Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature
Ben
Wang†
ab,
Weixin
Liang†
ab,
Zhiguang
Guo
*ab and
Weimin
Liu
b
aHubei Collaborative Innovation Centre for Advanced Organic Chemical Materials and Ministry of Education Key Laboratory for the Green Preparation and Application of Functional Materials, Hubei University, Wuhan 430062, People's Republic of China. E-mail: zguo@licp.cas.cn; Fax: +86-931-8277088; Tel: +86-931-4968105
bState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, People's Republic of China
First published on 14th October 2014
Abstract
Oil spills and industrial organic pollutants have induced severe water pollution and threatened every species in the ecological system. To deal with oily water, special wettability stimulated materials have been developed over the past decade to separate oil-and-water mixtures. Basically, synergy between the surface chemical composition and surface topography are commonly known as the key factors to realize the opposite wettability to oils and water and dominate the selective wetting or absorption of oils/water. In this review, we mainly focus on the development of materials with either super-lyophobicity or super-lyophilicity properties in oil/water separation applications where they can be classified into four kinds as follows (in terms of the surface wettability of water and oils): (i) superhydrophobic and superoleophilic materials, (ii) superhydrophilic and under water superoleophobic materials, (iii) superhydrophilic and superoleophobic materials, and (iv) smart oil/water separation materials with switchable wettability. These materials have already been applied to the separation of oil-and-water mixtures: from simple oil/water layered mixtures to oil/water emulsions (including oil-in-water emulsions and water-in-oil emulsions), and from non-intelligent materials to intelligent materials. Moreover, they also exhibit high absorption capacity or separation efficiency and selectivity, simple and fast separation/absorption ability, excellent recyclability, economical efficiency and outstanding durability under harsh conditions. Then, related theories are proposed to understand the physical mechanisms that occur during the oil/water separation process. Finally, some challenges and promising breakthroughs in this field are also discussed. It is expected that special wettability stimulated oil/water separation materials can achieve industrial scale production and be put into use for oil spills and industrial oily wastewater treatment in the near future.
1. Introduction
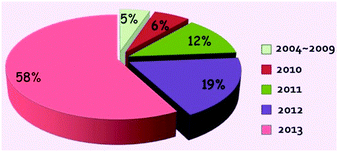
The leakage of oil into the ocean is a big catastrophe in the world, which will bring many toxic compounds to the ocean and thus threaten every species along with the marine food chain, from low grade algae to higher mammals even including human beings.1,2 Historical oil spill accidents have frequently occurred and never ended, from the 1967 Torrey Canyon oil spill to the latest 2011 Bohai Bay oil spill. Therefore the treatment of oil spills urgently needs to be addressed. In addition to the burning of oil, artificial separation of oily water is a more favoured way since the as-spilled oil can be re-collected without causing any environmental pollution and may be reused in various industries. The traditional oil/water separation technologies include gravity separation, filtration, centrifuge, flotation, and electrochemical methods.3–5 However, most of the traditional oil water separation methods often take a long time and need tedious manual operation. Moreover, the separation process of an oil-and-water mixture is empirical to a great extent, thus making the separation incomplete with either oil remaining in water or water remaining in oil.The rapid development of colloid and interface science, and bionics in the past decades, especially for superhydrophobic surfaces6–13 and deuterogenic underwater superoleophobic surfaces,14–17 has offered a brand-new idea for developing efficient, automated and recyclable oil/water separation materials that can thoroughly separate a water-and-oil mixture without exhausting any external energy. Compared to the traditional separation methods, the special wettability-controlled oil water separations show big advantages in both the separating speed and the efficiency of separation. As a type of special wettability, superhydrophobicity/superoleophobicity is commonly defined as possessing a water/oil contact angle (CA) higher than 150° but a sliding angle less than 10° under a certain external environment.18–22 Superhydrophobic surfaces are of great interest in various applications, including self-cleaning windows/windshields,23–26 anti-fouling,27 anti-drag microfluidic devices,28 and oil/water separation materials.29–31 As the most widely applied method, gravity driven oil/water separation material was first discovered by Jiang et al. in 2004 and further developed by Jiang et al. in 2011, from the initial oil/water separation strategy that allows oil to permeate the as-prepared materials to a more advanced strategy that allows water to pass through the as-prepared materials. The interest in oil/water separation materials inspired by special wettability in nature from 2004 onwards has risen rapidly for all classes of substrates. Up to 2013, according to literatures retrieval from the RSC, ACS, Wiley, Elsevier and Springer, there were in total about 5% of records in 2004–2009, 6% of records in 2010, 12% of records in 2011, 19% of records in 2012 and 58% of records in 2013, denoting its rapid development in recent years (Fig. 1). To utilize the special wettability to separate a water-and-oil mixture, the oil/water separation materials commonly possess an opposite wettability to water and oil. In general, oil/water separation materials can be created in two ways, either fabricating superhydrophobic/superoleophilic materials or developing superoleophobic/superhydrophilic materials in a definite external circumstance. The as-reported approaches commonly tend to prepare the former ones that can filter oils from water. Our group has prepared superhydrophobic fabrics and sponges on which a series of transition metal nanocrystals (Fe, Co, Ni, Cu and Ag) coated using a dip coating32 and in situ growth methods.33 In addition, our group also prepared hierarchical structured stainless steel mesh films/fabrics/sponges via the oxidative chemical polymerization of aniline34 and hydrothermal methods.35 The former method was more popular in the early studies, and the later was emerging till 2011 due to the difficulty in obtaining superoleophobic and superhydrophilic properties in air. Therefore, scientists ingeniously designed the underwater superoleophobic and superhydrophilic surfaces to realize oil/water separation via a simple pre-wetting process of the membranes.
In this feature article, we have reviewed the latest research in the field of oil/water separation stimulated from special surface wettability. Four typical materials with special wettability were specifically focused on that can be applied for efficient separation of the common layered mixture of oil and water in Section 2, which in turn are superhydrophobic and superoleophilic materials (Section 2.1), superhydrophilic and under-water superoleophobic materials (Section 2.2), superhydrophilic and superoleophobic materials (Section 2.3), and smart materials with switchable wettability (Section 2.4). Particularly, in Section 3, emerging materials with special wettability for oil/water emulsion separation are discussed. Next, the comprehensive understanding of all types of oil/water separation materials are put forward in Section 4. Moreover, related theories that are proposed to understand the various mechanisms of the oil/water separation process in both air and underwater (Section 5). Finally, conclusions about this review and an outlook for this area of research in the future are described (Section 6).
2. Separation of layered oil-and-water mixtures
2.1. Superhydrophobic–superoleophilic materials
Superhydrophobic–superoleophilic materials are typical oil removing materials. The superhydrophobic and superoleophilic properties will make an oil phase spread easily, absorbed (for porous bulk materials) and penetrate (for porous filter materials) on the material while the water phase will be repelled, thus separating oils from an oil and water mixture. As is well known, surface wettability is determined by the combined effects of surface chemical composition and topography.6–13 Accordingly, superhydrophobic–superoleophilic materials can be fabricated in two strategies: the first is to construct a rough structure on a hydrophobic surface; the second is to modify chemicals with low surface energy on a rough surface.10 The ideal oil removing materials are generally considered to exhibit superhydrophobic and superoleophilic properties, high oil absorption capacity as well as low water pickup, low density, environmental friendless, self-propelled exhausting external energy, and good recyclability for a wide range of oils/organics. The prevailing oil removing materials are fabric-based materials, sponge-based materials, metallic mesh-based materials, carbon and its derived materials, and particles, which are discussed in detail in the following sections.Stainless steel mesh was the first material for fabricating superhydrophobic and superoleophilic surfaces via a spray-and-dry method by Jiang et al. in 2004 (Fig. 2a and b).40 The pre-mixed aqueous emulsion containing Teflon, adhesive (polyvinyl acetate), dispersant (polyvinyl alcohol), and surfactant (sodium dodecyl benzene sulfonate) was sprayed evenly on copper mesh with compressed air and then underwent a high-temperature drying process (350 °C) to decompose the adhesive, dispersant and surfactant. The prepared mesh is so water-repellent that a water droplet is unstable on such mesh and easily rolls off (Fig. 2c). However, oil spreads quickly on the mesh and permeates thoroughly within only 240 ms (Fig. 2d). It is demonstrated that the as-prepared mesh could separate a mixture of diesel oil and water. Moreover, the results showed that the hydrophobicity of the coating mesh films was severely affected by the pore diameter of mesh. Since then, various methods and material coatings were employed to fabricate superhydrophobic and superoleophilic oil–water separation films, not only on stainless steel mesh substrates,41–44 but also on the other substrates, which will be introduced in the following sections. Feng and her team43 reported a polydopamine coated-stainless steel mesh with high hydrophobicity (the water CA of 144°) and superoleophilicity (the oil CA of 0°) through combining mussel-inspired chemistry (self-polymerization of dopamine at pH = 8.5) and a Michael addition reaction of n-dodecyl mercaptan (NDM) with polydopamine (PDA). The as-prepared mesh could separate a series of oil–water mixtures including gasoline and diesel (Fig. 3). Moreover, the separation efficiency remained high (99.95% for a hexane–water mixture, Fig. 3c) after 30 recycle numbers (Fig. 3d). More importantly, the relatively high intrusion pressure (2.2 kPa) gave the opportunity to use it for the separation of oil and water mixtures. Due to the easy oxidation and polymerization of dopamine under mild environment, the method is also applicable for other substrates such as fabric45 and sponge.46
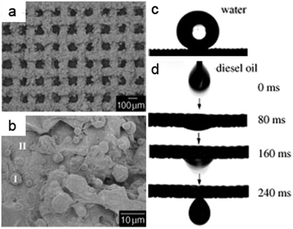 | ||
| Fig. 2 (a) Scanning electron microscopy (SEM) images of the stainless steel mesh coating (average pore diameter is about 115 μm); (b) high-resolution view of (a). (c) The shape of a water droplet on the resulting mesh film with a water contact angle (WCA) of 156.2° ± 2.8°; (d) the diesel oil spreads and penetrates the copper mesh film quickly within only 240 ms, indicating an oil contact angle (OCA) of 0°.40 Reprinted with permission from ref. 40. Copyright 2004 Wiley. | ||
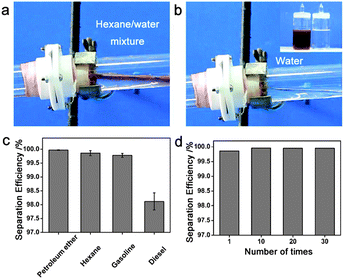 | ||
| Fig. 3 (a, b) The process of oil/water separation based on the highly hydrophobic PDA–NDM mesh. (c) The separation efficiency of the PDA–NMD mesh for a selection of oil and water mixtures. (d) The separation efficiency remains high after 30 times' repeated use.43 Reprinted with permission from ref. 43. Copyright 2013 ACS. | ||
In addition to stainless steel mesh, copper mesh is another frequently used substrate for oil/water separation.47–52 Lin et al.49 reported a superhydrophobic and superoleophilic copper mesh prepared using solution-immersion in HNO3 and sequential modification with 1-hexadecanethiol. This as-prepared mesh could be used to separate a mixture of oil and water. Importantly, the superhydrophobicity of the mesh was stable even in a corrosive environment, which enlarged the scope of its applications to harsh aqueous environments. Moreover, other properties such as catalysis can be combined with the oil/water separation process. Feng et al.50 reported a novel and multifunctional double-layer TiO2-based copper mesh with superhydrophobicity and superoleophilicity, which could not only achieve oil/water separation but also degrade the organic pollutants in water.
Surface modification with low surface energy materials is required for the vast majority of mesh used to fabricate a superhydrophobic (or hydrophobic) and superoleophilic (or oleophilic) surface. However, secondary pollution might be produced by the modifiers, especially fluoride. Recently, the superhydrophobic (hydrophobic) and superoleophilic (oleophilic) stainless steel meshes were obtained in the absence of modification of low surface energy materials or fluorinated chemicals.53–56 Lee et al.53 synthesized vertically carbon nanotubes (vertically-CNTs) on a stainless steel mesh for the separation of oil and water. The dual-scale structure (with nano-scale needle-like tubes on the mesh with microscale pores) was coordinated with the low surface energy of carbon, resulting in both enhanced hydrophobicity and oleophilicity. The CAs for diesel and water were 0° and 163° ± 4°, respectively. Similarly, the superhydrophobic and superoleophilic carbon nanotubes coated-stainless steel mesh was fabricated though thermal chemical vapour deposition for oil–water separation.54 Very interestingly, the as-prepared mesh has the ability to dewater the water–oil emulsion. It is obvious that the modification by low surface energy materials without fluorine is more environmentally friendly since it decreases the use of poisonous chemicals.
Except for the above two-dimensional meshes, recently, three-dimensional (3D) metallic meshes have also reported.57–59 Sun et al.57 described a simple approach for the fabrication of superwetting mesh films (SMFs) by engineering commercially available stainless-steel grids based on a layer-by-layer graphene assembly. Due to the good mechanical flexibility of the mesh, the SMFs can be simply folded or bent to prepare closed 3D-SMF for large-scale transportation. The cubic 3D-SMF could selectively absorb and store the oil from an oil-and-water mixture in its inner chamber (Fig. 4). Deng et al.58 provided a stainless steel mesh with hydrophobicity and oleophilicity for oil spill recovery devices by a dip-coated process using a xylene solution of low-density polyethylene. The wettability performance of the 3D mesh was considerably improved in comparison with traditional oil/water separation technologies.
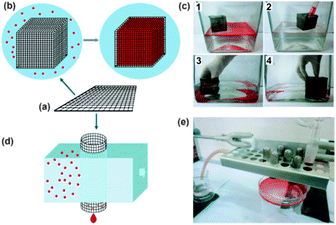 | ||
| Fig. 4 (a) Schematic model of a SMF. (b) Schematic model of cubic 3D-SMF prepared from the SMF and the selective absorption process of oil/water mixture (the red spots represent oil). (c) Optic images demonstrating the selective absorption of octane (1, 2) and chloroform (3, 4) from water phase using a cubic 3D-SMF (6 × 6 × 6 cm3). (d) Schematic model of columnar 3D-SMF prepared from the SMF for oil/water separation. (e) Photograph of the equipment assembled for separation of chloroform/water mixture, showing the water by-passed the columnar 3D-SMF whereas the chloroform was permeate and collected by the container underneath.57 Reprinted with permission from ref. 57. Copyright 2013 Wiley. | ||
In short, various superhydrophobic and superoleophilic metallic meshes, including two-dimensional or three-dimensional meshes, have been successfully fabricated, which can be applied towards the efficient separation of a mixture of oil and water. However, there are some questions and challenges. First, the rough structure of these superhydrophobic and superoleophilic metallic meshes can be easily destroyed, which leads to the loss of superhydrophobicity and oil/water separation ability. Secondly, these superhydrophobic meshes cannot be applied to in situ oil removal since the oil-contaminated water must be firstly collected and then filtered, which is not suitable for large-scale oil spills. Thirdly, the vast majority of oils and organic solvents show smaller density than that of water. Thus, superhydrophobic–superoleophilic meshes are not suitable for large-scale oil/water separation since oils floating on water makes it difficult for the oils to keep in touch with mesh surface, resulting in low separation efficiency and speed. Fourthly, so far, the metallic mesh films used for oil/water separation are mainly stainless steel and copper meshes, other types of metallic meshes are seldomly reported. Thus, the development of other types of metallic mesh should be also considered since they may provide various advantages in complex and specific environments.
