Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mesomeric betaine – N-heterocyclic carbene interconversions of 1,2,4-triazolium-phenolates. Sulfur, selenium, and borane adduct formation†
Ming
Liu
a,
Martin
Nieger
b and
Andreas
Schmidt
*a
aClausthal University of Technology, Institute of Organic Chemistry, Leibnizstrasse 6, D-38678 Clausthal-Zellerfeld, Germany. E-mail: schmidt@ioc.tu-clausthal.de
bLaboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, P.O. Box 55 (A.I. Virtasen aukio 1), FIN-00014 Helsinki, Finland
First published on 24th October 2014
Abstract
The conjugated mesomeric betaines 2-(1-phenyl-4H-1,2,4-triazolium-4-yl)phenolates are masked N-heterocyclic carbenes of 1,2,4-triazole which can be trapped as thiones and selenones. Reaction with triethylborane and triphenylborane resulted in the formation of first representatives of a new zwitterionic heterocyclic ring system, benzo[e]1,2,4-triazolo[3,4-c][1,4,2]oxazaborinium-4-ide, as a formal trapping product of an anionic N-heterocyclic carbene.
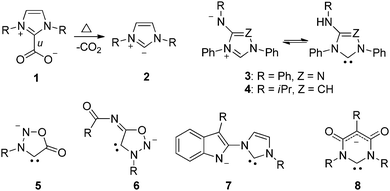
Recently, mesomeric betaines (MB) have come into the focus of N-heterocyclic carbene (NHC) research as it was recognized that mesomeric betaines not only comprise versatile starting materials for the preparation of a broad variety of NHCs, but also stimulate the designs of new architectures and variations in their charge properties. The chemical and physical properties of mesomeric betaines are strongly dependent on their types of conjugation which define five distinct classes of mesomeric betaines. Two of them were not recognized until 2013.1 As summarized in the first review articles,2 the types of conjugation of mesomeric betaines also determine their transformations into N-heterocyclic carbenes. Thus, some pseudo-cross-conjugated mesomeric betaines (PCCMB) are widely applied as stable precursors of normal N-heterocyclic carbenes (nNHC).3Scheme 1 shows the PCCMB imidazolium-2-carboxylate 1, which gives 2 upon decarboxylation.4 Other suitable PCCMBs for the generation of nNHCs are pyrazolium-3-carboxylates,5 indazolium-3-carboxylates6 and pyridinium-2-carboxylates.7 N-heterocyclic carbenes as well as anions derived thereof can also be generated from conjugated mesomeric betaines (CMB), as exemplified by 3–7. Thus, nitron 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and imidazolium-4-aminide 4
8 and imidazolium-4-aminide 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 are in tautomeric equilibrium with their corresponding NHCs. The anionic N-heterocyclic carbenes 5
9 are in tautomeric equilibrium with their corresponding NHCs. The anionic N-heterocyclic carbenes 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 6
10 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 are deprotonated sydnones and sydnonimines, respectively, whereas the carbene 7 was generated from an ylide.12 By contrast, carbene 8
11 are deprotonated sydnones and sydnonimines, respectively, whereas the carbene 7 was generated from an ylide.12 By contrast, carbene 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 is a deprotonated cross-conjugated mesomeric betaine (CCMB).
13 is a deprotonated cross-conjugated mesomeric betaine (CCMB).
In continuation of our research interests (polycations,14 mesomeric betaines,5,6,12 synthesis and catalysis with NHCs15) we report here on the first hetarenium-phenolates (1,2,4-triazolium-phenolates) which are in equilibrium with their tautomeric carbenes. They form the first representatives of a new ring system upon treatment with boranes.
