of multifunctional coatings, such as anti-fogging, anti-abrasion and self-cleaning coatings.7,10,11For outdoor and solar energy-related applications, anti-reflection and self-cleaning (AR-SC) are two practical properties, which have been achieved on glass substrate by SiO2 and TiO2 films, respectively.7,9,10,12–14 On the one hand, porous SiO2 was considered to achieved high anti-reflection property due to its tunable refractive index properties.6,7,15,16 On the other hand, TiO2 film showed self-cleaning property because of its two photo-induced phenomena: photocatalysis and photo-induced hydrophilicity.17–20 TiO2 could decompose the organic pollutant absorbed on the surface of film under UV light irradiation. And dirt particles could be washed off from the surface when water droplet rolled over the surface of the film.2,19,20
In the past few years, two main methods have been used to fabricate AR-SC coatings on glass substrate.7,9,10,12–14 In the first method, SiO2 and TiO2 particles were deposited on substrate by various techniques, such as dip-coating, spin-coating and spray pyrolysis techniques, to obtain double layer coating.9,10,13,14 The bottom SiO2 and the top TiO2 films were prepared to provide anti-reflection and self-cleaning properties, respectively. As the thickness of TiO2 layer increased, the transmittance of the coating decreased sharply with the self-cleaning capacity increased.10 In the second method, the co-deposition of TiO2 and SiO2 particles to form a single layer ARCs.7,12 However, the refractive index of TiO2 was high (n > 1.45), so the content of TiO2 and the structure of the coating has to be controlled.2 According to previous reports,2,6,7 the index of the coating could be lowered by introducing porosity and reducing the particle size of TiO2. Meanwhile, the more photo-active of TiO2 nanoparticle was due to the organic molecules were decomposed at or near the TiO2 surface and its wider band gap.18 So a coatings with a high porosity and TiO2 nanoparticle will maintain both the high transmittance and self-cleaning property for a long time, which was important when a coating was used in practical applications. But only a few research works has focused on the preparation of AR-SC coatings with TiO2 nanoparticles to maintain high antireflective and photocatalytic self-cleaning properties.21
Recently, there were mainly two approaches to investigate the self-cleaning property of these AR-SC coatings. Some authors investigated the self-clean capacity of coatings by measuring the degradation of organic films deposited on the surface of the coatings throughout water contact angle measurement13,22 and IR-spectroscopy.7,12 Degradation of some liquid pollutants, including rhodamine B14 and methylene blue,9,10 was also used to measure the self-cleaning property of the coatings. On the other hand, during practical application process, the surface of the anti-reflection coating was easily polluted by various pollutants in the air and the applied contaminants. Those pollutants would dramatically deteriorate the long term transmittance property of the anti-reflection coatings. However, few researchers measured the self-cleaning property of AR-SC coating by the degradation of gaseous pollutants and the applied contaminants.7 But, during practical application process, those contaminants would dramatically deteriorate the long term transmittance property of the AR-SC coatings. Therefore, it is an interesting and important work to fabricate a coating with self-cleaning property to maintain the high anti-reflection property for a long time.
In this study, TiO2–SiO2 anti-reflection coating with multifunctional characteristics, including high transmittance, self-cleaning and abrasion resistance properties, was prepared by spin-coating method. The effect of content of TiO2 nanoparticles on light transmittance, photocatalytic activity, hydrophilicity and abrasion resistance properties of the coatings was explored respectively. The combination of those properties provided promising outdoor uses, such as window of high rise buildings and solar thermal collectors.
Experimental
Materials
Tetraethyl orthosilicate (TEOS), ammonium hydroxide (NH4OH), hydrochloric acid (HCl, 37%) and ethanol (EtOH) were purchased from Sinopharm Chemical Reagent. Titanium n-butoxide (n-Buti, 99%) was obtained from Alfa Aesar. All chemicals were used as received.Preparation of sol–gel spin coatings
The base-catalyzed SiO2 sol was prepared by mixing TEOS, EtOH, H2O and NH4OH with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8. This solution was stirred for 4 h and aged at room temperature for 3 days. And then it was refluxed at 80 °C until the pH of the solution was about 6. TiO2 sol was prepared by mixing n-Buti and EtOH, followed by the dropwise addition of an aqueous solution of HCl. The chemicals were mixed at a molar ratio n-Buti
0.8. This solution was stirred for 4 h and aged at room temperature for 3 days. And then it was refluxed at 80 °C until the pH of the solution was about 6. TiO2 sol was prepared by mixing n-Buti and EtOH, followed by the dropwise addition of an aqueous solution of HCl. The chemicals were mixed at a molar ratio n-Buti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl of 1
HCl of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3. This sol was stirred for 4 h and aged at room temperature for 1 day. Both precursor solutions were mixed together to obtain sols with different TiO2 concentrations.
0.3. This sol was stirred for 4 h and aged at room temperature for 1 day. Both precursor solutions were mixed together to obtain sols with different TiO2 concentrations.
The glass substrates (1 mm thick, 30 × 30 mm soda-lime glass) were cleaned with acetone, ethanol and distilled water by ultrasonic cleaning method, and then dried at 80 °C. The coatings were single deposited onto pre-cleaned glass slides by spin coating (8000 rpm, 20 s). The coated glass samples were dried in air at room temperature and were calcined at 500 °C for 30 min and cooled down to room temperature in the closed furnace.
Photocatalytic activity
Photocatalytic performance of the coatings was evaluated by degrading gaseous benzene under irradiation of UV light at ambient temperature in a 2 L cubic reactor, before which the air tightness of the reactor was tested. The UV light was generated from a Xenon lamp (UV300, Aulight of China Co., Ltd) with a wavelength range of 200–400 nm. After six pieces of the coated glass substrates (3 × 3 cm, each piece) were placed into the reactor, an amount of aqueous benzene was also injected within by a syringe. The bottom of the reactor was slightly heated (below 40 °C) to ensure benzene all evaporated. The analysis of the concentration of benzene and CO2 was performed with a gas chromatography (GC9560, Shanghai Huaai Co., Ltd). Each set of experiments was monitored for 3 h.Characterization
The transmission spectra of the coated and uncoated glasses were measured by using UV-vis transmittance spectra (Unico UV-2012PC) in the wavelength range of 200–1000 nm. The surface morphology of the coatings was characterized by scanning electron microscopy (SEM, Sirion 200). Transmission electron microscope (TEM) and high-resolution transmission electron microscope (HRTEM) characterizations were performed with a FEI Tecnai G220 microscope working at 200 kV. Chemical composition of the coating was defined by X-ray photoelectron spectroscopy (XPS, AXIS-ULTRA DLD-600W). The surface wettability of the coatings was studied by a contact angle/interface system (SL-200) with ±1° accuracy in atmospheric air at room temperature. Water droplets of 1 μL were dropped carefully onto the coating surface. Nanoscratch test was carried out using the Tribolndenter system (Hysitron TI750) with a spherical diamond indenter tip.Results and discussion
Anti-reflection property
The effect of the content of TiO2 on the transmittance of coatings was shown in Fig. 1. According to the UV-vis spectra, the coated glass substrates showed high anti-reflection. As shown in Fig. 1a, in contrast to the transmittance of the base-catalyzed SiO2 coating, the transmittance of the coatings decreased gradually in the range of 400–1000 nm with the content of TiO2 increased from 2 to 20 wt%. The average transmittance of the coated glass substrate was decreased from 93.43% to 92.3% with the content of TiO2 increased from 0% to 20% in the wavelength range 300–1000 nm compared to that (90%) of bare glass substrate, as shown in Fig. 1b.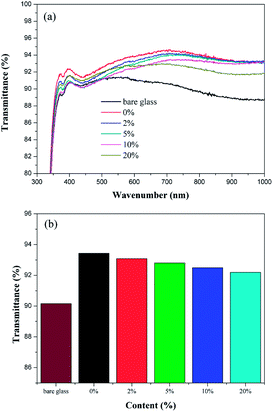 | ||
| Fig. 1 (a) Transmittance spectra and (b) average transmittance of the coatings with different contents of TiO2. | ||
The refractive index of coating (nc) was one parameter to its ideal anti-reflection property. According to the relevant research,23 the refractive index of porous coating can be calculated. The transmittance of the bare glass substrate is 90.6% at the wavelength of 701 nm, and the refractive index was estimated to be 1.55. The transmittance of the different coatings (0%, 2%, 5%, 10% and 20% TiO2–SiO2) at 701 nm were 94.6%, 94.2%, 94.0%, 93.5% and 93.1%, respectively. Then, the nc were estimated to be 1.47, 1.48, 1.49, 1.50 and 1.51, respectively.
