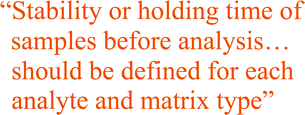Sample stability studies for environmental analysis
Analytical Methods Committee, AMCTB No 65
First published on 30th January 2015
Abstract
The concentration of an analyte in a sample may change with time through chemical or biological degradation. Analysts must therefore establish a set of conditions that ensure that any such change occurring between sampling and analysis is negligible. These conditions can be defined by specifying: (a) the sample container; (b) transport and storage requirements; and (c) maximum holding times in storage before analysis.
Samples cannot be expected to be stable indefinitely and it is important that the stability or holding time is known for each analyte and matrix type. The stability or holding time can be defined as the maximum allowable duration between the taking of the sample and the analysis. This will include transport to, and storage in, the laboratory. There are several factors that should be considered as part of the stability study design. Loss by volatilisation can be minimised by completely filling the container, sensitivity to light by use of amber glass or opaque bottles. Chemical or biological reactions can be slowed or halted by the use of preservatives and by cooling or freezing of samples. Some materials may require pre-treatment before measurement and it may be necessary to take that separately into account.
Approaches to determining stability
There are several publications that detail holding times for soils and waters.1–4 Some of these are based on validated stability studies and some on ‘best practice’. Whilst these are useful as guidance documents, particularly with respect to information regarding storage vessels, they can be inconsistent in the recommended storage conditions and holding times. These inconsistencies are probably due to differences in matrix composition of the samples used to define the storage conditions.Literature guidelines can be used for samples with well defined matrices (e.g., potable water) but the relevant sample vessels, storage conditions and holding times, as described in the cited publication, must be adhered to. An alternative approach would be for in-house stability study trials to be undertaken. The advantage of this approach is that the conditions established would be of guaranteed applicability. An in-house stability study can give useful information on the chemical characteristics of the samples and may allow for longer holding times than those quoted in the literature.
Stability study design
In-house stability studies should be designed to ensure that they provide the data to support the derived holding time. There are several publications that describe stability study design1,5–7 and other information is available in the international standards associated with the preparation of certified reference materials and proficiency testing, for example ISO Guide 35 and BS ISO 13528:2005 (Annex B). The statistical protocol used for assessment of the data is a critical component of the stability study. Often compromises will have to be made; the key factor is that the stability or holding time derived from the study should be defensible. There are a variety of possible approaches but there are some important factors that should be considered as part of any stability study trial.(a) Ideally the test materials used within the stability study should be typical of those routinely sampled/analysed. If more than one type of test material is analysed, then separate studies for each matrix type should be undertaken. Alternatively, where justified, stability studies can be undertaken on the most challenging type and applied generally as a ‘worst-case scenario’. Use of a synthetic matrix should be avoided where possible. Testing should be conducted on test materials with the analyte in its native (‘incurred’) species and close to the concentration of interest. Tests at other concentrations may be informative but are not sufficient. Tests involving fortification with the analyte (spiking) should be avoided, wherever possible.
(b) The stability should be assessed in the container, with the preservatives, and under the storage conditions typical for the analysis in question. If these factors are unknown then stability may need to be assessed under various conditions. It may also be expedient to check stability at additional temperatures to allow for variations in conditions of transportation.
(c) The design of the stability study should provide sufficient statistical power. It is usually advisable to employ a method of the highest precision available to achieve this. Routine precision may not be sufficient. The precision then determines the number and timing of points in the time series and the number of replicate measurements at each point. The number and spacing of time points should be sufficient to determine and confirm the stability time. It is unlikely that stability will be established and confirmed in less than three time points.
(d) The experimental design should be such as to avoid any ambiguity between changes in the test material and changes in the performance of the analytical method, for example, by using an isochronous design or including a quality control sample of known stability.
Conclusion
Stability or holding time of samples before analysis is an important aspect of any chemical or biological procedure and should be defined for each analyte and matrix type so that the integrity of the sample is maintained. Holding times can sometimes be obtained from the published literature but care should be taken to ensure that they are appropriate for the matrix type and that the conditions quoted are followed exactly. An alternative approach is to carry out an in-house stability study and details are given of the areas that need to be considered in the design of a stability study and in the assessment and evaluation of the data produced.Robert Bettinson (United Kingdom Accreditation Service).
This Technical Brief was prepared for the Analytical Methods Committee and approved on 01/09/14.
References
- BS EN ISO 5667–3:2012, Water Quality – Sampling, Part 3: Preservation and handling of water samples, (Contains holding times for waters in Table A-1 and stability trials information in Appendix C).
- United States Environmental Protection Agency (USEPA), Analytical Support Branch, Laboratory Operations and Quality Assurance Manual, 2014, http://www.epa.gov/region4/sesd/asbsop/). Contains details on holding times for water, soil, waste and tissue for various determinands in Table 3.1.
- Sampling and Analysis of Waters, Wastewaters, Soils and Wastes, IWRG 701, Environmental Protection Authority (epa), Victoria, Australia, 2009, http://www.epa.vic.gov.au/%7E/media/Publications/IWRG701.pdf.
- United States Environmental Protection Agency (USEPA), Sample Holding Time Re-evaluation, EPA/600/R-05/124, October 2005, http://www.epa.gov/esd/cmb/research/bs_033cmb06.pdf.
- Drinking Water Inspectorate (DWI), Guidance on Sample and Sample Extract Stability Trials, 22 August 2005 http://dwi.defra.gov.uk/stakeholders/guidance-and-codes-of-practice/.
- A Manual on Analytical Quality Control for Water Industry (NS30), R.V Cheeseman and A.L Wilson, Revised M.J Gardiner, June 1989, ISBN Number 0902156853, Appendix A6.1 – Design of tests for the investigation of sample stability.
- Standard Practice for Estimation of Holding Time for Water Samples Containing Organic and Inorganic Constituents, ASTM D4841–88, 2013, http://www.astm.org/Standards/D3694.htm.
| This journal is © The Royal Society of Chemistry 2015 |