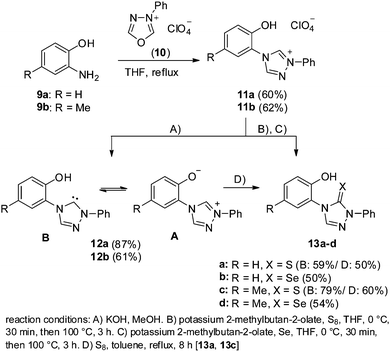
We started the preparation of the title compounds from 2-aminophenol 9a and its 4-methyl derivate 9b which induced a nucleophilic ring transformation of 3-phenyl-1,3,4-oxadiazolium salt 10 to give the salts 11a,b in reasonable yields, respectively (Scheme 2). The OH group can be detected between δ = 11.22 (11a) and 10.95 ppm (11b) in DMSO-d6. Potassium hydroxide in methanol (method A) converted the salts 11a,b at rt into the 2-(1,2,4-triazolium)phenolates 12a,b the tautomers A of which are members of the class of conjugated heterocyclic mesomeric betaines (CMB). Upon formation of betaine, the resonance frequency of the OH groups of 11a,b disappears while the signals of 3-H of the triazolium at δ = 11.27/11.25 ppm broaden considerably. The largest shift differences are observable for the phenolate protons which shift by Δδ = 0.15–0.25 ppm to a higher field. The betaine tautomers 12a,b(A) proved to be stable in polar protic solvents such as methanol (ENT = 0.762). In aprotic, less polar solvents such as DMSO (ENT = 0.444) or THF (ENT = 0.207), however, decomposition occurs. The carbene tautomers 12a,b(B) can be trapped as triazolethiones 13a,c by reaction with sulfur in toluene in 50% and 60% yield, respectively (method D). The carbene tautomers were also trapped starting from the salts 11a,b in a one-step reaction (methods B and C): deprotonation of 11a,b with potassium 2-methylbutan-2-olate in THF in the presence of sulfur and selenium, respectively, gave the triazolethiones 13a,c and triazoleselenones 13b,d in acceptable to good yields.
Suitable single crystals of the salt 11b were obtained by slow evaporation of a concentrated solution in ethanol (Fig. 1). The phenol substituent is twisted by 56.24(19)° (C5–N4–C6–C7) from the plane of the triazolium ring, whereas the phenyl ring and the triazolium ring form an almost planar system in the crystal (see ESI†).
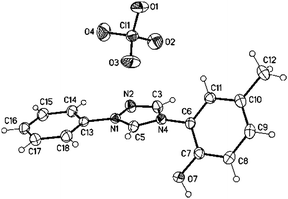 | ||
| Fig. 1 Molecular drawing of triazolium salt 11b (displacement parameters are drawn at the 50% probability level). | ||
Suitable single crystals of the corresponding betaine 12b were obtained by slow evaporation of a concentrated solution in EtOH–EtOAc. The betaine, which adopts tautomer 12b(A) in the crystal, is stabilized by water of crystallization (Fig. 2). One hydrogen bond is formed from 3-Htriazole (CH-5, crystallographic numbering) to the oxygen atom of water (H⋯O/O–H⋯O: 203 pm/172°), another from the phenolate oxygen atom (O18) to the hydrogen atoms of two water molecules (H⋯O/O–H⋯O: 188(2) pm/177(3)°; 192(2) pm/163(3)°). In contrast to the corresponding salt 11b, the phenol substituent is twisted only slightly by −7.4(3)° (C3–N4–C12–C17) from the plane of the triazolium ring. Upon deprotonation, the C–O bond of the phenol shortens by approximately 6 pm (see ESI†).
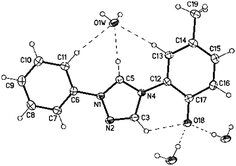 | ||
| Fig. 2 Molecular drawing of triazolium betaine 12b(A) showing the hydrogen bond pattern (displacement parameters are drawn at the 50% probability level). | ||
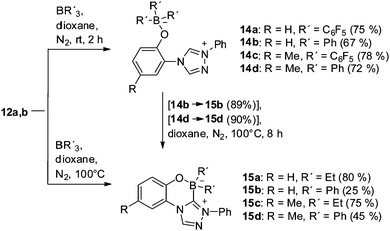
Borane adducts of N-heterocyclic carbenes have attracted considerable attention in the last few years.16 The betaine–carbene tautomers 12a,b reacted with triphenylborane and tris(pentafluorophenyl)borane in dioxane at rt to form the triazolium phenoxytriphenylborates 14a–d, respectively (Scheme 3). Triethylborane and triphenylborane converted 12a,b at elevated temperatures into first representatives of the new heterocyclic ring system benzo[e]1,2,4-triazolo[3,4-c][1,4,2]oxazaborininium-4-ide 15a–d which are formal trapping products of an anionic N-heterocyclic carbene possessing a phenolate moiety. Correspondingly, the phenylborates 15b,d are also available in excellent yields starting from 14b,d upon heating. The 11B NMR resonance frequencies of 14b,d shift from −6.56 and −6.57 ppm, respectively, to −0.08 and −0.80 ppm upon ring closure to 15b,d.
Suitable single crystals of 14c were obtained by slow evaporation of a concentrated solution in DMSO. The bond length between the phenolate oxygen and the boron atom (O19–B20) was determined to be 150.30(19) pm. The phenyl rings are twisted by −120.8(3)° (C5–N1–C6–C7) and 148.59(15)° (C5–N4–C12–C17) [−35.7(2)° (C5–N4–C12–C13)] from the plane of the triazolium ring (Fig. 3).
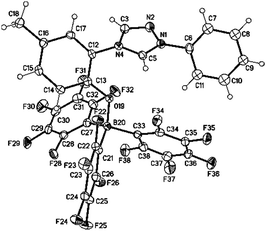 | ||
| Fig. 3 Molecular drawing of triazolium phenoxytriphenylborate 14c (displacement parameters are drawn at the 50% probability level). | ||
Finally, suitable single crystals of the new ring system 15a were obtained by slow evaporation of a concentrated solution in ethanol (Fig. 4). In the elemental cell two molecules are connected via one molecule of water which forms hydrogen bonds to the oxygen atoms of the central [1,4,2]oxazaborininium-4-ide cores, respectively [H⋯O/O–H⋯O: 188(2) pm/178(2)° and 195(2) pm/162(2)°] (cf. Fig. S5, ESI†). The phenyl rings are twisted by 46.13(18)° (N5–N4–C18–C23) −42.7(2)° (N105–N104–C118–C123) from the plane of the triazolium ring. The oxazaborininium ring is twisted. Thus dihedral angles of −28.57(16)° [24.48(17)°] (O1–B2–C3–N7) and 46.77(15)° [−46.84(16)°] (C13–O1–B2–C3) were measured. The bond lengths Ophenolate–B and Ccarbene–B were determined to be 154.26(17) pm [154.27(19) pm] and 163.9(2) pm [164.2(2) pm], respectively, whereas the bonds connecting the boron atom with the two remaining ethyl groups are shorter (160.6(2) and 162.7(2) pm [160.3(2) pm and 161.8(2) pm)]. In imidazodiazaboroloindoles, trapping products of anionic imidazole-2-ylidenes, the B–Ccarbene bond is longer [165.2(2) pm] than in 15a.17 The angle N–Ccarbene–N in 15a (N4–C3–N7) was determined to be 103.40(12)°. In the BH3 adduct of 2-phenyl-pyrrolo[2,1-c]-[1,2,4]triazol-3-ylidene the bond length Ccarbene–B is 161.1(3) pm, the angle N–Ccarbene–N is 102.0(2)° and the N–Ccarbene bond lengths 136.3(3) pm and 137.0(3) pm, respectively.18 Borane adducts of 1,2,4-triazolium-3-ylidenes are quite rare. One literature-known example is the trapping reaction of 2,4,5-triphenyl-[1,2,4]triazol-3-ylidenes with BH3·THF.19
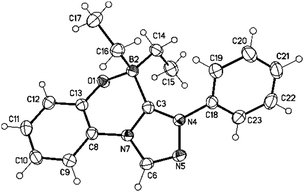 | ||
| Fig. 4 Molecular drawing of the new heterocyclic ring system 15a (displacement parameters are drawn at the 50% probability level). | ||
In summary, triazolium-phenolates represent an additional class of mesomeric betaines which are in equilibrium with their N-heterocyclic carbenes. This equilibrium gives the access to new borane-containing heterocyclic ring systems which are formed upon trapping with boranes.
Notes and references
- C. A. Ramsden, Tetrahedron, 2013, 69, 4146 CrossRef CAS PubMed; C. A. Ramsden and W. P. Oziminski, Tetrahedron, 2014, 70, 7158 CrossRef PubMed; W. D. Ollis, S. P. Stanforth and C. A. Ramsden, Tetrahedron, 1985, 41, 2239 CrossRef.
- A. Schmidt, S. Wiechmann and T. Freese, ARKIVOC, 2013, i, 424 CrossRef CAS; A. Schmidt and Z. Guan, Synthesis, 2012, 3251 CrossRef PubMed; A. Schmidt and A. Dreger, Curr. Org. Chem., 2011, 15, 2897 CrossRef; A. Schmidt and A. Dreger, Curr. Org. Chem., 2011, 15, 1423 CrossRef; A. Schmidt, A. Beutler and B. Snovydovych, Eur. J. Org. Chem., 2008, 4073 CrossRef.
- PCCMB are trapping products of nNHCs with heterocumulenes: L. Delaude, Eur. J. Inorg. Chem., 2009, 1681 CrossRef CAS.
- M. Fèvre, J. Pinaud, A. Leteneur, Y. Gnanou, J. Vignolle, D. Taton, K. Miqueu and J.-M. Sotiropoulos, J. Am. Chem. Soc., 2012, 134, 6776 CrossRef CAS PubMed; E. L. Kolychev, T. Bannenberg, M. Freytag, C. G. Daniliuc, P. G. Jones and M. Tamm, Chem. – Eur. J., 2012, 18, 16938 CrossRef PubMed; X. Sauvage, G. Zaragoza, A. Demonceau and L. Delaude, Adv. Synth. Catal., 2010, 352, 1934 CrossRef; J. Li, J. Peng, G. Zhang, Y. Bai, G. Lai and X. Li, New J. Chem., 2010, 34, 1330 RSC; T. Le Gall, S. Baltatu and S. K. Collins, Synthesis, 2011, 3687 Search PubMed; T. K. Olszewski and D. E. Jaskólska, Heteroat. Chem., 2012, 23, 605 CrossRef; M. Albrecht, P. Maji, C. Häusl, A. Monney and H. Müller-Bunz, Inorg. Chim. Acta, 2012, 380, 90 CrossRef PubMed; P. Bissinger, H. Braunschweig, T. Kupfer and K. Radacki, Organometallics, 2010, 29, 3987 CrossRef; L. Tommasi and F. Sorrentino, Tetrahedron Lett., 2009, 50, 104 CrossRef PubMed; A. Schmidt, A. Beutler, M. Albrecht and F. J. Ramírez, Org. Biomol. Chem., 2008, 6, 287 Search PubMed.
- A. Schmidt, N. Münster and A. Dreger, Angew. Chem., Int. Ed., 2010, 49, 2790 CrossRef CAS PubMed; A. Dreger, R. Cisneros Camuña, N. Münster, T. A. Rokob, I. Pápai and A. Schmidt, Eur. J. Org. Chem., 2010, 4296 CrossRef; A. Schmidt and T. Habeck, Lett. Org. Chem., 2005, 2, 37 CrossRef.
- Z. Guan, M. Gjikaj and A. Schmidt, Heterocycles, 2014, 10, 2356 Search PubMed; Z. Guan, S. Wiechmann, M. Drafz, E. Hübner and A. Schmidt, Org. Biomol. Chem., 2013, 11, 3558 Search PubMed; A. Schmidt, L. Merkel and W. Eisfeld, Eur. J. Org. Chem., 2005, 2124 CrossRef CAS.
- K. W. Ratts, R. K. Howe and W. G. Phillips, J. Am. Chem. Soc., 1969, 91, 6115 CrossRef CAS; H. Quast and E. Schmitt, Liebigs Ann. Chem., 1970, 732, 43 CrossRef; A. R. Katritzky and H. M. Faid-Allah, Synthesis, 1983, 149 CrossRef.
- C. Färber, M. Leibold, C. Bruhn, M. Maurer and U. Siemeling, Chem. Commun., 2012, 48, 227 RSC.
- V. César, J.-C. Tourneux, N. Vujkovic, R. Brousses, N. Lugan and G. Lavigne, Chem. Commun., 2012, 48, 2349 RSC; A. A. Danopoulos, K. Yu. Monakhov and P. Braunstein, Chem. – Eur. J., 2013, 19, 450 CrossRef CAS PubMed . Related systems have been described as well: L. Benhamou, N. Vujkovic, V. César, H. Gornitzka, N. Lugan and G. Lavigne, Organometallics, 2010, 29, 2616 CrossRef; L. Benhamou, V. César, H. Gornitzka, N. Lugan and G. Lavigne, Chem. Commun., 2009, 4720 RSC; A. T. Biju, K. Hirano, R. Fröhlich and F. Glorius, Chem. – Asian J., 2009, 4, 1786 Search PubMed.
- S. Wiechmann, T. Freese, M. H. H. Drafz, E. G. Hübner, J. C. Namyslo, M. Nieger and A. Schmidt, Chem. Commun., 2014, 50, 11822 RSC.
- V. N. Kalinin, S. N. Lebedev, I. A. Cherepanov, I. A. Godovikov, K. A. Lyssenko and E. Hey-Hawkins, Polyhedron, 2009, 28, 2411 CrossRef CAS PubMed; S.-T. Lin, H.-S. Cheo, L.-S. Liu and J.-C. Wang, Organometallics, 1997, 16, 1803 CrossRef.
- N. Pidlypnyi, S. Wolf, M. Liu, K. Rissanen, M. Nieger and A. Schmidt, Tetrahedron, 2014, 70, 8672 CrossRef CAS PubMed; N. Pidlypnyi, F. Uhrner, M. Nieger, M. H. H. Drafz, E. G. Hübner, J. C. Namyslo and A. Schmidt, Eur. J. Org. Chem., 2013, 7739 CrossRef . An N-(fluoren-9-yl)imidazol-2-ylidene was also described: L. Benhamou, S. Bastin, N. Lugan, G. Lavigne and V. César, Dalton Trans., 2014, 43, 4474 RSC.
- V. César, S. Labat, K. Miqueu, J.-M. Sotiropoulos, R. Brousses, N. Lugan and G. Lavigne, Chem. – Eur. J., 2013, 19, 17133 CrossRef PubMed; V. César, N. Lugan and G. Lavigne, J. Am. Chem. Soc., 2008, 130, 11286 CrossRef PubMed.
- A. Schmidt and T. Mordhorst, Synthesis, 2005, 781 CrossRef CAS PubMed; A. Schmidt, T. Mordhorst and T. Habeck, Org. Lett., 2002, 4, 1375 CrossRef PubMed.
- A. Schmidt and A. Rahimi, Chem. Commun., 2010, 46, 2995 RSC; A. Rahimi, J. C. Namyslo, M. Drafz, J. Halm, E. Hübner, M. Nieger, N. Rautzenberg and A. Schmidt, J. Org. Chem., 2011, 76, 7316 CrossRef CAS PubMed.
- D. P. Curran, A. Solovyev, M. M. Brahmi, L. Fensterbank, M. Malacria and E. Lacôte, Angew. Chem., Int. Ed., 2011, 50, 10294 CrossRef CAS PubMed.
- N. Pidlypnyi, J. C. Namyslo, M. H. H. Drafz, M. Nieger and A. Schmidt, J. Org. Chem., 2013, 78, 1070 CrossRef CAS PubMed.
- S.-H. Ueng, M. M. Brahmi, É. Derat, L. Fensterbank, E. Lacôte, M. Malacria and D. P. Curran, J. Am. Chem. Soc., 2008, 130, 10082 CrossRef CAS PubMed.
- D. Enders, K. Breuer, J. Runsink and J. H. Teles, Liebigs Ann., 1996, 2019 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data of 11b, 12b, 14c, and 15a in cif-format. CCDC 1027080 (11b), 1027081 (12b), 1027082 (14c) and 1027083 (15a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc08032g |
| This journal is © The Royal Society of Chemistry 2015 |