To realize the separation of a mixture of water and oils or organics, establishing rough structured surfaces on fabric, with both superhydrophobic and superoleophilic properties, is a good choice. A dip coating procedure is one of the simplest approaches and has been performed by many research groups66–68 Xue et al.66 prepared superhydrophobic cotton fabrics by immersing the pristine fabrics into a titania sol to generate a dual-size surface roughness, which was followed by hydrophobization with stearic acid. Wang et al.67 presented a superhydrophobic cotton textile with oil/water separating properties by dip coating a superhydrophobic modified-ZnO–polystyrene nanocomposite coating. In addition to the dip coating method, Ding et al.69,70 fabricated a superhydrophobic and superoleophilic nanofibrous membrane with oil/water separation properties using a combination of electrospun poly(m-phenyleneisophthalamide) (PMIA) nanofibers and a novel in situ polymerized fluorinated polybenzoxazine (F-PBZ) functional layer that incorporated SiO2 NPs. In addition, they71 reported a hydrophobic–oleophilic fibrous mat via a co-axial electrospinning method where a polystyrene (PS) solution was used as the shell solution and a polyurethane (PU) solution was used as the core solution. The prepared composite PU–PS fibrous mats showed a good oil absorbent capacity and excellent reusability. Other approaches, such as chemical vapour deposition,72 layer-by-layer technique,73 sol–gel method74 and electro-spinning method,75 were also reported. Zhang and Seeger72 proposed a superhydrophobic and superoleophilic polyester textile using a one-step growth of silicone nanofilaments onto the textile via chemical vapour deposition of trichloromethylsilane. Compared with the original hydrophilic textile (Fig. 5a), the resultant textile was water repellent (Fig. 5b and c) and could be applied for oil/water separation in the form of a filtration membrane. Frankly, fabric is not a good candidate for large-scale oil absorption owing to its thin, paper-like two-dimensional structure, which seriously limits its oil capture ability. However, the selective oil absorption ability of fabric was well performed by Zhang and Seeger.72 To utilize the good flexibility and mechanical stability, the fabric was designed as a bag (Fig. 5g) containing absorbent material. The fabric bag could function as a selective oil absorption material, showing good separation efficiency and excellent reusability (Fig. 5g–k). Moreover, some non-woven materials can also be applied as the substrate material for oil/water separation due to their extremely similar microstructure with plenty of cross-linked fibers.76
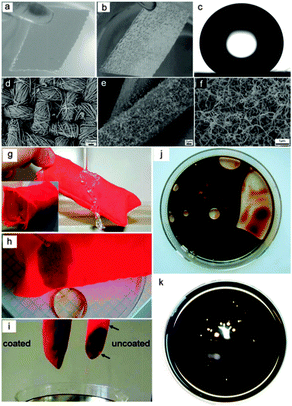 | ||
| Fig. 5 Photographs of the pristine textile in water (a) and the resultant textile in water for 8 weeks (b). The resultant textile is reflective underwater due to the existence of an air cushion between the textile and the water phase, denoting good water repellent properties. (c) CA image of the resultant textile. (d–f) SEM images at various magnifications of the resultant textiles (relative humidity (RH) 35%). (g) Optical images of a water column bounce off the resultant fabric bag. (h) Selective absorption of crude oil from water. (i) Optical images of pristine and the resultant fabric bags after oil absorption. (j) Oil absorbed by the pristine fabric bag. (k) Oil absorbed by the resultant fabric bag.72 Reprinted with permission from ref. 72. Copyright 2011 Wiley. | ||
In addition, the stability of the as-prepared superhydrophobic fabrics is also important since an excellent stability will endow the fabrics with more durable and robust properties, thus lengthening their lifetime and reducing production costs.77–85 Zhang et al.78–80 presented a durable and robust superhydrophobic textile with good mechanical (e.g., abrasion, laundering, scratching with a scalpel and adhesion of double side tape), chemical (e.g., exposure to acid and organic solvents) and environmental (e.g., exposure to UV irradiation and outdoor conditions) durability by simply dip-coating of a nanocomposite solution of fluoro-free organosilanes. The as-prepared textile can be efficiently applied to separate oil-and-water mixtures.79 Zhang et al.81 reported a robust superhydrophobic cotton fabric that can withstand severe environmental conditions such as high temperature (up to 120 °C), humid atmospheres (with a relative humidity up to 95%), corrosive substances, and mechanical forces (the water CA was higher than 150° and the separation efficiency remained above 93% after 600 scratch experiments) using an in situ vapour phase deposition process. Otherwise, as-prepared fabrics can be effectively and sustainably used for oil spill clean-up. Seeger et al.82 reported a simple one-step method to coat silicone nanofilaments onto various fabric substrates. The as-prepared fabrics showed unparalleled long-term water resistance and stability of superhydrophobicity that can maintain the superhydrophobicity even after continuous rubbing with a skin simulating friction partner under significant loads. In our group, we proposed general methods to fabricate stable superhydrophobic fabrics via both dip coating32 and chemical in situ growth33 of transition metal/metal oxide nanocrystals (including Fe, Co, Ni, Cu and Ag) with n-octadecylthiol modification (Fig. 6a). The results showed that both the wettability and the coating capacity increase after the in situ growth of nanoparticles, which correlated positively with the concentration of the precursor (Fig. 6b). The as-prepared fabrics could be efficiently used to separate oil-and-water mixtures (Fig. 6c). Interestingly, either the fabrics with different metal nanocrystals or the metal with different combined state (metal or metal oxide) grown on them show different colours (Fig. 6a), which would enhance both their potential applications and aesthetics.
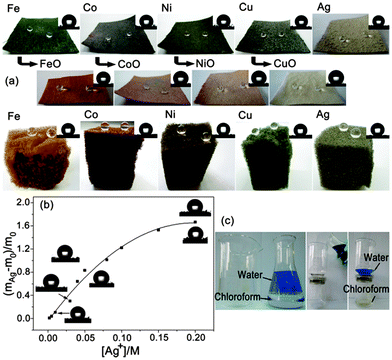 | ||
| Fig. 6 (a) Optical images of the superhydrophobic fabric/sponge from the in situ growth of Group VIII and IB metal/metal oxide nanoparticles. The inset images in the upper right-hand corner of each panel are images of the static water droplets (5 μL) (b) The relationship between the proportion of weight increase ((mAg − m0)/m0) and the Ag+ concentration. The images of the CA modified with both n-octadecylthiol (upper left) and perfluorodecanethiol (lower right) at different concentrations are presented near the curve. (c) Photographs of the fabric-based oil/water separation process of water and chloroform. The water was dyed with Methylene Blue for clear observation.33 Reprinted with permission from ref. 33. Copyright 2013 ACS. | ||
In general, the developed superhydrophobic and superoleophilic fabrics prepared using various physical and chemical routes, such as the dip coating method, chemical in situ growth approach, and electrospinning strategy, commonly can be applied to separate water-and-oil mixtures with high separation efficiency. However, a major drawback existing on the substrates themselves (such as metal mesh film and fabric) is that the as-prepared materials cannot be directly used to deal with oil spills in the ocean since they require the polluted water to be collected first and then filtered, which is inconvenient in the actual operation.
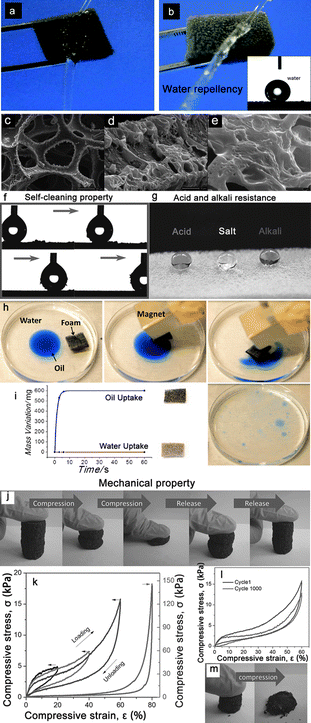 | ||
| Fig. 7 Photographs of a water column squirted on a piece of pristine sponge (a) and superhydrophobic sponge (b).94 The pristine sponge was immediately wetted by the water column whereas the superhydrophobic one strongly resisted the water column, indicating its water repellent properties. (c–e) show the typical rough structured superhydrophobic sponge from an integral view (c) to the enlarged views (d, e).94 (f) A demonstration of the self-cleaning properties of the superhydrophobic PU foam via the removal process of carbon black particles from the surface using a moving water droplet (5 μL).93 (g) Demonstration of the corrosive liquids resistance properties of the superhydrophobic PU foam by dropping aqueous hydrochloric acid (pH = 1), NaCl (pH = 7), and NaOH (pH = 14) solution droplets with spherical shapes on the superhydrophobic PU foam.93 (h) Magnetically driven floating foams for the removal of oil contaminants from water.97 (i) A graph showing the variation of the weight of the foam with time.97 (j–m) shows the mechanical properties of the PGFs with 6% graphene sheets. (j) Optical images showing the compression recovery process and that the PGFs recover their original shape after compression by more than 90%.99 (k) Stress–strain curves of PGFs with different set strain of 20%, 40%, 60% and 80%, respectively. (l) Cyclic stress–strain curves of PGFs at 60% strain at a compression speed of 10 mm min−1.99 (m) The compression-recovery process of the graphene foam obtained by direct reduction of GO, and it collapses after a compression by 50%.99 Reprinted with permission from ref. 93 and 99. Copyright 2013 Wiley; reprinted with permission from ref. 94. Copyright 2013 RSC. Reprinted with permission from ref. 97. Copyright 2012 ACS. | ||
Except for the frequently mentioned resistant properties to corrosive liquids, some other properties were also researched on the sponges/foams to realize “multifunctional integration,” which was first suggested by Jiang et al.96 Therein, the most concerned property above all is magnetism.97,98 Calcagnile et al.97 presented a composite magnetic material with highly efficient oil/water separation capability. They used a facile and easy scalable fabrication technique based on commercially available PU foams functionalized with colloidal superparamagnetic iron oxide nanoparticles and submicrometer CNT particles. After the separation procedure, the foam could be easily collected using a magnet (Fig. 7h and i). In addition to magnetism, conductivity is also considered by scientists, mainly based on carbon-coated sponge. Jiang et al.99 reported a novel strategy for the fabrication of ordered and flexible polymer-based graphene foams (PGFs) by the self-assembly of graphene sheets on the 3D skeleton of PU foam. The as-obtained PGFs possessed a polymer skeleton, which effectively supported the graphene foam, exhibiting high conductivity, hydrophobicity, outstanding mechanical properties, and excellent recyclability. Thereby, they could not only show considerable potential in oil/water separation field, but also served as a good candidate for use as a pressure dependent resistor (Fig. 7j–l). It is noteworthy that the 3D skeleton of PU foam played an important role to realize the pressure sensitivity. While the graphene foam was fabricated by direct reduction of GO, it could easily collapse without support of PU foam (Fig. 7m).
As the absorption efficiency of oils and organic solvents are commonly limited to the volume of air in the sponges/foams, some other strategies have been suggested to fabricate superhydrophobic and superoleophilic sponges/foams with high absorption efficiency, in other words, with high porosity. Li et al.100 proposed superhydrophobic activated carbon sponges by coating highly porous activated carbon particles onto sponge skeletons using a facile dip coating method, followed by PDMS treatment. The combination of highly porous activated carbon and the porous sponge skeleton endowed excellent absorption selectivity and absorbencies for various oils and organics. Moreover, a few sponge-templated carbon materials were developed by ultimately pyrolyzation of PU sponge but still retained the sponge skeleton to form a simultaneous super porous and ultralight material.101,102 This is specifically discussed in next section (Section 2.1.4). The sponge/foam-based superhydrophobic/superoleophilic materials show great potential for direct and large-scale removal of organic contaminants or oil spills from water.
The template method usually applies self-supported porous materials or particles as the template. The template will be removed after the target material is prepared. According to the post-treatment used to remove the template, two types can be classified: an etching method and pyrolysis method. The resultant carbon material possesses a hollow structure with only carbon skeleton retained. Koratkar et al.101 proposed a 3D graphene foam network by applying porous nickel foam as the template for the deposition of graphene. After modification with Teflon, the graphene foam became superhydrophobic with an advancing WCA of ∼163° while the receding WCA is ∼143°. Chen et al.102 fabricated a 3D graphene–CNT hybrid foam using chemical vapour deposition of graphene on nickel foam and subsequently in situ growth of CNT forest on it, followed by acid etching to remove the nickel skeleton. The low-density hybrid foam can be efficiently applied as a selective absorbent to remove oils and organic solvents from water. Li et al.103 synthesized a mesoporous graphene employing CaCO3 microspheres as hard templates and PDMS as the modifier. The mesoporous graphene showed abundant mesopores, which were crucial for realizing a high absorption capacity and exhibited excellent separation ability for various organic compounds from water.
Particularly, the pyrolysis method, as one type of template method, is the most widely used by researchers.104,105 Sun et al.106 fabricated twisted carbon fibers (TCF) aerogels by a facile pyrolysis method under an argon atmosphere using economic raw cotton. The TCF aerogels could absorb a wide range of organic solvents and oils (50–190 times the weight of pristine TCFs aerogel) and exhibited excellent recyclability. Similarly, Yu et al.107 reported a facile pyrolysis route to fabricate ultralight, flexible and fire-resistant carbon nanofiber aerogels on a large-scale from bacterial cellulose pellicles. The as-prepared carbon nanofiber aerogels showed excellent recyclability and selectivity for a wide range of organic solvents and oils (reaching up to 310 times the weight of pristine carbon nanofiber aerogels). They also108 reported PDMS-coated carbonaceous nanofibre hydrogels and aerogels, which can be used as oil removing materials and prepared by a direct burning process via a template-directed hydrothermal carbonization process. Generally, the pyrolyzation treated carbon-based materials can be divided into two kinds based on their elasticity, namely, flexible and inflexible. This makes a great difference on the oil reusability since the flexible carbon-based aerogels can use a direct squeezing method, a combustion method and the distillation method to recycle the materials.106,109 However, the squeezing method is inapplicable for an inflexible carbon material. On the contrary, in order to recycle the oils or organics, the distillation method (Fig. 8h and i)110 is applicable to replace the squeezing method (Fig. 8e in ref. 106).
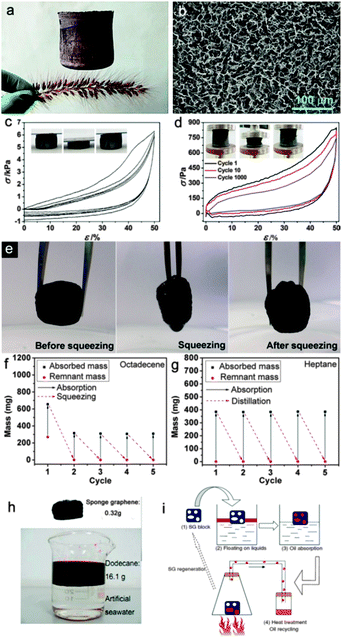 | ||
| Fig. 8 (a) Digital photograph of a 100 cm3 UFA cylinder standing on a flower-like dog's tail (Setairaviridis (L.) Beauv), denoting the ultra-flyweight properties of the UFA.109 (b) SEM image of the porous architecture of a UFA.109 (c) Stress–strain (σ–ε) curves of multi-cycle compressions on a UFA (ρ = 5.6 mg cm−3, f = 0.5), exhibiting recoverable deformation. The inset shows the optical images of the UFA under a compression and release cycle. The top head of the UFA was shuttling between a set fixed distance during compression at a speed of 2 mm min−1.109 (d) Stress–strain curves of several selected cycles (the 1st, 10th, and 1000th cycle) on a UFA (r = 1 mg cm−3, f = 0.5) during repeated compression. The inset shows the optical images of the UFA under a fatigue test for 1000 cycles. The compression and release speed is 600 mm min−1.109 (e) Photographs showing the process of recycling the TCF aerogel via a squeezing process.106 (f) The recyclability of the TCF aerogel for sorption of octadecene via a squeezing process.106 (g) The recyclability of the TCF aerogel for sorption of heptane via a distillation approach.106 (h) The optical image shows the sorption of oil.110 (i) Four-step schematic diagram of the distilled recycling process.110 The carbon-based aerogel can be regenerated and reused without affecting its performance when heated up to the boiling point of the absorbate. The as-evaporated liquid could be recollected elsewhere after a condensation procedure and the carbon-based aerogel was willing to be applied in the next cycle of absorption without any post-treatment.110 Reprinted with permission from ref. 106, 109, and 110. Copyright 2012, 2013 Wiley. | ||
A freeze-drying method usually uses the direct sublimation effect of the as-prepared carbon hydrogel to obtain the carbon aerogel.109–114 It is a frequently used approach to fabricate carbon aerogels with large specific surface area and porosity. Sun et al.110 prepared spongy graphene by reducing a suspension of graphene oxide (GO) platelets followed by moulding via a hydrothermal treatment and freeze-drying process. The as-obtained spongy graphene showed good oil removal ability for petroleum products, fats, alkanes, toluene, and other organic solvents without any further modification or treatment (20–86 times the weight of pristine spongy graphene) and could be repeatedly applied to removing oils using heat treatment to remove and collect the as-absorbed oils (Fig. 8i). Likewise, He et al.111 fabricated three kinds of porous reduced GO foams with different pore structures using freeze-drying methods, i.e. unidirectional freezing drying, non-directional freezing drying, and air freezing drying. Gao et al.109 fabricated all carbon ultra-flyweight aerogels (UFAs) by freeze-drying an aqueous solution of CNTs and giant GO sheets, followed by chemical reduction to transform GO into graphene with hydrazine vapour. The resultant aerogels were purely made of carbon and showed extremely low density (the density ρ ≥ 0.16 mg cm−3) and can even stand on a flower like a dog's tail (Fig. 8a), temperature-invariant super recyclable compressibility and elasticity-responsive conductivity. Importantly, the hydrophobic carbon aerogels had a porosity (Fig. 8b) of ∼99.9% and provided an ultra-high oil-absorption capacity (215–913 times of the weight of pristine carbon aerogel). More importantly, using its elasticity–responsive conductivity, this material could be used as a pressure sensitive resistor (Fig. 8c and d). Qu et al.112 developed a versatile, N-doped, ultralight 3D graphene framework with fire-resistant properties and an ultra-low density of 2.1 ± 0.3 mg cm−3, which is the lowest to date for a graphene architecture. The graphene framework exhibited a very high capacity for the reversible adsorption of oils and organic solvents (200–600 times the weight of the pristine graphene framework) and could be easily recycled many times.
Chemical vapour deposition (CVD) is also used to fabricate the porous carbon materials. Gui et al.115–117 reported a sponge-like bulk material composed of self-assembled, interconnected CNT skeletons with an ultralow density, a porosity of >99%, high structural flexibility and robustness, and was wettable to organics in its pristine form. It effortlessly floated on the surface of water and could quickly remove a spreading oil film on water with high efficiency (up to 180 times the weight of pristine carbon nanofibre aerogels). In addition, porous carbon materials can be also be deposited on other substrate materials via CVD. Moon et al.118 used a glow discharge deposition process to fabricate carbon NP networks with tuneable wettability and absorbability on various substrates such as silicon wafer, metals, paper and polymers.
Similar to the magnetic sponges/foams introduced in the previous section, magnetic carbon-based materials were also developed to endow manoeuvrability and thus improve their practical applications. Pan et al.119 fabricated ultralight magnetic Fe2O3/C, Co/C, and Ni/C foams on a centimeter scale by pyrolyzing PU sponge, which was grafted with polyelectrolyte layers at 400 °C with the corresponding metal acrylate, forming ultralight foams consisting of 3D interconnected hollow tubes. After siloxane modification, the foams can be used to separate oils from water with much higher oil-absorption capabilities than many other porous materials. Moreover, a three-dimensional macroporous Fe–C nanocomposite was also reported as a highly selective absorption material for removing oils from water surfaces by sintering a mixture of closely packed polystyrene microspheres and ferric nitrate precursor.120 The resultant nanocomposites exhibited superhydrophobic and superoleophilic properties without modification using low-surface-energy reagents. By applying a same method with ref. 115 in addition to an increase in the concentration of ferrocene, a magnetic CNT sponge with rough porous structure consisting of interconnected CNTs with rich Fe encapsulated in it was obtained by Gui et al.121 The magnetic CNT sponge obtained showed application as a sorbents for spilled oil recovery with a high mass sorption capacity (up to 56 g/g) and excellent recyclability (more than 1000 times).
Although these particles and powders can be used in oil spill clean-ups, they were difficult to transfer and recycle. Naturally, magnetic particles/powders were developed to overcome the recycling problem because particles/powders with magnetism can be easily collected using an external magnetic field. Wang et al.131 reported the fabrication of papilla-like magnetic particles with a dual-scale structure via thermal treatment of Fe microparticles (Fig. 9a and b). These particles were immersed in an aqueous solution of lauric acid to modify the papilla-like particles (LA-papilla-like particles) with desired wettability. The LA-papilla-like particles-composed surface had a water CA as high as 164.5° ± 1.6° (Fig. 9c), whereas the CA of oil was close to 0° (Fig. 9d). As shown in Fig. 9e, the LA-papilla-like particles could absorb oils and were separated from water using a magnetic field. Then, these LA-papilla-like particles with absorbed oils were transferred into ethanol to release the oils, which made the oil remover recyclable. Through the adsorption and desorption of oil and magnetic motion, the oil contaminants in water were completely removed and the LA-papilla-like particles were easily regenerated and then reused many times.
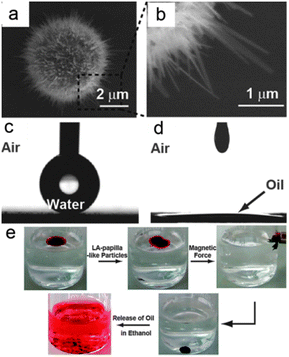 | ||
| Fig. 9 (a) SEM image of the papilla-like particle; (b) enlarged view of a nano-flake on the papilla-like particle. Still photographs of a water droplet (c) and a corn oil droplet (d) residing on the LA-papilla-like particles film. (e) The separation process of the oil phase from an oil-and-water mixture using the LA-papilla-like particles.131 Reprinted with permission from ref. 131. Copyright 2013 RSC. | ||
Apart from the homogeneous magnetic Fe microparticles, more research was focused on composite magnetic particles/powders.132–137 Zhu et al.134 reported the fast and selective removal of oils from the surface of water using core–shell Fe2O3@C nanoparticles with highly hydrophobic and superoleophilic properties under a magnetic field. These nanoparticles could selectively absorb lubricating oil up to 3.8 times the particles weight while completely repelling water. In particular, the nanoparticles showed unsinkable properties under agitation conditions, good stability towards corrosive media, and excellent recyclability. Zhang et al.135 presented superhydrophobic core–shell-satellite carbonyl iron–polydopamine–silver composite particles to separate oil from an oil–water mixture, which could be easily transported via an external magnet field. Moreover, they also found that an oil sphere could be spontaneously formed under water encapsulated by the superhydrophobic magnetic particles under the external magnet field.
It is obvious that hydrophobic and oleophilic particles and powders can be effectively used on a large scale to treat severe water pollution caused by oil spills because they are portable and can be used to selectively remove oil in situ. However, there exist two drawbacks on this kind of materials, which become a crucial obstacle for their practical production and applications. First, the oil absorption capacity of superhydrophobic and superoleophilic particles and powder is low. Secondly, most magnetic hydrophobic and oleophilic particles are easily destroyed in acidic solutions, resulting in the loss of their recoverability, and even their superhydrophobicity.
Interestingly, paper can also be applied as an oil/water separation material just like fabric.142,143 Superhydrophobic and superoleophilic filter paper was successfully prepared upon treating commercial filter paper with a mixture of hydrophobic silica nanoparticles and polystyrene solution in toluene by Wang and co-workers. The SiO2-coated filter paper could selectively adsorb oil that floats on the surface of water or in aqueous emulsions. Interestingly, the filter paper could also extract oil from homogeneous aqueous solutions.142 The above-mentioned diversified substrates provide much more options to separate oil-and-water mixtures.
Moreover, most of the reported substrate materials are non-degradable, directly discarded or burnt after use, which will cause ground contaminants or create poisonous gases resulting in secondary pollution to the environment.144 To overcome the non-degradable properties of the most reported substrates, Feng et al.144 developed biodegradable poly(lactic acid) oil absorption and filtration materials with superhydrophobic and superoleophilic properties via a phase separation process. The as-prepared materials can separate an oil and water mixture with high efficiency and easy recyclability. Furthermore, the used materials can be easily decomposed because of their good biodegradable characteristics.
Moreover, integrated oil/water separation devices prepared from superhydrophobic and superoleophilic materials were also proposed,145–147 which is significant and instructional for the industrial large-scale oil/water separation. Cheng et al.145 reported a multifunctional device, which was fabricated using superhydrophobic and superoleophilic nickel foam, for highly efficient and inexpensive oil spill clean-up. The device integrated the functions of oil containment booms, oil-sorption materials, oil skimmers, and water–oil separating devices. The device can be used for many types of oil/water mixtures, even for emulsions of petroleum and water, with high efficiency and reproducibility. Moreover, they designed a functional integrated system, which was magnetically responsive to directionally absorb and continuously collect the spilled oil underwater demonstrating re-collection efficiency as high as 98%.146
2.2. Superhydrophilic and underwater superoleophobic materials
As the vast majority of oil/water separation materials are applied to allow the oil phase to penetrate the surface (or absorb the oil phase) while repelling water, some scientists have tried to exert their wisdom to develop inverse oil/water separation materials that can let the water pass through freely however repelling oil totally. Fish scales, which are known to be well protected from contamination by oil pollution in the sea, have stimulated particular interest. Inspired by fish scales, membranes with hydrophilic and underwater superoleophobic properties have been developed and show promising application as water-removal materials.148–152 This novel idea overcomes the drawbacks that exist with oil-removal materials, i.e., easily fouled or even blocked by oils due to their intrinsic oleophilicity, thus limiting their recyclability. Moreover, water-removal materials avoid the formation of a water barrier between the substrate and the oil phase, which frequently occurs with oil-removal materials due to the fact that water commonly possesses a greater density than that of oils, thus preventing contact between the oil and separation substrates.153 In addition, these materials will cause waste from both the oils and the oil-removal materials, especially for oils with high viscosity. The as-absorbed oils are hard to clear away, which would easily cause secondary pollution during the post-treatment process of the materials.154 In 2011, Jiang et al.154 applied hydrophilic polyacrylamide (PAM) hydrogel-coated stainless steel mesh (Fig. 10a and b) to separate water from an oil and water mixture after pre-wetting the as-prepared mesh before the oil/water separation process. The as-prepared mesh showed both underwater superoleophobic (Fig. 10c, d and f) and low oil-adhesion (Fig. 10e and f) characteristics in an oil–water–solid three-phase system. The separation efficiency was above 99% for a series of oils, as given in Fig. 10g. This was the first oil/water separation material that allows water to pass through (Fig. 10h and i).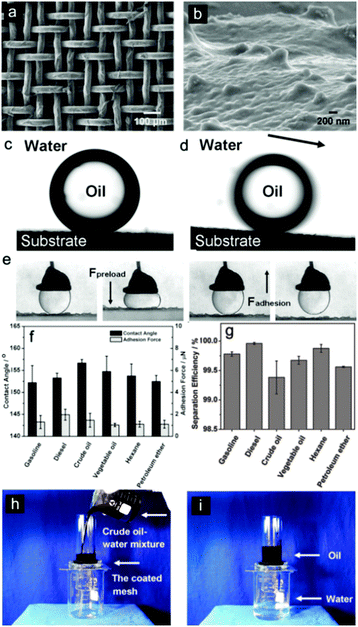 | ||
| Fig. 10 SEM images of the PAM hydrogel-coated mesh prepared from a stainless steel mesh with an average pore diameter of about 50 μm. (a) The integral view and (b) the magnified view of one single wire on the hydrogel-coated stainless steel mesh. (c) The still image shows the underwater oil droplet (1,2-dichloroethane, 2 μL) on the hydrogel-coated stainless steel mesh (OCA = 155.3° ± 1.8°). (d) The underwater sliding angle of oil droplet on the stainless steel mesh (oil sliding angle = 2.6° ± 0.5°). (e) Optical images of the dynamic underwater oil adhesion measurements on the mesh. A 5 μL oil droplet was applied as the detecting probe to contact the mesh surface and was released. The mesh shows an ultralow affinity to the water droplet. (f) Underwater oil wettability and oil adhesion of the resultant mesh for a series of oils in terms of CA and oil-adhesion force. (g) The oil/water separation efficiency of the resultant mesh for a selection of oils. (h, i) The oil/water separation process of the resultant mesh. The mesh film was fixed between two glass tubes. (h) A snapshot of the removal process of crude oil and water. (i) A snapshot after the removal process.154 Reprinted with permission from ref. 154. Copyright 2011 Wiley. | ||
After that, based on the same principle, some researchers also proposed superhydrophilic and underwater superoleophobic materials via various methods.153,155–159 Yu et al.156 reported an outstanding superhydrophilic and underwater superoleophobic film that can separate water from oil prepared by growing pure-silica zeolite crystals on stainless steel mesh. Wang et al.153 proposed an all-inorganic-coating-based steel mesh via a layer-by-layer assembly strategy. The silicate/TiO2 coated steel mesh showed superhydrophilic and underwater superoleophobic properties, which could be efficiently applied to separate water from an oil phase. Moreover, the as-coated TiO2 showed UV-responsive properties, which endowed the mesh with degradative and self-cleaning abilities. The mesh showed equivalent functions with the double layered TiO2-based mesh membrane fabricated by Feng et al.50 However, it simplified the two separate meshes into one. Jin et al.157 reported a superhydrophilic and underwater superoleophobic copper mesh film with ultra-low adhesive superoleophobicity for water removal from an oil-and-water mixture via a facile chemical-based oxidation method. Moreover, Feng et al.158 used the one-step chemical oxidation of a smooth copper mesh to obtain a superhydrophilic and underwater superoleophobic Cu(OH)2-covered mesh with hierarchical structure, which can selectively separate water from oil–water mixtures with high efficiency and excellent recyclability. Xu et al.159 synthesized a biomineralized polypropylene–CaCO3 composite non-woven mesh by UV-induced poly(acrylic acid) grafting and alternate soaking process.