Therefore, according to the results of the experiments, the coatings could maintain the high transmittance when the content of TiO2 was no more than 20%.
Surface morphology
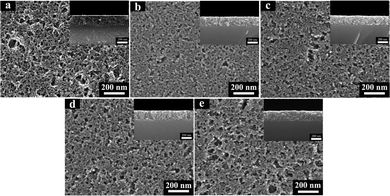
FESEM images of the base-catalyzed SiO2 and TiO2–SiO2 coatings were shown in Fig. 2. As shown in Fig. 2a–e, porous structures were generated on the surface of the coatings and the surface was changed with the increase in content of TiO2. When the TiO2 content was no more than 10% (Fig. 2a–d), the size of most pores were less than 80 nm. The porosity of the coatings was helpful to trap and restrain the scattered and reflected light.15 As a result; more light could transmit through the coating. Then, the coatings showed high transmittance property in the wavelength range (400–1000 nm). But, when the TiO2 content was 20%, there were some large pores (about 100 nm) on the surface of the coating (Fig. 2e). The pore size was about 10% of the incident light wavelength, which could lead to surface scattering.16 This was maybe the main reason for the decrease of transmittance of this coating.The thickness of TiO2–SiO2 coatings were also observed by cross section morphology of the coatings from FSEM images (Fig. 2). The thickness of the coatings was about 200 nm. On the other hand, the thickness of the coating was corresponding to the maximum transmittance of those coatings.16 As shown in Fig. 1, the wavelength of the maximum transmittance of those coating has not changed significantly. This result also indicated the uniform thickness of those coatings.
Chemical composition
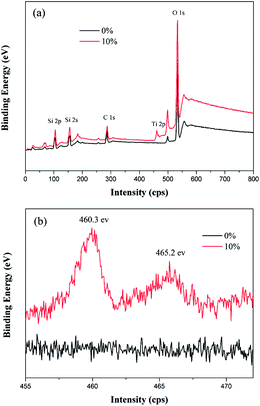
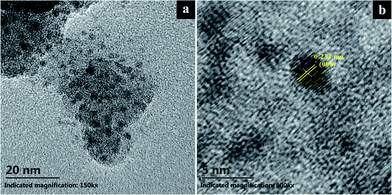
XPS was used to determine the chemical composition of the surface of 10% TiO2–SiO2 coating, as shown in Fig. 3. The appearance of the Ti2p peak in the 10% TiO2–SiO2 coating confirmed the introduction of TiO2 (Fig. 3b). The two peaks at 460.3 and 465.2 eV were the 2p3/2 orbital and the 2p1/2 orbital of the Ti atom,24,25 respectively. The reported binding energy of Ti2p3/2 for pure TiO2 coating was 458.2 eV.24,25 But, in this article, the Ti2p3/2 of the 10% TiO2–SiO2 coating was 460.3 eV. It showed a 1.1 eV of chemical shifts to the pure TiO2 coating. In the similar research work,24 the Ti2p3/2 binding energy of (TiO2)10(SiO2)90 coating shifted 1.25 eV as compared to that of pure TiO2 coating. The chemical shifts of Ti2p gave an evidence of the formation of interaction between SiO2 and TiO2 particles.To get further indication of TiO2 nanoparticles, the microstructures and crystalline of 10% TiO2–SiO2 powder were studied. TEM and HRTEM micrographs were shown in Fig. 4. It was obvious that the sample was consisting of good crystal TiO2 nanoparticles, which were dispersed in amorphous SiO2 particles, as shown in Fig. 4a. The average particle sizes of TiO2 estimated from HRTEM micrographs were about 5 nm (Fig. 4b). The spacing d = 0.232 nm of 10% TiO2–SiO2 powders corresponded to the (004) plane of anatase TiO2 phase.26,27 The nano-crystalline TiO2 particles in this coating provided high photocatalytic property (Fig. 5 and 6).25 And this size of TiO2 nanoparticles could be made optically clear enough for commercial window use.18
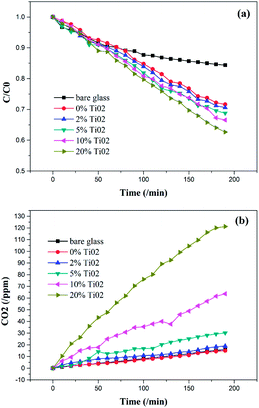 | ||
| Fig. 5 The curves of (a) conversion of C6H6 and (b) the amount of produced CO2 of different coatings under UV light irradiation. | ||
Self-cleaning properties
In this study, we investigated the self-cleaning property of coating by the degradation of air borne organic contaminants (gaseous benzene) and applied contaminants (fingerprints) under UV irradiation.On the one hand, photocatalytic activities of different coatings for degradation of gaseous benzene under UV irradiation at ambient temperature were investigated. The conversion of benzene and the production of CO2 were not observed when these samples were in dark. Fig. 5 showed the photocatalytic activities of coatings with different concentrations of TiO2 nanoparticles under UV light irradiation. As shown in Fig. 5a, the amount of benzene still decreased when there was only glass substrate and SiO2 coating in the reactor. This was due to the adsorption of gaseous benzene. But the amount of adsorption is limit. On the other hand, the coatings in this article were porous coating, which can be seen in the SEM images (Fig. 2). Thus, as shown in Fig. 5a, all porous coatings have the good absorption effect of gaseous benzene. But the conversion amount of benzene was different. Then the conversion of benzene was not enough to evaluate the photocatalytic activity of the coatings. So the curves of the production of CO2 of different coatings were also shown (Fig. 5b). The production of CO2 of bare glass and SiO2 coating were merely about 15 ppm which was almost same as that of the blank test (no glass substrate, only with benzene and UV-light irradiation), as shown in ESI (Fig. 10†). This increasing may be due to the increasing temperature of the reactor which was caused by the UV-light irradiation during the test (about from 25 °C to 36 °C). And the concentration of CO2 was affected by the temperature of the gas when it was measured with a gas chromatography. This result also proved that the decrease of benzene of bare glass and SiO2 coating was due to the absorption effect. As shown in Table 1, the production of CO2 increased remarkably from 19 ppm to 121 ppm (Fig. 5b and Table 1) and the contrast of generated carbon dioxide to the amount of degradation of benzene increased from 0.31 to 1.59, when the content of TiO2 nanoparticles increased from 2% to 20%. Those results shown the amount of production of CO2 was correlated to the total amount of TiO2. The photocatalytic activity of the coatings was corresponding to the formation of anatase TiO2 nanoparticles (Fig. 4b).26 From the above discussions, as the content of TiO2 nanoparticles increased in the coatings, their photocatalytic activities gradually increased.
| Glass | 0% | 2% | 5% | 10% | 20% | |
|---|---|---|---|---|---|---|
| ΔC6H6/ppm | 32 | 60 | 61 | 64 | 68 | 76 |
| ΔCO2/ppm | 16 | 15 | 19 | 30 | 63 | 121 |
| ΔCO2/ΔC6H6 | 0.5 | 0.25 | 0.31 | 0.46 | 0.93 | 1.59 |
On the other hand, photocatalytic activities of 10% TiO2–SiO2 coating for decomposing of fingerprint under UV irradiation at ambient temperature was investigated. During practical application, fingerprints will inevitably stick on the surface of coating. Then the anti-reflection property of the coating will be seriously affected. As shown in Fig. 6, the fingerprint disappeared completely from the surface of the coating after irradiation with UV light for 150 min. This result indicated that the self-cleaning property of this coating was useful to maintain high transmittance for a long time.
Surface wettability
Water droplets could spread out to form a thin water film on a hydrophilic surface and then, dusts on the surface could be taken away easily, showing the self-cleaning ability.2,19,20 Table 2 showed the contact angle of 1 μL water on the coatings with different content of TiO2 (0%, 2%, 5%, 10%, 20% and 100%), respectively. The water contact angle of the SiO2 coating was about 1°. The increase of the contact angle was proportional to the increase of the content of TiO2 nanoparticles in the coatings. However, the wettability of the coatings became almost same after UV irradiation. This was due to the special photo-induced hydrophilicity of TiO2.13,19,22 All coatings showed hydrophilic property with contact angle less than 1°, as shown in Table 2. On the other hand, we measured the duration of the photo-induced hydrophilicity of those TiO2–SiO2 coatings. The change of the contact angle in dark was shown in ESI (Fig. 11†). It seemed that the photo-induced hydrophilicity of those coatings was not permanent. The highly hydrophilic surface produced by UV light irradiation gradually converted to less hydrophilic state in several days. But the hydrophilicity state can easily be recovered by exposing the coatings to UV-light.| TiO2% | Glass | 0% | 2% | 5% | 10% | 20% | 100% |
|---|---|---|---|---|---|---|---|
| CA (before) | 38° | <1° | <1° | 9° | 15° | 21° | 51° |
| CA (after) | 37° | <1° | <1° | <1° | <1° | <1° | <1° |
Adhesion property
During practical application, the coated glass will be cleaned usually, thus the coating should have good abrasion resistance property. In this study, nanoscratch was performed to evaluate the abrasion resistance of the coating (10% TiO2–SiO2 coating), as shown in Fig. 7 and 8. The critical load, Pc, is defined as the lateral load when the lateral load fluctuation occurs in the scratch test. It is a comprehensive index that it represents the carrying capacity of the coating/substrate.28 It was mainly affected by the adhesive and hardness of the coating and substrate, the structure and thickness of the film. Fig. 7 showed the load-distance curves of nanoscratch test of base-catalyzed SiO2 coating and 10% TiO2–SiO2 coating. The Pc of the base-catalyzed SiO2 coating and 10% TiO2–SiO2 coating were 198 μN and 305 μN, respectively. This result pointed to excellent abrasion resistance property of this coating. It also could be identified in the AFM images of the scratches on the surface of two coatings (Fig. 8). It was obviously that the 10% TiO2–SiO2 coating exhibited better scratch resistance property.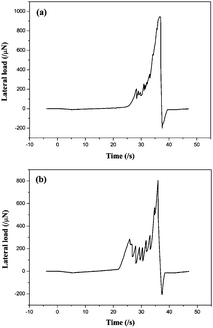 | ||
| Fig. 7 Loading-time curves of (a) the base-catalyzed SiO2 coating and (b) the 10% TiO2–SiO2 coating. | ||
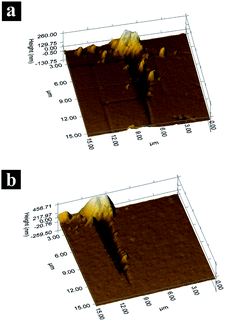 | ||
| Fig. 8 AFM images of scratch on the surface of (a) the base-catalyzed SiO2 coating and (b) the 10% TiO2–SiO2 coating. | ||
The preparation and the self-cleaning phenomena of the AR-SC coating were presented in Fig. 9. The base-catalyzed SiO2 particles were negatively charged and those particles repelled each other to form particulate sol.8,16 In the base-catalyzed SiO2 coating, individual SiO2 particles kept intact only by point contact forces,16 pointing to the poor mechanical robustness. On the other hand, the negatively charged SiO2 particles in silica sol could selectively adsorb positive charged TiO2 nanoparticles from the surrounding solution.29 TiO2 nanoparticles seemed to act as viscous glue between the silica particles. During the heat treatment process, ethanol solvent was evaporated and alkanol was burned; then pores with different sizes were appeared. In order to enhance the mechanical property, the neighboring particles were chemically bound to each other and to the surface through Ti–O–Si bonds (Fig. 3b and 9).2 As shown in Fig. 9, in particle application, organic pollutants and dirt particles will be stuck on the surface of coatings inevitably. The dirt particles will be easily aggregated in the organic pollutants, which may affect the anti-reflection effect of the coating. But, after UV irradiation, the TiO2 nanoparticles in the coating could exhibit the desired simultaneous property of photocatalysis and photo-induced hydrophilic. Then, the absorbed organic contaminants could be decomposed and the dust on the surface could be removed easily by the rainwater.
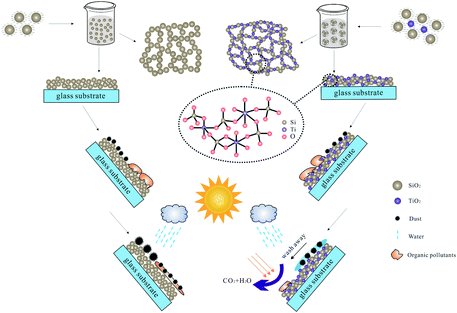 | ||
| Fig. 9 Schematic showing the preparation and the self-cleaning phenomena of the anti-reflection self-cleaning coating on glass substrate. | ||
Conclusions
In this study, glass substrates were coated with TiO2 and SiO2 nanoparticles to achieve coating with both anti-reflection, self-cleaning and abrasion resistance properties by spin-coating method. The coated glass substrate exhibit high transmittance even the concentration of TiO2 was up to 20%. The average transmittance of the coating (10% TiO2–SiO2) increased more than 2.3% as compared to uncoated glass substrate. Moreover, the photocatalytic activity of this coatings will lead to the decomposition of contaminants (gaseous benzene and fingerprints) keeping the surface clean and maintaining the high transmittance property. The photocatalytic and hydrophilic properties of coating provided the self-cleaning function. Further, this coating exhibited abrasion resistance property. The Pc of the coatings enhanced from 198 μN (0%) to 305 μN (10%), pointing to excellent abrasion resistance of this coating. The combined anti-reflection, self-cleaning and abrasion resistance properties should substantially improve the long-term optical properties of porous AR-SC coatings.Acknowledgements
This work was supported by the National Basic Research Program of China (Project no. 2009CB939705 and 2009CB939702). Also, we thank the technology support by the Analytic Testing Center of HUST for carrying out FESEM and XPS analyses.Notes and references
- H. E. Çamurlu, Ö. Kesmez, E. Burunkaya, N. Kiraz, Z. Yeşil, M. Asiltürk and E. Arpaç, Chem. Pap., 2012, 66, 461–471 CrossRef PubMed.
- L. Yao and J. H. He, Prog. Mater. Sci., 2014, 61, 94–143 CrossRef PubMed.
- L. Q. Ye, S. M. Zhang, Q. Wang, L. H. Yan, H. B. Lv and B. Jiang, RSC Adv., 2014, 4, 35818–35822 RSC.
- X. X. Zhang, M. Y. Zhuang, X. Miao, W. M. Su, M. Y. Lin, L. X. Lin, L. Q. Ye, L. H. Yan, W. B. Yang and B. Jiang, RSC Adv., 2014, 4, 48872–48875 RSC.
- G. S. Vicente, R. Bayon, N. German and A. Morales, Sol. Energy, 2011, 85, 676–680 CrossRef PubMed.
- Q. Q. Mao, D. W. Zeng and C. S. Xie, Mater. Lett., 2014, 132, 214–217 CrossRef CAS PubMed.
- S. Guldin, P. Kohn, M. Stefik, J. Song, G. Divitini, F. Ecarla, C. Ducati, U. Wiesner and U. Steiner, Nano Lett., 2013, 13, 5329–5335 CrossRef CAS PubMed.
- X. X. Zhang, B. B. Xia, H. P. Ye, Y. L. Zhang, B. Xiao, L. H. Yan, H. B. Lv and B. Jiang, J. Mater. Chem., 2012, 22, 13132–13140 RSC.
- R. Prado, G. Beobide, A. Marcaide, J. Goikoetxea and A. Aranzable, Sol. Energy Mater. Sol. Cells, 2010, 94, 1081–1088 CrossRef CAS PubMed.
- L. Yao and J. H. He, J. Mater. Chem. A, 2014, 2, 6994–7003 CAS.
- Y. K. Lai, Y. X. Tang, J. J. Gong, D. G. Gong, L. F. Chi, C. J. Lin and Z. Chen, J. Mater. Chem., 2012, 22, 7420–7426 RSC.
- G. Helsch and J. Deubener, Sol. Energy, 2012, 86, 831–836 CrossRef CAS PubMed.
- Z. Y. Liu, X. T. Zhang, T. Murakami and A. Fujishima, Sol. Energy Mater. Sol. Cells, 2008, 92, 1434–1438 CrossRef CAS PubMed.
- E. Burunkaya, Ö. Kesmez, N. Kiraz, H. E. Çamurlu, M. Asiltürk and E. Arpaç, Mater. Chem. Phys., 2010, 120, 272–276 CrossRef CAS PubMed.
- Z. Geng, J. H. He, L. G. Xu and L. Yao, J. Mater. Chem. A, 2013, 1, 8721–8724 CAS.
- A. Vincent, B. Suresh, E. Brinley, A. Karakoti, S. Deshpande and S. Seal, J. Phys. Chem. C, 2007, 111, 8291–8298 CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Nature, 1997, 388, 431–432 CrossRef CAS PubMed.
- I. P. Parkin and R. G. Palgrave, J. Mater. Chem., 2005, 15, 1689–1695 RSC.
- L. W. Zhang, R. Dillert, D. Bahnemann and M. Vormoor, Energy Environ. Sci., 2012, 5, 7491–7507 CAS.
- K. S. Liu, M. Y. Cao, A. Fujishima and L. Jiang, Chem. Rev., 2014, 114, 10044–10094 CrossRef CAS PubMed.
- X. Y. Li and J. H. He, ACS Appl. Mater. Interfaces, 2013, 5, 5282–5290 CAS.
- J. G. Cai, J. F. Ye, S. Y. Chen, X. W. Zhao, D. Y. Zhang, S. Chen, Y. R. Ma, S. Jin and L. M. Qi, Energy Environ. Sci., 2012, 5, 7575–7581 CAS.
- L. J. Gao and J. H. He, J. Colloid Interface Sci., 2013, 400, 24–30 CrossRef CAS PubMed.
- M. W. Gaultois and A. P. Grosvenor, J. Mater. Chem., 2011, 21, 1829–1836 RSC.
- N. Guo, Y. M. Liang, S. Lan, L. Liu, G. J. Ji, S. C. Gan, H. F. Zou and X. C. Xu, Appl. Surf. Sci., 2014, 305, 562–574 CrossRef CAS PubMed.
- W. J. Ong, L. L. Tan, S. P. Chai, S. T. Yong and A. R. Mohamed, Nanoscale, 2014, 6, 1946–2008 RSC.
- X. F. Wang, Q. Y. Xiang, B. Liu, L. J. Wang, T. Luo, D. Chen and G. Z. Shen, Sci. Rep., 2013, 3, 2007 Search PubMed.
- X. Obradors, T. Puig, M. Gibert, A. Queralto, J. Zabaleta and N. Mestres, Chem. Soc. Rev., 2014, 43, 2200–2225 RSC.
- J. J. Jing, Y. Zhang, W. Y. Li and W. W. Yu, J. Catal., 2014, 316, 174–181 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10424b |
| This journal is © The Royal Society of Chemistry 2014 |