Open Access Article
Open Access ArticleActivity enhancement of zeolite MCM-22 by interlayer expansion enabling higher Ce loading and room temperature CO oxidation†
Wieslaw J.
Roth
a,
Waclaw
Makowski
a,
Bartosz
Marszalek
a,
Piotr
Michorczyk
b,
Weronika
Skuza
a and
Barbara
Gil
*a
aFaculty of Chemistry, Jagiellonian University in Kraków, Ingardena 3, 30-060 Kraków, Poland. E-mail: gil@chemia.uj.edu.pl; Fax: +48 12 634 0515
bFaculty of Chemical Engineering and Technology, Cracow University of Technology, Warszawska 24, 31-155 Kraków, Poland
First published on 8th August 2014
Abstract
Layered zeolite precursor MCM-22P can be stabilized as interlayer expanded structure MWW-IEZ with enlarged pores by silylation producing –O–Si(OH)2–O– bridges. It adsorbs two times more cerium than MCM-22 and becomes activated for CO oxidation to CO2 at room temperature. This represents one of the most notable activity enhancements upon modification of layered zeolites.
Zeolites are valuable catalytic materials1 widely used in industrial processes2 with exceptional activity and selectivity produced by their framework structures with ordered micropores and strong acid sites resulting from Al incorporation.3,4 These inherent zeolite characteristics can be further expanded and enhanced by modification with metal ions as exemplified by the lanthanide stabilization of zeolite Y making it a superb cracking catalyst5 and Ti-ZSM-5, which is very active for oxidations.6 However, the very qualities that make zeolites exceptional, i.e. rigid frameworks with fixed micropores, often prevent their functionalization with transition metals because of size constraints or charge incompatibility. New opportunities appeared with the discovery of zeolite framework formation as layered materials.7 It enabled spatial modification,8 especially expansion, similar to the well known behavior of 2D solids.9–12 As shown below, structural expansion can produce new results, which may be prohibited by the rigidity of 3D frameworks, by combining zeolitic activity with new metal functionality. The field of layered, 2D zeolites13 is expanding14 including remarkable findings like the first top down synthesis of a new zeolite15 and preparation of ZSM-5 as nanosheets by design.16 The title zeolite of this work MCM-22, with the framework denoted MWW, is the archetype and leading representative of 2D zeolites. Its layers contain internal 10-ring sinusoidal channels and 12-ring cavities on the surface. The latter form 0.7 × 1.8 nm supercages accessible through elliptical 10-ring apertures in the 3D framework MCM-22.7 Expansion of the interlayer distance increases access to these surface cavities containing Brønsted acid sites. MCM-22 is a commercial catalyst for aromatic alkylation with exceptional selectivity attributed to its surface cavities.17 More than ten frameworks demonstrated formation of layered forms18 and their ranks continue to grow.11,19 Many more,20 possibly all zeolites9 may allow formation of 2D species.
The precursor MCM-22P contracts upon calcination producing the complete MWW framework but it can be stabilized as expanded zeolite by silylation21 with insertion of O2Si(OH)2 interlayer bridges. The product, called interlayer expanded zeolite, MWW-IEZ, has 12-ring openings between layers.22 It is shown here to allow increased adsorption of Ce3+ with generation of new activity – CO oxidation at room temperature (RT), which has been monitored quantitatively by IR. The standard 3D MCM-22 has much lower capacity for Ce and is inactive showing no CO adsorption at RT. The observed activation seems to be related to increased Ce access to Al centers and generation of metal species in the formal 4+ state.
The oxidation of CO is currently of great interest because of the desire and mandates to remove toxic components from exhaust gases. Many metal23 and oxide catalysts24 are active for this process. Gold nanoparticles are very active at low temperatures and better than platinum metals.23 Recent examples of active materials include cerium/ceria with noble metals25,26 and ZSM-5,27,28 Zn in ZSM-5,29 rare earth in MCM-4130 and ceria hollow nano-spheres.31 Rare earth incorporation into zeolites provided the first breakthrough in zeolite catalysis5 but proved problematic with important medium pore high silica zeolites. The present result with MWW-IEZ demonstrates that expansion of zeolite structures may enhance incorporation of metal ions and expand catalytic potential, especially oxidation, which for zeolites is still below expectations.
Results and discussion
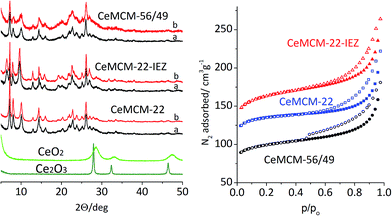
Detailed description of experimental procedures is provided in the ESI.† MCM-22P and MCM-56/49 were prepared using gels with Si/Al = 24 and 12, respectively. MCM-22P was silylated with CH3Si(OCH2CH3)2. The XRD, N2 sorption and spectroscopic characterizations were based on standard procedures.Three MWW materials were investigated in calcined forms: the standard MCM-22 zeolite (Si/Algel = 24), its IEZ derivative with discrete O2Si(OH)2 interlayer bridges, and high Al MCM-56/49 (Si/Algel = 12) containing randomly packed MWW layers with some 3D ordering concluded from XRD. The identity and good quality of materials were confirmed by XRD and N2 sorption (Fig. 1 and 2). MWW-IEZ showed a distinct peak at 6.4° in XRD, proving an interlayer distance of 2.7 nm and increased micropore volume due to the expanded structure. For MWW-IEZ the N2 adsorption isotherm shows a considerable slope at intermediate pressures indicating increased access to the interlayer space, i.e. the external surface of the layers. Basic composition and acidity properties are listed in Table 1.
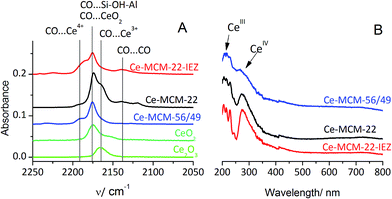 | ||
| Fig. 2 (A) IR spectra of CO adsorbed at −100 °C on cerium–zeolites, commercial Ce2O3 and CeO2. (B) UV-Vis-DRS spectra of cerium–zeolites. | ||
| Zeolite | Si/Al, XRF | BET, m2 g−1 | Ce (La), wt% | BAS μmol g−1 | LAS, μmol g−1 |
|---|---|---|---|---|---|
| MCM-22 | 13 | 436 | — | 664 | 46 |
| MCM-22-IEZ | 13 | 531 | — | 621 | 112 |
| MCM-56/49 | 8 | 324 | — | 678 | 56 |
| Ce-MCM-22 | 13 | — | 0.40 | 714 | 95 |
| Ce-MCM-22-IEZ | 13 | — | 0.88 | 357 | 70 |
| Ce-MCM-56/49 | 8 | — | 0.74 | 792 | 72 |
| La-MCM-22 | 12 | — | 0.28 | 721 | 84 |
| La-MCM-22-IEZ | 13 | — | 0.52 | 457 | 110 |
| La-MCM-56/49 | 7 | — | 0.45 | 963 | 48 |
The pyridine adsorption measured by FTIR indicated high Brønsted acid site concentrations (BAS) of over 600 μmol g−1, consistent with previously reported data.32 The unmodified zeolites MCM-22 and MCM-56/49 showed a slight increase of the BAS after incorporation of Ce3+.
The expanded IEZ form adsorbed 2 times more cerium ions with about the same Al content as MCM-22. A significant reduction in the BAS ensued. The enhanced Ce adsorption must be related to the expanded interlayer distance, which appears to allow diffusion of ions between the layers. The incorporation of Ce into high silica zeolites (Si/Al > 5) cannot occur by the standard balancing of the 3+ or 4+ charge by 3 to 4 Al centers, which are unlikely to be grouped close enough. The overall effective charge on such ions may be reduced33 through interactions with the oxide lattice, proton losses from coordinated water and other not fully accounted for phenomena.
The XRD patterns show the absence of either CeO2 or Ce2O3 oxides suggesting that the sizes of postulated oxide-like clusters are below the detection limit for this method.
The nature of incorporated Ce was established by IR after CO adsorption at low temperature (−100 °C). The spectra, shown in Fig. 2A, indicated only Ce3+ species in the MCM-22 zeolite while MCM-56 and MWW-IEZ contained Ce in both 3+ and 4+ oxidation states in different proportions. Ce4+ enables oxidation of CO as temperature increases. In all cases, the band at 2175 cm−1 due to CO on OH groups is present. CO adsorbed on MCM-22 reveals a shoulder at 2165 cm−1, the same as when adsorbed on bulk Ce2O3. It suggests that most of the Ce is located at the external surfaces of the zeolite crystals, in the form of small Ce2O3-like clusters. The Ce-MCM-22-IEZ and Ce-MCM-56/49 show an additional IR band at 2190 cm−1, which can be assigned34 to CO adsorbed on Ce4+. The position is at higher frequency than in commercial CeO2 (2175 cm−1) suggesting greater coordinative unsaturation of Ce4+.35
The increasing adsorption of Ce ions, apparently caused by a greater number of Al sites accessible to them in different MWW forms, results in increasing oxidation to Ce4+ and activation of CO. MCM-56/49 is more open than MCM-22 and has a higher Al content, which is presumably conducive to generation of Ce4+ centers that retain adsorbed CO and cause its oxidation. For Ce-MCM-22-IEZ the share of Ce4+ is the highest, probably because the increased separation of the zeolite layers produces enough room for the Ce4+ cations to be located in exchangeable positions replacing some acidic protons.
This is suggested by the decreased concentration of BAS. Most likely some small CeO2-like clusters are formed at the surface of MWW layers, both internal in the crystal and external, including surface pockets. The geminal silanols in the interlayer bridges appear unchanged and do not interact with cerium.
The proposed nature of Ce centers on MWW zeolites was supported by UV-Vis spectra (Fig. 2B), which indicated the presence of small clusters of oxygen-coordinated Ce4+ and Ce3+ ions. A band near 300 nm is due to CeIV,36 while the band below 260 nm is assigned to CeIII. Both are very sensitive to the environment and dispersion of Ce ions.37
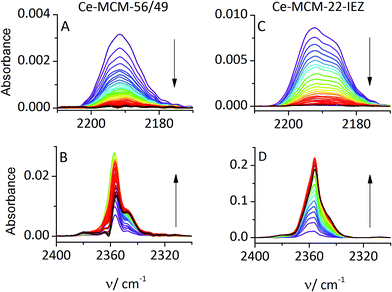
The oxidation of CO was observed upon warming of the IR cell to RT, after the initial adsorption at −100 °C and desorption of excess gas. The band for CO interacting with Ce4+ cations decreased with time, and the adsorbed CO2 band (2360 cm−1) increased, as shown in Fig. 3. Ce-MCM-22 did not show adsorbed CO at RT indicating an insufficient content of Ce4+ for detectable reaction. Both Ce-MCM-22-IEZ and Ce-MCM-56/49 showed oxidation of CO, attributed to the presence of Ce4+. The expanded IEZ structure was more active, which reflects its higher content of Ce4+.
The participation of Ce4+ is supported by control studies with La-MWW materials, where 3+ to 4+ metal oxidation is not viable. Only La-MCM-22-IEZ shows significant uptake of CO at −100 °C but no CO is retained at RT and consequently no oxidation is taking place (cf. ESI†). These results are important in supporting the critical role of redox metal centers represented by Ce in contrast to La.
The restoration of Ce4+ was demonstrated for Ce-MCM-22-IEZ. After evacuation of CO2 at RT the temperature was lowered to −100 °C but new exposure to CO did not restore the Ce4+–CO band at 2190 cm−1. This confirms that reduction to Ce3+ occurred upon CO oxidation at RT. When the sample was activated in pure oxygen (40 min, 450 °C) and the adsorption of CO was repeated at −100 °C, the band 2190 cm−1 (CO–Ce4+) was restored to its original intensity (Fig. 4). This demonstrates potential for the catalytic process, which has been confirmed by preliminary studies.
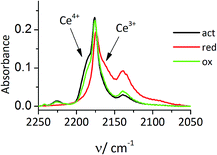 | ||
| Fig. 4 IR spectra of CO adsorbed at −100 °C on activation, reduced by CO reaction and oxidized Ce-MCM-22-IEZ. | ||
Conclusions
The reported results confirm that layered zeolite materials provide new opportunities because of potential for post-synthesis modification. The expansion of the interlayer space enabled increased adsorption of larger metal cations, such as Ce, resulting in novel enhanced activity. The approach may be general and can be expanded to other active metals and processes. MCM-22 is particularly attractive because of convenient availability in diverse active forms.Acknowledgements
This work was financed with the funds from the Narodowe Centrum Nauki provided on the basis of decision number DEC-2011/03/B/ST5/01551. The IR measurements were carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08).Notes and references
- A. F. Masters and T. Maschmeyer, Microporous Mesoporous Mater., 2011, 142, 423–438 CrossRef CAS PubMed.
- I. Fechete, Y. Wang and J. C. Védrine, Catal. Today, 2012, 189, 2–27 CrossRef CAS PubMed.
- C. Baerlocher, L. B. McCusker and D. H. Olson, Atlas of Zeolite Framework Types, Elsevier, Amsterdam, 2007, http://www.iza-structure.org Search PubMed.
- J. Cejka, H. van Bekkum, A. Corma and F. Schueth, Introduction to Zeolite Molecular Sieves, Elsevier, Amsterdam, 2007 Search PubMed.
- C. J. Plank, E. J. Rosinski and W. P. Hawthorne, Ind. Eng. Chem. Prod. Res. Dev., 1964, 3, 165–169 CrossRef CAS.
- G. Bellussi and V. Fattore, Stud. Surf. Sci. Catal., 1991, 69, 79–92 CrossRef CAS.
- M. E. Leonowicz, J. A. Lawton, S. L. Lawton and M. K. Rubin, Science, 1994, 264, 1910–1913 CAS.
- W. J. Roth, C. T. Kresge, J. C. Vartuli, M. E. Leonowicz, A. S. Fung and S. B. McCullen, Stud. Surf. Sci. Catal., 1995, 94, 301–308 CrossRef CAS.
- W. J. Roth, Stud. Surf. Sci. Catal., 2007, 168, 221–239 CrossRef CAS.
- W. J. Roth and J. Cejka, Catal. Sci. Technol., 2011, 1, 43–53 CAS.
- F. S. O. Ramos, M. K. De Pietre and H. O. Pastore, RSC Adv., 2013, 3, 2084–2111 RSC.
- A. Corma, U. Diaz, M. E. Domine and V. Fornés, Angew. Chem., Int. Ed., 2000, 39, 1499–1501 CrossRef CAS.
- W. J. Roth and D. L. Dorset, Microporous Mesoporous Mater., 2011, 142, 32–36 CrossRef CAS PubMed.
- W. J. Roth, P. Nachtigall, R. E. Morris and J. Čejka, Chem. Rev., 2014, 114, 4807–4837 CrossRef CAS PubMed.
- W. J. Roth, P. Nachtigall, R. E. Morris, P. S. Wheatley, V. R. Seymour, S. E. Ashbrook, P. Chlubná, L. Grajciar, M. Položij, A. Zukal, O. Shvets and J. Čejka, Nat. Chem., 2013, 5, 628–633 CrossRef CAS PubMed.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246–249 CrossRef CAS PubMed.
- T. F. Degnan Jr, C. M. Smith and C. R. Venkat, Appl. Catal., A, 2001, 221, 283–294 CrossRef.
- W. J. Roth, B. Gil and B. Marszalek, Catal. Today, 2014, 227, 9–14 CrossRef CAS PubMed.
- B. Marler and H. Gies, Eur. J. Mineral., 2012, 24, 405–428 CrossRef CAS.
- K. Na, C. Jo, J. Kim, K. Cho, J. Jung, Y. Seo, R. J. Messinger, B. F. Chmelka and R. Ryoo, Science, 2011, 333, 328–332 CrossRef CAS PubMed.
- P. Wu, J. F. Ruan, L. L. Wang, L. L. Wu, Y. Wang, Y. M. Liu, W. B. Fan, M. Y. He, O. Terasaki and T. Tatsumi, J. Am. Chem. Soc., 2008, 130, 8178–8187 CrossRef CAS PubMed.
- W. B. Fan, P. Wu, S. Namba and T. Tatsumi, Angew. Chem., Int. Ed., 2004, 43, 236–240 CrossRef CAS PubMed.
- Y. Yu, J. Huang, T. Ishida and M. Haruta, in Modern Heterogeneous Oxidation Catalysis, ed. N. Mizuno, Wiley, Weinheim, 2009 Search PubMed.
- S. Royer and D. Duprez, ChemCatChem, 2011, 3, 24–65 CrossRef CAS PubMed.
- W. Han, P. Zhang, Z. Tang, X. Pan and G. Lu, J. Sol–Gel Sci. Technol., 2013, 66, 526–532 CrossRef CAS.
- S. Zeng, W. Zhang, M. Śliwa and H. Su, Int. J. Hydrogen Energy, 2013, 38, 3597–3605 CrossRef CAS PubMed.
- W. Han, Z. Tang, P. Zhang and G. Lu, Catal. Surv. Asia, 2013, 17, 147–155 CrossRef CAS.
- W. Han, P. Zhang, Z. Tang and G. Lu, Process Saf. Environ. Prot., 2013 DOI:10.1016/j.psep.2013.04.003.
- G. Qi, J. Xu, J. Su, J. Chen, X. Wang and F. Deng, J. Am. Chem. Soc., 2013, 135, 6762–6765 CrossRef CAS PubMed.
- O. A. González Vargas, J. A. De Los Reyes Heredia, A. Montesinos Castellanos, L. F. Chen and J. A. Wang, Mater. Chem. Phys., 2013, 139, 125–133 CrossRef PubMed.
- Y. Jiao, F. Wang, X. Ma, Q. Tang, K. Wang, Y. Guo and L. Yang, Microporous Mesoporous Mater., 2013, 176, 1–7 CrossRef CAS PubMed.
- B. Gil, W. Makowski, B. Marszalek, W. J. Roth, M. Kubu, J. Čejka and Z. Olejniczak, J. Chem. Soc., Dalton Trans., 2014, 43, 10501–10511 RSC.
- B. Gil, P. Pietrzyk, J. Datka, P. Kozyra and Z. Sojka, Stud. Surf. Sci. Catal., 2005, 158A, 893–900 CrossRef.
- A. Bensalem, F. Bozon-Verduraz, M. Delamar and G. Bugli, Appl. Catal., A, 1995, 121, 81–93 CrossRef CAS.
- C. Binet, M. Daturi and J. C. Lavalley, Catal. Today, 1999, 50, 207–225 CrossRef CAS.
- R. Si, Y. W. Zhang, L. P. You and C. H. Yan, Angew. Chem., Int. Ed., 2005, 44, 3256–3260 CrossRef CAS PubMed.
- A. Bensalem, J. C. Muller and F. Bozon-Verduraz, J. Chem. Soc., Faraday Trans., 1992, 88, 153–154 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experiments, H2-TPR profiles, CO adsorption on La-MWW and enlarged versions of XRD patterns with MWW zeolites before cerium-exchange. See DOI: 10.1039/c4ta03308f |
| This journal is © The Royal Society of Chemistry 2014 |