2.3. Superhydrophilic–superoleophobic materials
In addition to the superhydrophilic and underwater superoleophobic materials introduced in the former section, another superhydrophilic and in-air superoleophobic material was developed that could also remove the water phase from an oil and water mixture and followed a quite different mechanism. Usually, a superhydrophilic (hydrophilic) surface exhibits superoleophilicity (oleophilicity) because oils commonly show lower surface free energy than water.160,161 In addition, for a superoleophobic surface, it generally shows superhydrophobicity.162–164 However, a few researchers find that some stimuli-responsive surfaces can simultaneously show hydrophilic and oleophobic properties based on a favourable interaction with polar liquids (e.g. water) and an unfavourable interaction with non-polar liquids (e.g. hexadecane).165–167 To utilize the complexation of a polyelectrolyte and oppositely charged surfactants, polyelectrolyte–fluorosurfactant complexes can be assembled on substrates. The stimuli-responsivity is caused by the layer of polyelectrolyte–fluorosurfactant complexes that simultaneously contain hydrophilic (the polyelectrolyte) and oleophobic (the fluorosurfactant) constituents. On these surfaces, oil is repelled by the surface because the fluorosurfactant is exposed outwards. However, water is wettable because the interaction between polar water molecules and the surface polyelectrolyte can induce molecular rearrangement such that the hydrophilic moieties are located at the solid–liquid interface.Although some moderate hydrophilic–oleophobic films have been reported,165–167 extreme superhydrophilic–superoleophobic films are still a challenge, and never been reported until 2012. To combine hydrophilic–oleophobic surfaces with a hierarchical rough structure, Zhang et al.168 firstly fabricated poly(diallyldimethylammoniumchloride)–perfluorooctanoate/SiO2 (PDDA–PFO/SiO2) coatings with both superhydrophilicity and superoleophobicity. The high surface concentration of fluorinated groups together with carboxyl and quaternary ammonium groups lead to a hydrophilic–oleophobic surface, whose mechanism is similar to that previously reported stimuli-responsive surfaces.165–167 Moreover, SiO2 nanoparticles created micro- and nanoscaled hierarchical structures, which could enhance the wettability to obtain superhydrophilicity–superoleophobicity (Fig. 11a and b). The PDDA–PFO/SiO2-coated mesh with an average diameter of about 200 μm showed water permeation (Fig. 11c) and oil repellent (Fig. 11d) behaviors, which indicates that the coated mesh could be applied to separate an oil and water mixture. Moreover, the meshes could be cleaned with water and then dried for reuse.
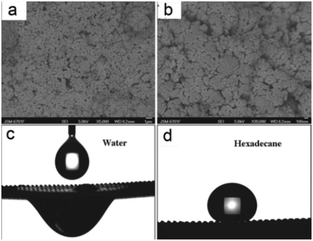 | ||
| Fig. 11 (a) SEM images of the PDDA–PFO/SiO2 coating showing plenty of protrusions and pores on its surface. (b) An enlarged view of the coating. (c) The water droplet spreads and penetrates through the resultant mesh. (d) The shape of a hexadecane droplet on the mesh with an OCA of 157° ± 2°. The PDDA–PFO/SiO2-coated mesh showed both superhydrophilicity and superoleophobicity.168 Reprinted with permission from ref. 168. Copyright 2012 RSC. | ||
In addition, hygro-responsive membranes with both superhydrophilic and superoleophobic properties in air and under water were reported by Tuteja et al.169 The membranes were fabricated by a dip coating method with fluorodecyl polyhedral oligomericsilsesquioxane (POSS) and cross-linked poly(ethylene glycol) diacrylate (x-PEGDA), forming a POSS + x-PEGDA complex. In air, the membrane surface is superoleophobic with several fluorodecyl POSS aggregates. While the membrane is immersed in an aqueous environment, the fluorodecyl POSS aggregates disappear because of surface reconfiguration caused by water molecules. The membrane could separate an oil-in-water emulsion (Fig. 12a and b) and a water-in-oil emulsion (Fig. 12d and e) by virtue of a solely gravity-driven process. The thermogravimetric analyses (TGA) demonstrated that the separation efficiency was more than 99% (Fig. 12c).
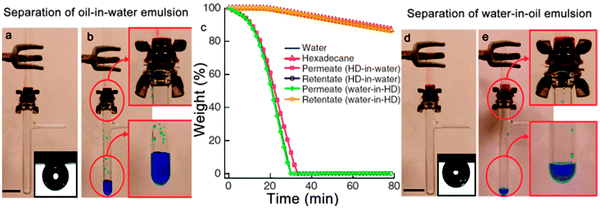 | ||
Fig. 12 (a, b) Photographs showing the separation of a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v 50 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v hexadecane-in-water emulsion. Inset in (a) presents the wettability underwater (with SDS of 1 mg ml−1) of a hexadecane droplet on a surface spin-coated with a 20 wt% fluorodecyl POSS + x-PEGDA blend. (c) TGA data showing the permeates and the retentates. Therein, HD is short for hexadecane. (d, e) The separation process of a 30 v hexadecane-in-water emulsion. Inset in (a) presents the wettability underwater (with SDS of 1 mg ml−1) of a hexadecane droplet on a surface spin-coated with a 20 wt% fluorodecyl POSS + x-PEGDA blend. (c) TGA data showing the permeates and the retentates. Therein, HD is short for hexadecane. (d, e) The separation process of a 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v 70 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v water-in-hexadecane emulsion. Inset in (d) presents the wettability of a hexadecane droplet on the surface spin-coated with a 20 wt% fluorodecyl POSS + x-PEGDA blend.169 Reprinted with permission from ref. 169. Copyright 2013 NPG. v water-in-hexadecane emulsion. Inset in (d) presents the wettability of a hexadecane droplet on the surface spin-coated with a 20 wt% fluorodecyl POSS + x-PEGDA blend.169 Reprinted with permission from ref. 169. Copyright 2013 NPG. | ||
The novel superhydrophilic and superoleophobic surfaces have potential to be a good candidate in industrial oil-polluted water treatments, clean-up of oil spills, and fuel purification. However, the preparation methods of the superhydrophilic–superoleophobic materials used are complicated. To date, superhydrophilic–superoleophobic surfaces and their applications still lack investigation, which remains a great challenge in their development.
2.4. Smart materials with switchable wettability
Materials with stimuli-responsive wettability have attracted increasing interest because of their importance in fundamental study and industrial applications.170,171 The controllable surface wettability can be achieved by applying an external stimulus such as light illumination,172 temperature,173 electrical potential,174 and pH.175,176 Very recently, based on an extreme wettability switch, controllable oil/water separation materials have been developed, which have important significance on the treatment of oil contaminated water for different demands.89,177,178Polymers containing acid or basic functional groups usually possess pH-responsive wetting behaviour since their conformation and charges are dramatically influenced by different pH solutions.179 Based on this view, Zhang et al.89 firstly developed smart materials that could be used for highly controllable oil/water separation processes. These smart materials with switchable superoleophilicity and superoleophobicity in aqueous media were prepared by grafting a BCP comprising of pH-responsive poly(2-vinylpyridine) and oleophilic/hydrophobic polydimethylsiloxane (i.e. P2VP-b-PDMS) on commonly used materials (Fig. 13a), such as textiles (Fig. 13b and c) and sponges. Therein, the P2VP block could alter its wettability and conformation via protonation and deprotonation upon changing the pH of the aqueous media, which provides a controllable and switchable access of oil by the PDMS block, resulting in switchable surface oil wettability in aqueous media (Fig. 13f). Fig. 13d and e show the highly controllable oil/water separation process of the resultant textile. When a mixture of gasoline and water at pH 6.5 was poured into the upper glass tube, the gasoline quickly passed though the textile membrane, but the water did not. However, when the textile membrane was at first, simply wetted by acidic water with a pH of 2.0 without subsequent drying and used under the same conditions, the opposite separation process was realized, with water passing through the membrane this time. Unlike the above strategy, Our group177 adopted a mixture of carboxyl-terminated thiol and methyl-terminated thiol as the modifying agent on hierarchical structured copper mesh to obtain a pH-responsive mesh. The protonation and deprotonation process was controlled via altering the pH of the aqueous media and endowed controllable and switchable access of both oil and water by the apolar and polar thiol on the mesh, which resulted in switchable surface in-air water wettability and underwater oil wettability. The as-prepared copper mesh had proved to be a smart material that could be applied to controllably and bi-directionally separate an oil–water mixture.
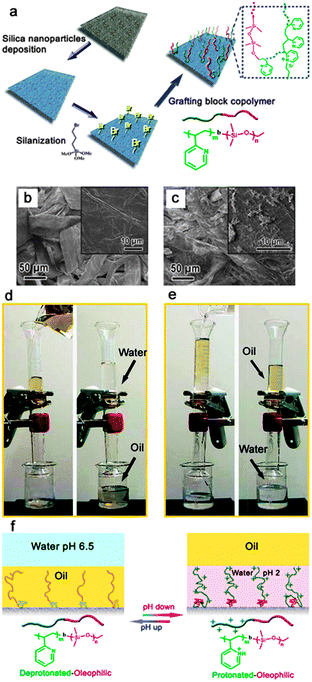 | ||
| Fig. 13 (a) A schematic illustration of the preparation procedure of a surface with switchable water and underwater oil wettability on a non-woven textile substrate. (b) and (c) show the SEM images of the raw textile and the textile after deposition of silica nanoparticles and BCP grafting. The insets in (b) and (c) show the enlarged view of single fibers. (d) and (e) show the controllable oil/water separation using the functionalized textile. (d) Separation of oil from the water phase. (e) Separation of water from the oil phase. Therein, the functionalized textile was pre-wetted with acidic water (pH = 2.0). (f) Schematic diagrams for the switchable oil wettability of the P2VPb–PDMS-grafted textile.89 Reprinted with permission from ref. 89. Copyright 2012 NPG. | ||
Light is another widely reported external stimuli. Photo-responsive wettability switching between superhydrophobicity and superhydrophilicity has been studied and reported.172,180 Recently, inspired by the extreme wettability switch, Zhai et al.178 firstly reported a photo-induced water–oil separation film, based on switchable superhydrophobicity–superhydrophilicity and underwater superoleophobicity, using a aligned ZnO nanorod array-coated stainless steel mesh. The ZnO-coated mesh showed excellent controllability for separating different water–oil mixtures in an oil–water–solid three-phase system.
Thermal response was also developed to control the wettability of water on a solid surface in the early years. Poly(N-isopropylacrylamide) (PNIPAAm), as a thermal responsive polymer with a lower critical solution temperature (LCST) of about 32–33 °C, was applied to fabricate a surface with switchable wettability.173 To utilize the thermal responsive polymer, Gao et al.181 reported a polymethylmethacrylate-b-PNIPAAm BCP with reversible switching between wettability states of hydrophilicity/oleophobicity and hydrophobicity/oleophilicity at different temperatures. Namely, water could permeate the BCP-coated mesh, but oil could not when the temperature was below the LCST; however, oil could penetrate the mesh and water could not when the temperature was above the LCST. Therefore, this film offered promising applications in the controllable separation of water and oil mixtures.
An electric field response was also developed by Tuteja et al.182 and used to switch the wettability and separate the oil–water mixture in a smart way. They developed the first-ever membrane-based single unit operation that could separate all types of oil–water mixtures with high separation efficiency upon applying a high electric field (higher than 1000 V).
On the basis of these strategies, functional materials with surfaces that have controlled water wettability (responsive to acidic or basic water) in air and oil wettability when submerged in a aqueous media, are expected to be used in many practical applications and help people design and fabricate smart functionalized interfacial materials for both in-air and underwater applications. So far, there are a few reports with respect to responsive materials for controllable oil/water separation. In addition, the responsive materials only involved pH-responsive materials, photo-responsive materials, thermally responsive materials and high electric field-responsive materials. Therefore, further research for controllable oil/water separation materials should be mainly focused on synthesizing new types of responsive materials, for instance, low electric field-responsive materials, stress-responsive materials, dual- and multi-responsive materials.
3. Separation of water-and-oil emulsions
As is well known, real oil–water mixtures are not always well layered. However, a large amount of oil–water mixtures to be processed, in fact, exist in the form of an emulsion. According to the report,169 an emulsion is defined as a drop in the diameter of the dispersed phase (water or oil) lower than 20 μm. The oil and water are generally considered as automatic layers in most of the literature reported since oil and water are immiscible and their densities are different. Sometimes a significant quantity of hydrocarbons may be introduced into the water phase due to the solubility of light fractions (the solubility of hydrocarbons in water generally decreases as the carbon number increases183), as well as emulsification processes. At present, although porous materials with special wettability, such as superhydrophobic–superoleophilic and superhydrophilic–superoleophobic surfaces, have been applied towards the separation of an oil–water mixture, these materials are commonly not suitable for emulsified oil/water separation, especially for surfactant-stabilized emulsions, because the droplet size of the emulsion (usually less than 20 μm) are smaller than the pore sizes of these materials (∼tens of micrometers). The emulsion separation materials can be also divided into the three types like the layered oil/water separation materials: superhydrophobic and superoleophilic materials, superhydrophilic and underwater superoleophobic materials, and superhydrophilic and superoleophobic materials.Jin et al.184 prepared a superhydrophobic–superoleophilic poly(vinylidene fluoride) (PVDF) membrane, with a water CA of 158° and oil CA of less than 1°, using a facile modified-phase inversion approach (Fig. 14). The as-prepared PVDF membrane was composed of spherical microparticles and the individual microparticles were isolated while being linked together through a fibre-like connection (Fig. 14a–c). This membrane could separate various water-in-oil emulsions including surfactant-free and surfactant-stabilized emulsions with droplet sizes from the micro- to nanometer range (Fig. 14d). Driven by gravity, the membrane exhibited a high separation efficiency with >99.95% oil purity after filtration and a high flux (Fig. 14e and f). It was worth noting that the membrane exhibited good antifouling properties, outstanding recyclability, thermal and mechanical stability, and durability. In addition, they also reported an ultrathin single-walled CNT (SWCNT) for the ultrafast separation of emulsified oil–water mixtures.185 A high permeation and nanometer scale pore size were combined in the SWCNT films, resulting in a surprising flux of up to 100000 L m−2 h−1 bar−1 and high separation efficiency >99.95%. Tao et al.186 presented a hierarchically hydrophobic porous silica monolith (HPSM) with macro- and meso-pores. The HPSM was synthesized using a sol–gel and phase separation process, and was subsequently modified by organosilanes. Oil droplets in a simple emulsion system (without emulsifier) were easily removed via filtration (Fig. 15a). In addition, when the emulsion was stabilized by a surfactant, a “reverse membrane emulsification” process is often used, HPSM exhibited excellent demulsification ability via adsorbing the emulsifier from the emulsion, leading to the complete breakdown of surfactant-stabilized emulsions. (Fig. 15b) The demulsification ratio reached 99.95% and the materials were reusable. Wang and co-workers187 reported a robust superhydrophobic and superoleophilic CNT/poly (dimethylsiloxane)-coated PU sponge for the continuous absorption and expulsion of oils from water surfaces. Surprisingly, when applied in conjunction with a vacuum system, this sponge could separate great amounts of oil, up to 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times its own weight and could also separate surfactant-free water-in-oil emulsions with high efficiency (oil purity: >99.97%).
000 times its own weight and could also separate surfactant-free water-in-oil emulsions with high efficiency (oil purity: >99.97%).
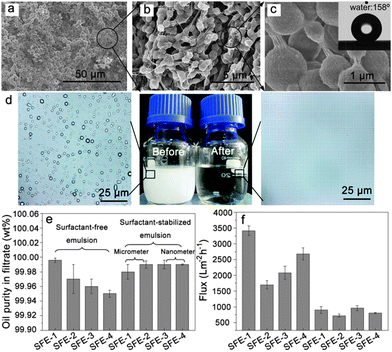 | ||
| Fig. 14 (a–c) SEM images of the PVDF membrane at different magnifications. The inset in (c) shows the image of a water droplet on the PVDF membrane (WCA = 158°). The drop sizes of the surfactant-free water-in-oil emulsions (SFE) were in the range of 5–20 μm. (d) Images of the SFE-3 emulsion before and after the filtration process. (e) Oil purity in the filtrate after the penetration process via the PVDF membrane for a selection of emulsions. (f) Fluxes of a selection of emulsions when passed through the PVDF membrane. Therein, the labels SFE-1, SFE-2, SFE-3 and SFE-4 represent the different kinds of oils, i.e. petroleum ether, toluene, isooctane, and dichloromethane respectively.184 Reprinted with permission from ref. 184. Copyright 2013 Wiley. | ||
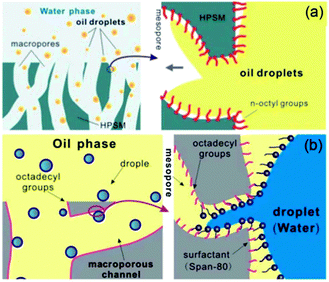 | ||
| Fig. 15 (a) A conjecture of the adsorption process of oil droplets by HPSM. When the oil droplets flowed through the macropores in an aqueous environment, they could easily reach the inner pore surface and be absorbed into the mesopores. (b) The mechanism of the demulsification by HPSM. The emulsifier-stabilized droplet was first pinned and captured on the surface of HPSM. After that, the tiny droplet would break (due to the flow impulsion) and the surfactant would be released. Accordingly, the tiny droplets without emulsifier would easily join together to form continuous phase.186 Reprinted with permission from ref. 186. Copyright 2013 RSC. | ||
Conversely, to separate an oil-in-water emulsion, a novel superhydrophilic and underwater superoleophobic zwitterionic polyelectrolyte grafted PVDF membrane was successfully fabricated by Jin et al.188 using a surface-initiated atom transfer radical polymerization method. This membrane could thoroughly separate the dispersed oil from water with an ultra-high separation efficiency (>99.999%), even with surfactant-stabilized oil-in-water emulsions with a droplet size in the micrometer scale. However, it was not suitable for emulsions with nanometer-scale droplets because of the pore-size effect. Furthermore, the membrane showed excellent antifouling property to organic liquids due to its ultralow oil adhesiveness. Afterwards, they reported189 a salt-induced phase-inversion approach to fabricate poly(acrylic acid)-grafted PVDF with superhydrophilic and underwater superoleophobic properties, showing great potential in separating oil-in-water emulsions with high separation efficiency and fluxes. Another work was suggested by Xu et al.190 who reported a hydrophilization approach through co-deposition of mussel-inspired polydopamine and polyethyleneimine on a polypropylene microfiltration membrane. The modified membranes exhibited good wettability and could be applied for oil-in-water emulsion separation.
In addition to the above superhydrophobic–superoleophilic and superhydrophilic-underwater superoleophobic materials, superhydrophilic–superoleophobic materials could also realize the separation of an oil/water emulsion. As exhaustively illustrated in Section 2.3, Tuteja et al.169 fabricated a POSS + x-PEGDA blend-coated hygro-responsive membrane with both superhydrophilic and superoleophobic properties in air and under water. This membrane could be employed towards the separation of an oil/water emulsion with droplet sizes larger than 1 μm with a separation efficiency ≥99.9%.
Membranes with special wettability have been demonstrated to be capable of overcoming the defects of pressure-driven filtration membranes for oil/water emulsions.191,192 These defects mainly include low flux and a quick decline in separation efficiency, which leads to a severe fouling issue and a clean-up problems.193–195 Therefore, it is expected that membranes with special wettability will replace the pressure-driven filtration membranes in the separation of oil–water emulsions.
4. Comprehensive understanding of all kinds of oil/water separation materials
A wide range of oil/water separation materials have been discussed in the former sections. Here, a comprehensive understanding and comparison of these materials are also discussed to acquaint their advantages and disadvantages. Table 1 shows some typical oil/water separation materials depending on the as-introduced substrates in the former sections. From the table, the oil/water separation features of all kinds of materials can be systematically understood, including substrate materials, preparation methods, wettability of water and oils, separation or absorption substances, separation efficiency of film-like material, absorption capacities of bulk materials, recyclability, and cost. Therein, the separation efficiency of film-like material and absorption capacities of bulk materials are commonly the mostly concerned.| Oil/water separation materials | Preparation methods | WCA (in air) (°) | OCA (in air) (°) | OCA (in water) | Separation or absorption substances | Separation efficiency (%) | Absorption capacities (times) | Recyclability | Cost | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polypropylene and poly(butylmethacrylaceto-hydroxyethylmethacrylate) non-woven material | Melt blown spinning method | ≥127 | 0 | n.a. | Crude oil, diesel, toluene | n.a. | 7–10 | Good | Inexpensive | 76 | |||
| Polyaniline coated cotton fabric | In situ vapor phase deposition | 156 | 0 | n.a. | Hexadecane | 97.8 | n.a. | Very good | Expensive | 81 | |||
| F-PBZ modified nanofibrous membranes | Electrospun and in situ polymerization | 161 | 0 | n.a. | Dichloromethane | n.a. | n.a. | n.a. | Inexpensive | 69, 70 | |||
| Composite PU–polystyrene fibrous mats | Co-axial electrospinning | Hydrophobic | Oleophilic | n.a. | Motor oil, sunflower seed oil | n.a. | 47.48–64.40 | n.a. | Inexpensive | 71 | |||
| Reduced GO@ZnO nanocomposite coated textiles | Layer-by-layer technique | 136 | 0 | n.a. | Oil, dodecane, decane, octane | n.a. | Up to 23 | Good | Fair | 73 | |||
| Silica nanoparticles coated kapok fibers | Sol–gel method | 151 | 0 | n.a. | n-Hexane, toluene, chloroform, gasoline, diesel, soybean oil | n.a. | 41.8–59.8 | Good | Inexpensive | 74 | |||
| Polystyrene fibers | Electrospinning method | 151.3 | 0 | n.a. | Diesel oil, silicon oil, peanut oil, motor oil | n.a. | 7.13–131.63 | n.a. | Inexpensive | 75 | |||
| SiO2/polystyrene nanocomposite fabric/filter paper | One-step dipping process | 154 (fabric), 156 (filter paper) | 0 | n.a. | Petroleum ether | >93 for fabric, > 91 for filter paper | n.a. | n.a. | Inexpensive | 68 | |||
| Transition-metal (Fe, Co, Ni, Cu, Ag) coated sponge | In situ growth method | >150 | 0 | n.a. | Hexane, hexadecane, edible oil, chloroform | n.a. | 18–35 | Good | Inexpensive | 33 | |||
| Polypyrrole-1H,1H,2H,2H-Perfluorooctyltriethoxysilane-sponge | Vapor-phase deposition process | 153.7 | 0 | n.a. | Motor oil, lubricating oil, pump oil, silicone oil, soybean oil | n.a. | >20 | Good | Expensive | 91 | |||
| Graphene-based sponges | Dip coating method | 162 | 0 | n.a. | Motor oil, soybean oil, pump oil, used pump oil, methanol, ethanol, acetone, hexane, chloroform | n.a. | 54–165 | Good | Expensive | 86 | |||
| Copper–C11H23COOAg coated sponges | Solution-immersion processes | 171 | 0 | n.a. | Lubricating oil, octane, decane, dodecane | n.a. | >13 | Good | Inexpensive | 88 | |||
| Fluoroalkylsilane modified PU sponge | Chromic acid etching | 155 | 0 | n.a. | Gasoline, crude oil, hexane, petroleum ether | >95 | n.a. | Good | Inexpensive | 93 | |||
| Methyltrichlorosilane modified PU sponge | Chemical etching method | 157 | 0 | n.a. | Decane, dodecane, octane, crude oil, gasoline, bean oil, lubricating oil | Good | 15–25 | Good | Inexpensive | 94 | |||
| Activated carbon-coated sponges | Dip-coating method | 150.2 | 0 | n.a. | Decane, octane, phenoxin, benzene, chloroform, kerosene, dichlorobenzene, nitrobenzene, ethanol, acetone, tetrahydrofuran (THF), n-hexane | Good | 27.0–85.9 | Good | Expensive | 100 | |||
| Conjugated microporous polymers coated sponge | Dip-coating after homocoupling polymerization | 167 | 0 | n.a. | Vegetable oil, pump oil, dodecane, decane, octane, hexane, phenol, nitrobenzene, chloroform, 1,2-dichlorobenzene, ethylbenzene, toluene, benzene, dimethylsulfoxide (DMSO), THF, dimethylformamide (DMF), acetone, ethanol, methanol | Good | 6–23 | Good | Inexpensive | 87 | |||
| 1,12-Dodecane diamine–graphene-oxide modified PU foam | Amidation procedure followed with grafting | 159.1 | Superoleophilic | n.a. | Toluene, gasoline, diesel oil | 93.8 | 26–41 | n.a. | Expensive | 95 | |||
| Silane (octyltrichlorosilane) modified SiO2/PTFE coated PU sponges | Chemical vapor deposition after dip coating procedure | 165 | 0 | n.a. | n-Hexane, pentane, heptane, benzene, toluene, silicone oil | n.a. | 10–12 | Fair | Expensive | 92 | |||
| Colloidal superparamagnetic iron oxide and submicrometer CNT particles coated PU foams | Electrostatic deposition technique | 160 | 0 | n.a. | Mineral oil | n.a. | >12 | Good | Inexpensive | 97 | |||
| Boron nitride nanosheets | Dynamic templating approach | 165 | 0 | n.a. | Ethanol, toluene, pump oil, used engine oil, ethylene glycol | n.a. | 20–33 | Good | Inexpensive | 128 | |||
| PDA–NDM mesh | Mussel-inspired chemistry and Michael addition reaction. | 144 | 0 | n.a. | Petroleum ether, hexane, gasoline, diesel. | 98.12–99.95 | n.a. | Good | Inexpensive | 43 | |||
| ZnO nanorod-coated mesh | Chemical vapor deposition | 157 ± 1 | 0 | n.a. | Gasoline, petroleum ether, hexane, diesel | 92–97.5 | n.a. | n.a. | Inexpensive | 56 | |||
| CNT-coated mesh | Thermal chemical vapor deposition | 163 ± 4 | 0 | n.a. | Emulsion (diesel, lubricating oil) | High (needs five iterations) | n.a. | Good | Inexpensive | 54 | |||
| Silicone elastomer-coated mesh | Aerosol assisted chemical vapor deposition | 152–167 | 0 | n.a. | Toluene, hexane | >99 | n.a. | Good | Inexpensive | 52 | |||
| Magnetic nickel foams | Electroless metal deposition | Superhydrophobic | 0 | n.a. | Dichloromethane, bromobenzene, carbon disulfide, mixed oil | >90 | n.a. | able | Expensive | 146 | |||
| PS–H–SiO2 filter paper | Dip-coating | 157 ± 2 | 4 | n.a. | Diesel oil | 96 | n.a. | Good | Inexpensive | 142 | |||
| MTMS–DMDMS gel | Sol–gel process | 152.6 | 0 | n.a. | Toluene, TMB, n-hexane, cyclohexane, kerosene, chloroform, petroleum ether, n-octanol, diethylether, n-octane, tetradecane, mineral oil | n.a. | 60–150 | Good | Inexpensive | 141 | |||
| Fe2O3@C nanoparticles | Thermal decomposition | 162.9 ± 2 | 0 | n.a. | N100 Lubricating oil | n.a. | 3.7 | Good | Expensive | 134 | |||
| Calcium carbonate powder | Sol–gel process | 152 | 42 for diesel oil, 25 for crude oil | n.a. | Diesel oil, crude oil | 98.1–99.6 | n.a. | Unable | Inexpensive | 122 | |||
| PAM hydrogel-coated mesh | Photo-initiated polymerization process | 0 | n.a. | 155.3 ± 1.8 | Gasoline, diesel, crude oil, vegetable oil, hexane, petroleum ether. | >99 | n.a. | Good | Expensive | 154 | |||
| Zeolite-coated mesh | Hydrothermal method | 0 | n.a. | >150 | Petroleum ether, cyclohexane, soybean oil, diesel, crude oil. | High | n.a. | Good | Inexpensive | 156 | |||
| Cu(OH)2 nanowire-haired mesh | Solution-immersion | 0 | n.a. | 155 | Isooctane, diesel, hexane, petroleum ether, soybean oil. | High | n.a. | Good | Inexpensive | 157 | |||
| POSS + x-PEGDA membrane | Dip-coating | 0 | 152 | n.a. | Oil-in-water emulsion and water-in-oil emulsion | >99.9 | n.a. | n.a. | n.a. | 169 | |||
| SWCNT network film | Vacuum-filtering | 94 | 0 | n.a. | Water-in-oil emulsions (including surfactant-free and surfactant-stabilized emulsions) | >99.95 | n.a. | good | Expensive | 185 | |||
| CNT/PDMS-coated sponge | Dip-coating method | 162 ± 2 | 0 | n.a. | Soybean oil, used motor oil, diesel oil, n-hexadecane, gasoline, n-hexane, water-in-oil emulsions | >99.97 for water-in-oil emulsion | 15–25; up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 from conjunction with a vacuum system 000 from conjunction with a vacuum system |
n.a. | Inexpensive | 187 | |||
| PVDF membrane | Modified-phase inversion process | 158 | <1 | n.a. | Water-in-oil emulsions (including surfactant-free and surfactant-stabilized emulsions) | >99.95 | n.a. | Good | Inexpensive | 184 | |||
| Zwitterionic polyelectrolyte grafted PVDF membrane | Surface-initiated atom transfer radical polymerization | 0 | n.a. | 158 | Dispersed oil (including isooctane, hexane, diesel, petroleum ether, soybean oil) | 99.999 | n.a. | Good | Expensive | 188 | |||
| Carbon nanofiber aerogels (ρ = 4–6 mg cm−3) | Pyrolysis method | 113.50–128.64 | 0 | n.a. | Methanol, ethanol, ethylene glycol, acetone, n-hexane, 1-octane, cyclohexane, chloroform, phenoxin, acetic ether, ethyl acetoacetate, oleic acid, acetaldehyde, ether, petroleum, THF, ethanediamine, toluene, bromobenzene, styrene, cyclohexene, 1-octadecene, soybean oil, diesel oil, sesame oil, gasoline oil, pump oil. | n.a. | 106–312 | Very good | Inexpensive | 107 | |||
| PDMS-coated Carbonaceous nanofiber hydrogels and aerogels (ρ = 3.3 mg cm−3) | Template-directed hydrothermal carbonization process | 158 ± 3 | 0 | n.a. | Gasoline, cyclohexane, ethanol, diesel oil, vegetable oil, chlorobenzene, phenoxin | n.a. | Up to 115 | Good | Fair | 108 | |||
| Twisted carbon fiber aerogel (ρ = 12 ± 5 mg cm−3) | Pyrolysis method | Hydrophobic | 0 | n.a. | Colza oil, olive oil, pump oil, chloroform, toluene, octadecylene, isopropyl alcohol, heptane, hexane, benzyl alcohol, DMF, acetone, ethanol, cyclohexane | n.a. | 50–190 | Good | Inexpensive | 106 | |||
| Spongy grapheme | |||||||||||||
| (ρ ∼ 12 mg cm−3) | Hydrothermal treatment and freeze-drying Hummers method | 114 ± 2 | 0 | n.a. | Methanol, ethanol, actetone, THF, DMSO, toluene, ethylbenzene, 1,2-dichlorobenzene, chloroform, nitrobenzene, hexane, heptane, octane, dodecane, pump oil, kerosene, castor oil, soybean oil. | n.a. | 20–86 | Good | Inexpensive | 110 | |||
| Magnetic Fe2O3/C, Co/C, and Ni/C foams (ρ < 5 mg cm−3) | Template method | 152 | 0 | n.a. | Bean oil, lubricating oil, crude oil, gasoline, diesel oil, hexane, octane, decane, dodecane | n.a. | Up to 100 | Good | Inexpensive | 119 | |||
| Mesoporous grapheme | Template method | >150 | 0 | n.a. | Phenoxin, NMP, DMSO, nitrobenzene, dodecane, dichlorobenzene, decane, benzene, octane, chloroform, toluene, DMF. | n.a. | 7.96–66 | Good | Expensive | 103 | |||
| Macroscopic graphene/iron oxide hydrogels | Self-assembly method | >150 | <30 | n.a. | Cyclohexane, toluene, gasoline, paraffin oil, vegetable oil, phenoxin. | n.a. | Up to 27 | Good | Inexpensive | 114 | |||
| CNT sponges (ρ = 5–10 mg cm−3) | Chemical vapor deposition | 156 | 0 | n.a. | Hexane, ethanol, gasoline, pump oil, DMF, ethylene glycol, chloroform, mineral oil, vegetable oil, diesel oil, octane, ethyl acetate. | n.a. | 80–180 | Good | Expensive | 115-117 | |||
| Magnetic CNT sponges (ρ = 15 mg cm−3) | Chemical vapor deposition | 145 | <4 | n.a. | Diesel oil, gasoline. | n.a. | Up to 56 | Very good | Expensive | 121 | |||
| 3D macroporous Fe/C nanocomposites | Template method | 157.2 | 0 | n.a. | Lubricating oil, bean oil, crude oil, dodecane, decane. | n.a. | 4.5–7.5 | Good | Inexpensive | 120 | |||
| Graphene–CNT hybrid foam | Two-step chemical vapor deposition | 152.3 | 0 | n.a. | Compressor oil, sesame oil, chloroform, dichlorobenzene, toluene, DMF | n.a. | About 80–130 | Good | Expensive | 102 | |||
| Carbon aerogel (ρ = 0.16 mg cm−3) | “Sol-cryo” method | 132.9 | 0 | n.a. | n-Hexane, ethanol, crude oil, toluene, motor oil, vegetable oil, 1,4-dioxane, ionic liquid, chloroform, phenoxin. | n.a. | About 220–750 | Good | Expensive | 109 |
The separation efficiency is generally concerned in film-like materials such as metallic mesh films and fabrics (sometimes sponge). The separation efficiency could generally reach >90% for all kinds of films in the reported work (Table 1). Particularly, the membranes used for the separation of emulsions could reach significantly higher separation efficiency due to the smaller pores on the membranes.
As for the absorption capacities, it is obvious that the hydrophobic particles and powders generally possess the lowest oil absorption capacities within several times its initial weight. But for the superhydrophobic sponges/foams, the oil absorption capacities are much larger than that of the particles and powders, ranging from a dozen times to dozens of times the weight of original materials. The carbon-based aerogels generally show the largest oil absorption capacities. The oil absorption capacities can commonly reach over 100 times the weight of the unused materials and the highest one could even achieve 750 times the weight of the unused material.109 Carbon-based aerogels are the most suitable for oil removal in the view of oil absorption capacity. However, their preparation methods are usually complicated and usually expensive when compared to those used for superhydrophobic sponges.
5. Theories behind oil/water separation behaviour
5.1. In-air contact angle
The oil/water separation, in essence, is the wettability behaviour that occurs at the interface of the solid, air, water and oil phase. When a liquid droplet was presented on an ideal smooth solid surface in air (Fig. 16a), the CA is determined by Young's equation:196cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 = (γSA − γSW)/γWA θ0 = (γSA − γSW)/γWA | (1) |
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW = rcos θW = rcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 θ0 | (2) |
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θCB = rffSWcos θCB = rffSWcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 + fSW − 1 θ0 + fSW − 1 | (3) |
5.2. Contact angle hysteresis and sliding angle
An oil/water separation material is either hydrophobic or oleophobic so that it can repel one phase of liquid while allow the other one permeate through it. To investigate hydrophobic and oleophobic surfaces, contact angle hysteresis (CAH) is another important concept that reflects the surface neterogeny.10,199 The contact angle hysteresis is quite common on rough and chemical heterogeneous surfaces. In these cases, a series of apparent static CA can be observed, the maximum is called advancing CA and the minimum is called the receding CA. The CAH is generally considered as the difference between advancing CA and receding CA. The sliding angle (SA), a threshold tilting value of the angle between the surface and horizon line, below which a liquid droplet starts to roll/slide upon elevating an end of the surface.10,199 The SA decrease with the decrease of CAH. However, SA is not equal to the difference between advancing CA and receding CA. Usually, superhydrophobic properties mean not only a large static CA but also a small CAH since the small CAH is responsible for the surface self-cleaning effect. Thus, CAH and sliding angle are also important indexes for the characterization of a super-lyophobic surface. For the two wetting states introduced above, the Cassie wetting state commonly possesses both high CA and small CAH due to the air trapped below reduced the sliding resistance.5.3. Transition between Wenzel and Cassie states
As shown in eqn (2) and (3), both the Wenzel relation and the Cassie relation give information indicating that the surface roughness can increase the surface hydrophobicity. Compared with the Wenzel equation (eqn (2)), the Cassie equation allows for the possibility of an apparent CA larger than 90°, even with θ0 < 90°. From the view of thermodynamics and energy barrier, the Wenzel and Cassie state are considered to be two separated energy minima with an energy barrier between the two states.200 Transition between these two states can occur when external energy is applied, such as pressure,201 vibration,202 and an electrical field.203 The threshold of the mutual transition occurs when these two states possess an equal apparent CA, i.e. θW = θCB.204 To combine the Wenzel equation and Cassie equation, the critical apparent CA can be obtained,204cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θC = (fSW − 1)/(r − fSW) θC = (fSW − 1)/(r − fSW) | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θC < 0, i.e. θC > 90°. While 90° < θ0 < θC, the air trapped is unstable, the Cassie state easily moves to the Wenzel state; while θ0 > θC, the Cassie state is stable. For a certain material, the Young's CA is constant. In order to obtain a stable and self-cleaning surface, we can adjust the surface geometric structure to minimize the θC.
θC < 0, i.e. θC > 90°. While 90° < θ0 < θC, the air trapped is unstable, the Cassie state easily moves to the Wenzel state; while θ0 > θC, the Cassie state is stable. For a certain material, the Young's CA is constant. In order to obtain a stable and self-cleaning surface, we can adjust the surface geometric structure to minimize the θC.
To fabricate an in-air superoleophobic surface is much more difficult than that of superhydrophobic surface because oils commonly have a smaller surface free energy than water. In addition, this is the fundamental reason why oils can be separated from water. To fabricate a superhydrophobic surface, as illustrated by the Wenzel and Cassie equations, an increase in the surface roughness and decrease in the surface free energy are efficient methods. However, to fabricate a superoleophobic surface, researchers commonly construct the re-entrant structure, such as an inverted trapezoidal structure, mushroom-like structure and convex structure.205–207 The re-entrant structures are more likely to trap air and form a stable Cassie wetting state. Theoretical studies revealed that this kind of structure can even form super-lyophobic properties on an inherent lyophilic surface.206,207
5.4. Underwater wettability of oil
While the wetting behaviour occurs underwater, we first take the CA of an ideally smooth surface into account (Fig. 16d). The wetting equation at the solid–water–oil interface can be shown by combining the Young's equation (eqn (1)) of a solid–air–water interface196 and a solid–air–oil interface as suggested by Jung and Bhushan.208 The apparent OCA (θOW) in an aqueous environment can be given as:208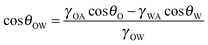 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θO and cos
θO and cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW are all positive. Since the surface tension of oil/organic liquids are much lower than that of water (γOA ≪ γWA), the value of γOA·cos
θW are all positive. Since the surface tension of oil/organic liquids are much lower than that of water (γOA ≪ γWA), the value of γOA·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θO − γWA·cos
θO − γWA·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW is commonly negative and thus it can be concluded that most hydrophilic surfaces in air show oleophobic properties underwater at the solid–water–oil interface. For a hydrophobic (θW > 90°) and oleophilic (θO < 90°) surface, the surface is always oleophilic in the aqueous media since the value of the numerator in the right-hand side of eqn (1) is always larger than 0. For a hydrophobic (θW > 90°) and oleophobic (θO > 90°) surface, an underwater oleophobic surface can be created if γOA·cos
θW is commonly negative and thus it can be concluded that most hydrophilic surfaces in air show oleophobic properties underwater at the solid–water–oil interface. For a hydrophobic (θW > 90°) and oleophilic (θO < 90°) surface, the surface is always oleophilic in the aqueous media since the value of the numerator in the right-hand side of eqn (1) is always larger than 0. For a hydrophobic (θW > 90°) and oleophobic (θO > 90°) surface, an underwater oleophobic surface can be created if γOA·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θO is bigger than γWA·cos
θO is bigger than γWA·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW. The wettability at different interfaces are summarized in Table 2.208
θW. The wettability at different interfaces are summarized in Table 2.208
| Solid–air–water interface | Solid–air–oil interface | Solid–water–oil interface |
|---|---|---|
| Hydrophilic (γSA > γSW) | Oleophobic if γOAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 < γWAcos θ0 < γWAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW θW |
|
Oleophilic if γOAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 > γWAcos θ0 > γWAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW θW |
||
| Hydrophobic (γSA < γSW) | Oleophobic if γSA < γSO | Oleophobic if γOAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 > γWAcos θ0 > γWAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW θW |
Oleophilic if γOAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ0 < γWAcos θ0 < γWAcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW θW |
||
| Oleophilic if γSA > γSO | Oleophilic |
Similar to the Wenzel and Cassie equations in air, the underwater Wenzel equation (Fig. 16e) and Cassie equation (Fig. 16f) can be obtained by introducing the surface roughness and contact phase fractions (solid–oil and solid–water interfaces)
Wenzel:197
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW = rcos θW = rcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θOW θOW | (6) |
Cassie:198
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θCB = rffSOcos θCB = rffSOcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θOW + fSO − 1 = rfcos θOW + fSO − 1 = rfcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θOW − fSW(rfcos θOW − fSW(rfcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θOW + 1) θOW + 1) | (7) |
6. Conclusions
Nowadays, oil leakage and oil-contaminated water needs to be addressed urgently since it has become a worldwide issue. Therefore, demands for synthesizing functional materials for efficient treatment of oily water is imperative. In this feature article, we have reviewed the development of various special wettability stimulated oil/water separation materials by sorting them into several categories based on the substrate materials used, from the early superhydrophobic–superoleophilic separation materials (oil-removal materials), to the superhydrophilic and underwater superoleophobic separation materials (water-removal materials), to smart separation materials with switchable wettability. Special wettability stimulated separation materials show big advantages when compared with conventional oil/water filter materials due to their high selective absorbing/filtering features. Amazingly, the separation of an oil-in-water emulsion, water-in-oil emulsion, and even surfactant-stabilized emulsions were successfully achieved very recently using special wettability membranes with a maximum separation efficiency larger than 99.999%. Distinguished from the former separation materials, which are usually applied to separate the layered oil-and-water mixture, these membranes possess a smaller and more compact pore size, which can be employed to separate oil–water emulsions with droplet sizes from the micrometer to the nanometer range. In the last section, the theories involved for both the oil-removal process and water-removal process are discussed from the viewpoint of a balanced condition of force. Here, particular attention is paid to summarizing and comparing the separation efficiency, absorption capacity and recyclability, which are crucial for practical use in oil spill accidents to oil/water separation materials.Although the special wettability stimulated oil/water separation materials show enormous potential in the treatment of oil spill accidents and industrial oily water, the investigations in this field are still facing a lot of challenges, and some of the problems still need to be solved before they can be used to replace traditional separation techniques, as well as for the further studies. First and foremost, the design and synthesis of stable and durable rough surface structures on materials with special wettability is a big challenge. Original porous substrate materials usually provide the pre-existing microscale rough structure, to obtain an extreme wetting state, a layer of nanoscale structures is needed to form a typically hierarchical structure. However, most of the surface fine structures can be easily damaged by external influences including mechanical stress and chemical contamination, which restricts the material's applications. Secondly, oil (water) filtering materials (such as fabric- and metallic mesh-based materials) are unrealistic to be directly used for oil leakage treatment for oily water, which should be collected in advance so as to realize the gravity driven oil/water separation. Thirdly, a large portion of the synthesis methods cannot be carried out on large-scale (such as the in situ growth method and hydrothermal method) and thus the mass-production techniques of oil/water separation materials for the large-area oil spills are still required. Fourthly, while the separation of an oil/water emulsion have been realized, the separation speed and the membrane pore sizes are considered to be contradictory. Therefore, how to realize effective and high-throughput separation of a wide range of oil/water emulsions with small droplet sizes from the micrometer to the nanometer range is an important issue. Finally, most of the previous work have focused on the separation of low-viscous oil-and-water mixtures; however, research on the separation of high-viscous oil-and-water mixtures are rare.
As is well known, the special wettability stimulated oil/water separation materials are commonly inspired from natural plants (e.g. lotus leaf) and animals (e.g. fish scales) that exhibit special wettability. To achieve the industrialization of bio-inspired oil/water separation materials at an early stage, future work will mainly concentrate on four aspects as follows: Firstly, by combining theoretical prediction, wear-resistant oil/water separation materials are aimed to be designed and synthesized to increase the materials' life time. Secondly, more and more simple and large-scale preparation methods are being researched to achieve industrialization in oil/water separation. Thirdly, efficient and rapid separation materials of oil/water emulsions with ultra-small droplets should be taken into consideration. Fourthly, specialized materials will be developed for the rapid separation of high-viscous oil-and-water mixtures. Finally, multifunctional (such as magnetic oil-removing materials) and external stimulus-responsive (from single to dual and even to multiple stimulus-responsive) materials are also needed to be considered to prepare smart interfacial materials for different oil/water separation purposes.
Acknowledgements
This work is supported by the National Nature Science Foundation of China (NO11172301 and 21203217), the “Funds for Distinguished Young Scientists” of Hubei Province (2012FFA002), the “Western Light Talent Culture” Project, the Co-joint Project of Chinese Academy of Sciences and the “Top Hundred Talents” Program of Chinese Academy of Sciences and the National 973 Project (2013CB632300).Notes and references
- J. W. Short, S. D. Rice, R. A. Heintz, M. G. Carls and A. Moles, Energy Sources, 2003, 25, 509–517 CrossRef CAS.
- B. Dubansky, A. Whitehead, J. T. Miller, C. D. Rice and F. Galvez, Environ. Sci. Technol., 2013, 47, 5074–5082 CrossRef CAS PubMed.
- A. B. Nordvik, J. L. Simmons, K. R. Bitting, A. Lewisa and T. S. Kristiansen, Spill Sci. Technol. Bull., 1996, 3, 107–122 CrossRef CAS.
- K. Gaaseidnes and J. Turbeville, Pure Appl. Chem., 1999, 71, 95–101 CrossRef CAS.
- B. Tansel and J. Regula, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2000, 35, 1557–1575 CrossRef.
- D. Quéré, Annu. Rev. Mater. Res., 2008, 38, 71–99 CrossRef.
- D. Bonn, J. Eggers, J. Indekeu, J. Meunier and E. Rolley, Rev. Mod. Phys., 2009, 81, 739–805 CrossRef CAS.
- (a) Y. B. Zhang, Y. Chen, L. Shi, J. Li and Z. G. Guo, J. Mater. Chem., 2012, 22, 799–815 RSC; (b) G. Y. Wang, Z. G. Guo and W. M. Liu, J. Bionic Engineer., 2014, 11, 325–345 CrossRef.
- (a) M. J. Liu, S. T. Wang and L. Jiang, MRS Bull., 2013, 38, 375–382 CrossRef CAS; (b) K. S. Liu, M. Y. Cao, A. Fujishima and L. Jiang, Chem. Rev., 2014, 114, 10044–10094 CrossRef CAS PubMed.
- B. Bhushan and Y. C. Jung, Prog. Mater. Sci., 2011, 56, 1–108 CrossRef CAS PubMed.
- X. J. Feng and L. Jiang, Adv. Mater., 2006, 18, 3063–3078 CrossRef CAS.
- N. Gao and Y. Y. Yan, Nanoscale, 2012, 4, 2202–2218 RSC.
- X. Zhang, F. Shi, J. Niu, Y. G. Jiang and Z. Q. Wang, J. Mater. Chem., 2008, 18, 621–633 RSC.
- Z. X. Xue, M. J. Liu and L. Jiang, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 1209–1224 CrossRef CAS.
- Y. Huang, M. J. Liu, J. X. Wang, J. M. Zhou, L. B. Wang, Y. L. Song and L. Jiang, Adv. Funct. Mater., 2011, 21, 4436–4441 CrossRef CAS.
- L. Lin, M. J. Liu, L. Chen, P. P. Chen, J. Ma, D. Han and L. Jiang, Adv. Mater., 2010, 22, 4826–4830 CrossRef CAS PubMed.
- M. J. Liu, Z. X. Xue, H. Liu and L. Jiang, Angew. Chem., Int. Ed., 2012, 51, 8348–8351 CrossRef CAS PubMed.
- Z. Burton and B. Bhushan, Ultramicroscopy, 2006, 106, 709–719 CrossRef CAS PubMed.
- A. Poynor, L. Hong, I. K. Robinson, S. Granick, Z. Zhang and P. A. Fenter, Phys. Rev. Lett., 2006, 97, 266101 CrossRef.
- T. Onda, S. Shibuichi, N. Satoh and K. Tsujii, Langmuir, 1996, 12, 2125–2127 CrossRef CAS.
- J. Bico, C. Marzolin and D. Quéré, Europhys. Lett., 1999, 47, 220–226 CrossRef CAS.
- W. Chen, A. Y. Fadeev, M. C. Hsieh, D. Öner, J. Youngblood and T. J. McCarthy, Langmuir, 1999, 15, 3395–3399 CrossRef CAS.
- R. Blossey, Nat. Mater., 2003, 2, 301–306 CrossRef CAS PubMed.
- L. W. Zhang, R. Dillert, D. Bahnemann and M. Vormoor, Energy Environ. Sci., 2012, 5, 7491–7507 CAS.
- Y. Chen, Y. B. Zhang, L. Shi, J. Li, Y. Xin, T. T. Yang and Z. G. Guo, Appl. Phys. Lett., 2012, 101, 033701 CrossRef PubMed.
- G. Y. Wang, W. X. Liang, B. Wang, Y. B. Zhang, J. Li, L. Shi and Z. G. Guo, Appl. Phys. Lett., 2013, 102, 203703 CrossRef PubMed.
- S. Nagappan, S. S. Park, E. J. Yu, H. J. Cho, J. J. Park, W.-K. Lee and C.-S. Ha, J. Mater. Chem. A, 2013, 1, 12144–12153 CAS.
- M. Elsharkawy, T. M. Schutzius and C. M. Megaridis, Lab Chip, 2014, 14, 1168–1175 RSC.
- B. Wang, Y. B. Zhang, W. X. Liang, G. Y. Wang, Z. G. Guo and W. M. Liu, J. Mater. Chem. A, 2014, 2, 7845–7852 CAS.
- J. Y. Lv, Y. L. Song, L. Jiang and J. J. Wang, ACS Nano, 2014, 8, 3152–3169 CrossRef CAS PubMed.
- H. R. Yang, H. J. Zhu, M. M. R. M. Hendrix, N. J. H. G. M. Lousberg, G. de With, C. C. Esteves and J. H. Xin, Adv. Mater., 2013, 25, 1150–1154 CrossRef CAS PubMed.
- J. Li, L. Shi, Y. Chen, Y. B. Zhang, Z. G. Guo, B. L. Su and W. M. Liu, J. Mater. Chem., 2012, 22, 9774–9781 RSC.
- B. Wang, J. Li, G. Y. Wang, W. X. Liang, Y. B. Zhang, L. Shi, Z. G. Guo and W. M. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 1827–1839 CAS.
- W. X. Liang and Z. G. Guo, RSC Adv., 2013, 3, 16469–16474 RSC.
- J. W. Zeng and Z. G. Guo, Colloids Surf., A, 2014, 444, 283–288 CrossRef CAS PubMed.
- Z. G. Guo, W. M. Liu and B. L. Su, Appl. Phys. Lett., 2008, 92, 063104 CrossRef PubMed.
- Z. G. Guo, F. Zhou, J. C. Hao and W. M. Liu, J. Am. Chem. Soc., 2005, 127, 15670–15671 CrossRef CAS PubMed.
- J. L. Song, S. Huang, K. Hu, Y. Lu, X. Liu and W. Xu, J. Mater. Chem. A, 2013, 1, 14783–14789 CAS.
- S. Sethi and A. Dhinojwala, Langmuir, 2009, 25, 4311–4313 CrossRef CAS PubMed.
- L. Feng, Z. Y. Zhang, Z. H. Mai, Y. M. Ma, B. Q. Liu, L. Jiang and D. B. Zhu, Angew. Chem., Int. Ed., 2004, 43, 2012–2014 CrossRef CAS PubMed.
- Q. J. Wang, Z. Cui, Y. Xiao and Q. M. Pan, Appl. Surf. Sci., 2010, 256, 4095–4102 CrossRef PubMed.
- H. Li, M. J. Zheng, L. Ma, C. Q. Zhu and S. Lu, Mater. Res. Bull., 2013, 48, 25–29 CrossRef CAS PubMed.
- Y. Z. Cao, X. Y. Zhang, L. Tao, K. Li, Z. X. Xue, L. Feng and Y. Wei, ACS Appl. Mater. Interfaces, 2013, 5, 4438–4442 CAS.
- J. An, J.-F. Cui, Z.-Q. Zhu, W.-D. Liang, C.-J. Pei, H.-X. Sun, B.-P. Yang and A. Li, J. Appl. Polym. Sci., 2014 DOI:10.1002/APP.40759.
- B. García, J. Saiz-Poseu, R. Gras-Charles, J. Hernando, R. Alibés, F. Novio, J. Sedó, F. Busqué and D. Ruiz-Molina, ACS Appl. Mater. Interfaces, 2014 DOI:10.1021/am503733d.
- B. C. Li, L. X. Li, L. Wu, J. P. Zhang and A. Q. Wang, ChemPlusChem, 2014, 79, 850–856 CrossRef CAS.
- S. T. Wang, Y. L. Song and L. Jiang, Nanotechnology, 2007, 18, 015103 CrossRef.
- B. Wang and Z. G. Guo, Appl. Phys. Lett., 2013, 103, 063704 Search PubMed.
- C. X. Wang, T. J. Yao, J. Wu, C. Ma, Z. X. Fan, Z. Y. Wang, Y. R. Cheng, Q. Lin and B. Yang, ACS Appl. Mater. Interfaces, 2009, 11, 2613–2617 Search PubMed.
- C. R. Gao, Z. X. Sun, K. Li, Y. N. Chen, Y. Z. Cao, S. Y. Zhang and L. Feng, Energy Environ. Sci., 2013, 6, 1147–1151 CAS.
- Y. Z. Yang, H. D. Li, S. H. Cheng, G. T. Zou, C. X. Wang and Q. Lin, Chem. Commun., 2014, 50, 2900–2903 RSC.
- C. R. Crick, J. A. Gibbins and I. P. Parkin, J. Mater. Chem. A, 2013, 1, 5943–5948 CAS.
- C. Lee and S. Baik, Carbon, 2010, 48, 2192–2197 CrossRef CAS PubMed.
- C. H. Lee, N. Johnson, J. Drelich and Y. K. Yap, Carbon, 2011, 49, 669–676 CrossRef CAS PubMed.
- D. L. Tian, X. F. Zhang, X. Wang, J. Zhai and L. Jiang, Phys. Chem. Chem. Phys., 2011, 12, 14606 RSC.
- H. Li, Y. S. Li and Q. Z. Liu, Nanoscale Res. Lett., 2013, 8, 183 CrossRef PubMed.
- H. X. Sun, A. Li, X. J. Qin, Z. Q. Zhu, W. D. Liang, J. An, P. Q. La and W. Q. Deng, ChemSusChem, 2013, 6, 2377–2381 CrossRef CAS PubMed.
- D. Deng, D. P. Prendergast, J. MacFarlane, R. Bagatin, F. Stellacci and P. M. Gschwend, ACS Appl. Mater. Interfaces, 2013, 5, 774–781 CAS.
- F. J. Wang, S. Lei, M. S. Xue, J. F. Ou, C. Q. Li and W. Li, J. Phys. Chem. C, 2014, 118, 6344–6351 CAS.
- B. Cortese, D. Caschera, F. Federici, G. M. Ingo and G. Gigli, J. Mater. Chem. A, 2014, 2, 6781–6789 CAS.
- B. Cortese, D. Caschera, G. Padeletti, G. M. Ingo and G. Gigli, Surf. Innovations, 2013, 1, 140–156 CrossRef CAS.
- Y. Y. Liu, J. H. Xin and C.-H. Choi, Langmuir, 2012, 28, 17426–17434 CrossRef CAS PubMed.
- Q. F. An, W. Xu, L. F. Hao, Y. S. Fu and L. X. Huang, J. Appl. Polym. Sci., 2013, 128, 3050–3056 CrossRef CAS.
- H. Zhou, H. X. Wang, H. T. Niu, A. Gestos and T. Lin, Adv. Funct. Mater., 2013, 23, 1664–1670 CrossRef CAS.
- B. X. Leng, Z. Z. Shao, G. de With and W. H. Ming, Langmuir, 2009, 25, 2456–2460 CrossRef CAS PubMed.
- C. H. Xue, S. T. Jia, H. Z. Chen and M. Wang, Sci. Technol. Adv. Mater., 2008, 9, 035001 CrossRef.
- M. Zhang, C. Y. Wang, S. L. Wang and J. Li, Carbohydr. Polym., 2013, 97, 59–64 CrossRef CAS PubMed.
- X. Zhang, T. Geng, Y. G. Guo, Z. J. Zhang and P. Y. Zhang, Chem. Eng. J., 2013, 231, 414–419 CrossRef CAS PubMed.
- X. M. Tang, Y. Si, J. L. Ge, B. Ding, L. F. Liu, G. Zheng, W. J. Luo and J. Y. Yu, Nanoscale, 2013, 5, 11657–11664 RSC.
- Y. W. Shang, Y. Si, A. Raza, L. P. Yang, X. Mao, B. Ding and J. Y. Yu, Nanoscale, 2012, 4, 7847–7854 RSC.
- J. Y. Lin, F. Tian, Y. W. Shang, F. J. Wang, B. Ding, J. Y. Yu and Z. Guo, Nanoscale, 2013, 5, 2745–2755 RSC.
- J. P. Zhang and S. Seeger, Adv. Funct. Mater., 2011, 21, 4699–4704 CrossRef CAS.
- J. F. Wang, T. Tsuzuki, B. Tang, L. Sun, X. J. Dai, G. D. Rajmohan, J. L. Li and X. G. Wang, Aust. J. Chem., 2013, 67, 71–77 CrossRef.
- J. T. Wang, Y. Zheng and A. Q. Wang, Chem. Eng. J., 2012, 213, 1–7 CrossRef CAS PubMed.
- J. Wu, N. Wang, H. Dong, Y. Zhao and L. Jiang, ACS Appl. Mater. Interfaces, 2012, 4, 3207–3212 CAS.
- J. Zhao, C. F. Xiao and N. K. Xu, Environ. Sci. Pollut. Res., 2013, 20, 4137–4145 CrossRef CAS PubMed.
- B. Deng, R. Cai, Y. Yu, H. Q. Jiang, C. L. Wang, J. Li, L. F. Li, M. Yu, J. Y. Li, L. D. Xie, Q. Huang and C. H. Fan, Adv. Mater., 2010, 22, 5473–5477 CrossRef CAS PubMed.
- J. P. Zhang, B. C. Li, L. Wu and A. Q. Wang, Chem. Commun., 2013, 49, 11509–11511 RSC.
- L. Wu, J. P. Zhang, B. C. Li and A. Q. Wang, J. Mater. Chem. B, 2013, 1, 4756–4763 RSC.
- L. Wu, J. P. Zhang, B. C. Li and A. Q. Wang, Polym. Chem., 2014, 5, 2382–2390 RSC.
- X. Y. Zhou, Z. Z. Zhang, X. H. Xu, F. Guo, X. T. Zhu and X. H. Men, ACS Appl. Mater. Interfaces, 2013, 5, 7208–7214 CAS.
- J. Zimmermann, F. A. Reifler, G. Fortunato, L.-C. Gerhardt and S. Seeger, Adv. Funct. Mater., 2008, 18, 3662–3669 CrossRef CAS.
- T. Darmanin and F. Guittard, J. Mater. Chem. A, 2014, 2, 16319–16359 CAS.
- Y. Yoo, J. B. You, W. Choi and S. G. Im, Polym. Chem., 2013, 4, 1664–1671 RSC.
- P. Ragesh, V. A. Ganesh, S. V. Nair and A. S. Nair, J. Mater. Chem. A, 2014, 2, 14773–14797 CAS.
- D. D. Nguyen, N.-H. Tai, S.-B. Lee and W.-S. Kuo, Energy Environ. Sci., 2012, 5, 7908–7912 CAS.
- A. Li, H. X. Sun, D. Z. Tan, W. J. Fan, S. H. Wen, X. J. Qing, G. X. Li, S. Y. Li and W.-Q. Deng, Energy Environ. Sci., 2011, 4, 2062–2065 CAS.
- Q. Zhu, Q. M. Pan and F. T. Liu, J. Phys. Chem. C, 2011, 115, 17464–17470 CAS.
- L. B. Zhang, Z. H. Zhang and P. Wang, NPG Asia Mater., 2012, 4, e8 CrossRef.
- S.-J. Choi, T.-H. Kwon, H. Im, D.-I. Moon, D. J. Baek, M.-L. Seol, J. P. Duarte and Y.-K. Choi, ACS Appl. Mater. Interfaces, 2011, 3, 4552–4556 CAS.
- X. Y. Zhou, Z. Z. Zhang, X. H. Xu, X. H. Men and X. T. Zhu, Ind. Eng. Chem. Res., 2013, 52, 9411–9416 CrossRef CAS.
- G. H. Jiang, R. B. Hu, X. G. Xi, X. H. Wang and R. J. Wang, J. Mater. Res., 2013, 28, 651–656 CrossRef CAS.
- X. Y. Zhang, Z. Li, K. S. Liu and L. Jiang, Adv. Funct. Mater., 2013, 22, 2881–2886 CrossRef.
- Q. Zhu, Y. Chu, Z. K. Wang, N. Chen, L. Lin, F. T. Liu and Q. M. Pan, J. Mater. Chem. A, 2013, 1, 5386–5393 CAS.
- H. D. Liu, Z. Y. Liu, M. B. Yang and Q. He, J. Appl. Polym. Sci., 2013, 130, 3530–3536 CrossRef CAS.
- K. S. Liu and L. Jiang, ACS Nano, 2011, 5, 6786–6790 CrossRef CAS PubMed.
- P. Calcagnile, D. Fragouli, I. S. Bayer, G. C. Anyfantis, L. Martiradonna, P. D. Cozzoli, R. Cingolani and A. Athanassiou, ACS Nano, 2012, 6, 5413–5419 CrossRef CAS PubMed.
- Q. Zhu and Q. M. Pan, ACS Nano, 2014, 8, 1402–1409 CrossRef CAS PubMed.
- C. Wu, X. Y. Huang, X. F. Wu, R. Qian and P. K. Jiang, Adv. Mater., 2013, 25, 5658–5662 CrossRef CAS PubMed.
- H. X. Sun, A. Li, Z. Q. Zhu, W. D. Liang, X. H. Zhao, P. Q. La and W. Q. Deng, ChemSusChem, 2013, 6, 1057–1062 CrossRef CAS PubMed.
- E. Singh, Z. Chen, F. Houshmand, W. Ren, Y. Peles, H.-M. Cheng and N. Koratkar, Small, 2013, 9, 75–80 CrossRef CAS PubMed.
- X. Dong, J. Chen, Y. Ma, J. Wang, M. B. Chan-Park, X. Liu, L. Wang, W. Huang and P. Chen, Chem. Commun., 2012, 48, 10660–10662 RSC.
- Z. L. Fan, X. J. Qin, H. X. Sun, Z. Q. Zhu, C. J. Pei, W. D. Liang, X. M. Bao, J. An, P. Q. La, A. Li and W. Q. Deng, ChemPlusChem, 2013, 78, 1282–1287 CrossRef CAS.
- X. D. Huang, B. Sun, D. W. Su, D. Y. Zhao and G. X. Wang, J. Mater. Chem. A, 2014, 2, 7973–7979 CAS.
- Y. Yang, Z. Tong, T. Ngai and C. Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 6351–6360 CAS.
- H. C. Bi, Z. Y. Yin, X. H. Cao, X. Xie, C. L. Tan, X. Huang, B. Chen, F. T. Chen, Q. L. Yang, X. Y. Bu, X. H. Lu, L. T. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- Z. Y. Wu, C. Li, H. W. Liang, J. F. Chen and S. H. Yu, Angew. Chem., Int. Ed., 2013, 52, 2925–2929 CrossRef CAS PubMed.
- H. W. Liang, Q. F. Guan, L. F. Chen, Z. Zhu, W. J. Zhang and S. H. Yu, Angew. Chem., Int. Ed., 2012, 51, 5101–5105 CrossRef CAS PubMed.
- H. Y. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- H. C. Bi, X. Xie, K. B. Yin, Y. L. Zhou, S. Wan, L. B. He, F. Xu, F. Banhart, L. T. Sun and R. S. Ruoff, Adv. Funct. Mater., 2012, 22, 4421–4425 CrossRef CAS.
- Y. Q. He, Y. Liu, T. Wu, J. K. Ma, X. R. Wang, Q. J. Gong, W. N. Kong, F. B. Xing, Y. Liu and J. P. Gao, J. Hazard. Mater., 2013, 260, 796–805 CrossRef CAS PubMed.
- Y. Zhao, C. G. Hu, Y. Hu, H. H. Cheng, G. Q. Shi and L. T. Qu, Angew. Chem., Int. Ed., 2012, 51, 11371–11375 CrossRef CAS PubMed.
- C. B. Chen, R. Li, L. M. Xu and D. Y. Yan, RSC Adv., 2014, 4, 17393–17400 RSC.
- H.-P. Cong, X.-C. Ren, P. Wang and S.-H. Yu, ACS Nano, 2012, 6, 2693–2703 CrossRef CAS PubMed.
- X. C. Gui, J. Q. Wei, K. L. Wang, A. Y. Cao, H. W. Zhu, Y. Jia, Q. K. Shu and D. H. Wu, Adv. Mater., 2010, 22, 617–621 CrossRef CAS PubMed.
- X. H. Gui, H. B. Li, K. L. Wang, J. Q. Wei, Y. Jia, Z. Li, L. L. Fan, A. Y. Cao, H. W. Zhu and D. H. Wu, Acta Mater., 2011, 59, 4798–4804 CrossRef CAS PubMed.
- K. Zhu, Y. Y. Shang, P. Z. Sun, Z. Li, X. M. Li, J. Q. Wei, K. L. Wang, D. H. Wu, A. Y. Cao and H. W. Zhu, Front. Mater. Sci., 2013, 7, 170–176 CrossRef.
- W. Dai, S. J. Kim, W.-K. Seong, S. H. Kim, K.-R. Lee, H.-Y. Kim and M.-W. Moon, Sci. Rep., 2013, 3, 2524 Search PubMed.
- N. Chen and Q. M. Pan, ACS Nano, 2013, 7, 6875–6883 CrossRef CAS PubMed.
- Y. Chu and Q. M. Pan, ACS Appl. Mater. Interfaces, 2012, 4, 2420–2425 CAS.
- X. C. Gui, Z. P. Zeng, Z. Q. Lin, Q. M. Gan, R. Xiang, Y. Zhu, A. Y. Cao and Z. K. Tang, ACS Appl. Mater. Interfaces, 2013, 5, 5845–5850 CAS.
- T. Arbatan, X. Y. Fang and W. Shen, Chem. Eng. J., 2011, 166, 787–791 CrossRef CAS PubMed.
- B. Akhavan, K. Jarvis and P. Majewski, ACS Appl. Mater. Interfaces, 2013, 5, 8563–8571 CAS.
- S. J. Liu, Y. J. Li, Y. M. Wang, X. Liu and E. S. Yeung, J. Colloid Interface Sci., 2013, 407, 243–249 CrossRef CAS PubMed.
- X. S. Wang, J. Liu, J. M. Bonefont, D. Q. Yuan, P. K. Thallapally and S. Q. Ma, Chem. Commun., 2013, 49, 1533–1535 RSC.
- X. H. Li, Y. C. Guo, J. Zhang and L. Zhang, J. Appl. Polym. Sci., 2013, 128, 2994–2999 CrossRef CAS.
- F. Novio and D. Ruiz-Molina, RSC Adv., 2014, 4, 15293–15296 RSC.
- W. W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- A. Sarkar and S. Mahapatra, J. Mater. Chem. A, 2014, 2, 3808–3818 CAS.
- K. Dutta and A. Pramanik, Chem. Commun., 2013, 49, 6427–6429 RSC.
- L. P. Xu, X. W. Wu, J. X. Meng, J. T. Peng, Y. Q. Wen, X. J. Zhang and S. T. Wang, Chem. Commun., 2013, 49, 8752–8754 RSC.
- A. Banerjee, R. Gokhale, S. Bhatnagar, J. Jog, M. Bhardwaj, B. Lefez, B. Hannoyer and S. Ogale, J. Mater. Chem., 2012, 22, 19694–19699 RSC.
- W. X. Liang, G. Y. Wang, B. Wang, Y. B. Zhang and Z. G. Guo, Acta Chim. Sin., 2013, 71, 639–643 CrossRef CAS.
- Q. Zhu, F. Tao and Q. M. Pan, ACS Appl. Mater. