Reversible biomechano-responsive surface based on green fluorescent protein genetically modified with unnatural amino acids†
Johan
Longo‡
a,
Chunyan
Yao‡
b,
César
Rios
a,
Nguyet Trang Thanh
Chau
a,
Fouzia
Boulmedais
ac,
Joseph
Hemmerlé
d,
Philippe
Lavalle
d,
Stefan M.
Schiller
*befgh,
Pierre
Schaaf
*ad and
Loïc
Jierry
ac
aICS (UPR22-CNRS), 23 rue du Loess, 67034, Strasbourg, France. E-mail: schaaf@unistra.fr
bUniversität Freiburg, Albertstrasse 19, Freiburg im Breisgau, Germany. E-mail: Stefan.Schiller@ZBSA.Uni-Freiburg.de
cUSIAS, 5 allée du Général Rouvillois, 67083 Strasbourg, France
dINSERM, UMR-S 1121, 11 rue Humann, 67085 Strasbourg Cedex, France
eInstitute for Pharmaceutical Sciences, Albertstr. 25, D-79104, Germany
fFRIAS, School of Soft Matter Research, Albertstr. 19, D-79104, Germany
gBIOSS Centre for Biological Signalling Studies, Univ. of Freiburg, Schänzlestrasse 18, D-79104 Freiburg, Germany
hDep. of Microsystems Engineering, Univ. of Freiburg, Georges-Köhler-Allee 102, D-79110 Freiburg, Germany
First published on 6th November 2014
Abstract
GFP has been genetically modified at two specific positions of its molecular architecture. These modifications allow its covalent attachment onto PEG brushes grafted on functionalized silicone surfaces. The stretching of this material leads to a reversible decrease of the fluorescence intensity due to stretch-induced forces applying on GFP molecules.
Chemo-mechanoresponsive processes can be defined as the transfer of information initiated by a mechanical force and resulting in a chemical signal. This topic is a fast-growing field of study which covers biology, chemistry and more recently material science. From a chemical point of view there are two possible approaches to tackle this subject: one is to investigate the effect of a mechanical force on the behavior of covalent bonds to induce bond breaking and/or intramolecular reactions. It is, for example, illustrated by the work of the groups of Moore and Sottos1 who showed that a mechanical force can induce chemical modifications in materials containing “mechanophore” compounds, leading to a color change in material regions of high mechanical strain. Currently, a number of chemo-mechano-responsive materials based on an intramolecular reaction resulting in a color change of the material have been reported.2 A second approach is to mimic the mechanotransduction processes used by nature to transform a mechanical signal into a chemical one. Such processes are used by cells for sensing the mechanical properties of their environment with widespread consequences on their fate. One way found by nature to transform a mechanical signal into a chemical response is through conformational changes of proteins subjected to a mechanical force.3 Fibronectin, an adhesion protein present in the extracellular matrix represents a prominent example.4 Since the last five years, our group has developed approaches mimicking nature to design mechano-responsive materials based on the hiding of ligands5 or enzymes,6 becoming accessible to their environment through mechanical stress.
Up to now, many computational simulations and single-molecule studies have demonstrated that the application of a mechanical force on a protein can result in a deformation of its tertiary structure.7,8 In particular, green fluorescent protein (GFP), a model protein extensively studied because of its photophysical properties and used for detection of biomolecule localization or expression, has its fluorescence emission due to its particular folding where a chromophore is located in the center of a β-barrel structure. It has been shown that mechanical stresses applied to this β-barrel alter their resulting fluorescence.7
Herein, we present a general method to construct biomechano-responsive surfaces based on stretch-induced forces on proteins that should induce conformational changes. To make a proof of principle, and in particular to prove that stretching a material can induce protein conformational changes, we use GFP as mechano-sensitive element able to undergo a fluorescence change upon conformational changes. GFP has been specifically modified at two opposite positions on the barrel surface of its three dimensional structure to be covalently grafted onto a functionalized silicone substrate. A uniaxial stress of this material at various strains is applied to affect the conformation of GFP resulting in changes in fluorescence emission.
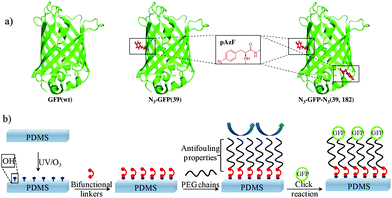
The green fluorescent protein (GFP) from Aequorea Victoria is a protein composed of 238 amino acid residues (26.9 kDa) forming an eleven-stranded β-barrel structure with an α-helix running through the center of the barrel.9 Along its central helix, inside the hydrophobic core, a para-hydroxybenzylidene imidazolidone chromophore (HBI) is located. This chromophore is formed through an internal cyclisation of Ser65, Tyr66 and Gly67 into a conjugated structure. Single-molecule measurements have shown that the three dimensional deformation response of GFP varies with the direction of the force applied. In particular, a minimal force-extension is required along the direction of the amino acid positions 3 and 212 to partially unfold GFP, compared to other directions of stress tested.7 Truncated GFP mutant has been produced by Saeger et al. to mimic GFP elongated along roughly the same direction (3, 212). These authors observed a decrease of the fluorescence intensity but no shift of the fluorescence maxima.8 Therefore, we decided to introduce unnatural amino acids with bioorthogonal reactivity10 at positions of the GFP surface, sensitive to structural forces exerted. Emphasis was placed on several boundary conditions: (1) the positions to be replaced with the phenyl-azide site-chain should be close structural homologues favoring Tyr-positions. (2) The force steps needed to disturb GFP and its elongation length should be small. (3) The positions should be placed on opposite sites of the GFP-barrel directly on the β-strand to effectively exert stretching forces. Taking these considerations into account two tyrosines, Tyr39 and Tyr182, present in the GFP wild type (wt) sequence appeared as appropriate candidates. Both amino acids are known for not playing a major role in protein folding and are furthermore accessible from the outside of the structure. para-Azidophenylalanin (pAzF) was chosen to replace Tyr39 and Tyr182. Indeed, pAzF is an unnatural amino acid having a similar chemical structure as tyrosine except that an azido group in para position of the aromatic ring replaces the natural phenolic hydroxyl group. The azide group allows to covalently bind GFP through Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) onto a silicone surface modified beforehand in a suitable way. Representation of the molecular structure of GFP (wt) and GFP mutants N3-GFP (39) and N3-GFP-N3 (39, 182) are shown in Fig. 1a. All details concerning their preparations are given in Part 1 of the ESI.†
Covalent grafting of azide containing GFP mutants onto a substrate required the modification of its surface by the introduction of alkyne groups. The elastomer chosen was the polydimethylsiloxane (PDMS) named Sylgard-184 (Part 2, ESI†). This PDMS is a filler-free elastomer, transparent and chemically inert. Genzer et al. have developed a mild and efficient method of PDMS oxidation based on UV irradiations and ozone treatment (UVO).11 This treatment generates the formation of silanol groups on the surface in a controlled way. We used this method to initiate the modification of the silicone before the ultimate grafting step of azide-containing GFP mutants. The three steps of silicone treatment are illustrated in Fig. 1: (i) once the UVO oxidation is realized, (ii) a homobisfunctional linker such as the 4,4′-methylenebis(phenyl)isocyanate (MDI) can be used to modify the activated surface. This linker, generally used for the modification of polyurethane, is covalently anchored onto the silicone surface allowing to introduce free and reactive isocyanate groups. (iii) Nucleophilic addition between these groups and amine terminated PEG chains modified with alkyne groups at the other terminus leads to functionalized PEG brushes surfaces for the covalent attachment of azide functionalized GFP via click-chemistry.
One hour of UVO exposition of a 30% stretched PDMS leads to a hydrophilic surface. Stretching of the substrate was done using a home-made device (Fig. S1, ESI†) and thus allows higher density of polar groups generated on the PDMS surface.11 Water contact angle measurements reveal a value of 71° compared to 105° of the native silicone sheet (Part 3.1 in ESI†). The presence of hydroxyl groups (silanol) all over the surface is confirmed by ATR FTIR spectroscopy. Then, this highly hydroxylated surface is quickly brought in contact with 0.2 mg mL−1 of 4,4′-methylenebis(phenyl isocyanate) in acetone solution during two hours leading to a contact angle of 90°. PEG chains (3000 Da), terminated with amine on one side and alkyne on the other side, are brought in contact with the silicone surface. Nucleophilic addition of the amino-PEG chains provides a highly hydrophilic surface with 5° of water contact angle. All chemical modifications of PDMS surfaces have been confirmed by XPS analysis (Part 3.4 in ESI†). Next, the GFP mutants are grafted onto this substrate through CuAAC click chemistry in presence of Cu(II) sulfate and sodium ascorbate.
Proteins are sensitive biomacromolecules which need to be handled under appropriate conditions to maintain their whole structural and functional integrity. In particular, it is known that copper salts have to be avoided because of their ability to denature proteins due to complexation and induction of redox processes. To circumvent this problem we used tris(3-hydroxypropyltriazolylmethyl)amine (THPTA), a water soluble ligand of Cu(I).12 This ligand complexes Cu(I) and thus avoids side-reactions and increases the yield of the click reaction. The following click reactions between GFP (wt) or mutants, N3-GFP (39) and N3-GFP-N3 (39, 182), onto the modified silicone substrate were thus realized in presence of copper sulfate, sodium ascorbate and THPTA in buffered solution (pH 7.4), at 25 °C and over 60 minutes.
As expected, the presence of the PEG brushes on the substrate suppresses non-specific binding of GFP (wt) (Fig. 2a). N3-GFP (39) and N3-GFP-N3 (39, 182) grafting onto the substrate through the reaction between the alkyne-terminated PEG-chains and the azide moieties introduced into both GFP mutants leads to surfaces with almost similar fluorescence emissions (Fig. 2b and c). This indicates that the click reaction was effective. Stretching unidirectionally the PDMS surface modified with the N3-GFP-N3 (39, 182) (GFP molecule with two anchoring sites) by 10%, 20% and 30% leads respectively to 23%, 42% and 60% decrease of the initial fluorescence (see Fig. 3a). All the experiments were performed while maintaining the surfaces in the hydrated state with buffer. Beyond 30% of stretching, we observed tearing of the substrate. We thus limited our study to this percentage of elongation. A full restoration of the initial fluorescence emission is measured after the material is relaxed to 0% strain. Furthermore, three stretching–relaxation cycles (we could not perform more cycles due to breakage of the silicone sheets) show that the influence of the stretching on the fluorescence emission is fully reversible which is remarkable (Fig. 3c). In order to verify that this fluorescence decrease induced by stretching is not related to a dilution of the fluorescence molecules over the surface during stretching we performed similar experiments but with mono-azide mutant N3-GFP (39). Logically, no stretched-induced force and no conformational modification of these N3-GFP molecules should occur with stretching. We measured, as expected, a decrease of the fluorescence related to a decrease in density, but to a much smaller extend than for the di-azide mutant N3-GFP-N3 (39, 182) molecules: 8%, 15% and 22% (instead of 23%, 42% and 60% respectively) for 10%, 20% and 30% stretching degrees respectively (Fig. 3a). This clearly indicates that most of the fluorescence decrease observed with the di-azide mutant upon stretching can be attributed to stretched-induced forces applying on the GFP molecules affecting their fluorescence, in agreement with single molecule measurement and simulations previously reported.7,8 In fact during stretching, the PEG anchor chains will become elongated and play the role of springs whose forces onto the GFP molecules should lead to conformational changes that are small enough to be reversible but large enough to induce fluorescence decrease. Due to the fact that not all GFP molecules are attached through the two anchors we can only get a lower estimate of the fluorescence decrease assigned to stretch-induced forces applied on GFP molecules which is of the order of 15%, 27% and 38% for respectively 10%, 20% and 30% stretching degrees.
Mechanoresponsive materials based on biomacromolecules are rare. Bruns et al. reported the development of mechanical nanosensor based on FRET within a thermosome.13 More recently, Brantley et al. have described the development of biocomposite materials including GFP modified by mutagenesis.14 This material exhibits a mechanically induced decrease of fluorescence by compression (high compression of 40 MPa is needed). A gradual deformation of the three dimensional structure of the GFP was hypothesized to explain this effect. When reaching 40 MPa, a non-reversible quenching of the fluorescence was observed. In our case, we report the first example of a system where a GFP changes its fluorescence due to a uniaxial stretching of the functionalized material and most importantly our process is fully reversible. Moreover, adjusting the stretching degree allows for a gradual control of the process.
To sum up, we designed a biomechano-responsive surface based on conveniently functionalized silicon substrate covalently functionalized with GFP, site-selectively genetically modified with unnatural amino acids bearing site chains with bioorthogonal chemical reactivity. The position of the unnatural amino acids introduced into the primary sequence of GFP have been selected on the basis of simulation and single-molecule works reported.7,8 When a uniaxial mechanical stress is applied to our system, we observe a linear decrease of the fluorescence intensity which is mainly attributable to stretch-induced forces applied on the GFP molecules (38% when stretched up to 30%) that should induce conformational changes of GFP. Relaxation to 0% of stretching allows to fully restore the initial fluorescence which is a crucial point in future developments of stress–strain mechano-responsive biosystems and materials.
J.L. and C.Y. thank IRTG for granting doctoral fellowships. C.R. thanks icFRC and ANR (project “Biostretch” ANR-10-BLAN-0818) for granting doctoral fellowship. S. M. S. the (DFG) EXC 294 BIOSS and the Rectorate of the University of Freiburg for support. This research was supported by grants from icFRC, IUF and USIAS.
Notes and references
- D. A. Davis, A. Hamilton, J. L. Yang, L. D. Cremar, D. Van Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martinez, S. R. White, J. S. Moore and N. R. Sottos, Nature, 2009, 459, 68 CrossRef CAS PubMed.
- K. Ariga, T. Mori and J. P. Hill, Adv. Mater., 2012, 24, 158 CrossRef CAS PubMed.
- V. Vogel and M. Sheetz, Nat. Rev. Mol. Cell Biol., 2006, 7, 265 CrossRef CAS PubMed.
- V. Vogel, Annual Review of Biophysics and Biomolecular Structure, Annual Reviews, Palo Alto, 2006, vol. 35, p. 459 Search PubMed.
- J. Davila, A. Chassepot, J. Longo, F. Boulmedais, A. Reisch, B. Frisch, F. Meyer, J. C. Voegel, P. J. Mesini, B. Senger, M. H. Metz-Boutigue, J. Hemmerle, P. Lavalle, P. Schaaf and L. Jierry, J. Am. Chem. Soc., 2012, 134, 83 CrossRef CAS PubMed; J. Bacharouche, F. Badique, A. Fahs, M. A. Spanedda, A. Geissler, J.-P. Malval, M.-F. Vallat, K. Anselme, G. Francius, B. Frisch, J. Hemmerlé, P. Schaaf and V. Roucoules, ACS Nano, 2013, 7, 3457 CrossRef PubMed.
- D. Mertz, C. Vogt, J. Hemmerle, J. Mutterer, V. Ball, J. C. Voegel, P. Schaaf and P. Lavalle, Nat. Mater., 2009, 8, 731 CrossRef CAS PubMed.
- H. Dietz, F. Berkemeir, M. Bertz and M. Rief, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12724 CrossRef CAS PubMed.
- J. Saeger, V. P. Hytönen, E. Klotzsch and V. Vogel, PLoS One, 2012, 7, e46962 CAS.
- J. J. van Thor, Chem. Soc. Rev., 2009, 38, 2935 RSC.
- C. J. Noren, S. J. Anthony-Cahill, M. C. Griffith and P. G. Schultz, Science, 1989, 244, 182 CAS.
- K. Efimenko, W. E. Wallace and J. Genzer, J. Colloid Interface Sci., 2002, 254, 306 CrossRef CAS.
- V. Hong, S. I. Presolski, C. Ma and M. G. Finn, Angew. Chem., Int. Ed., 2009, 48, 9879 CrossRef CAS PubMed.
- N. Bruns, K. Pustelny, L. M. Bergeron, T. A. Whitehead and D. S. Clark, Angew. Chem., Int. Ed., 2009, 31, 5776 CrossRef.
- J. N. Brantley, C. B. Bailey, J. R. Cannon, K. A. Clark, D. A. Vande Bout, J. S. Brodbelt, A. T. Keatinge-Clay and C. W. Bielawski, Angew. Chem., Int. Ed., 2014, 53, 5088 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Material and methods, preparation and characterization of GFP wt and mutants, PDMS preparation and surface modifications. See DOI: 10.1039/c4cc07486f |
| ‡ Both first authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |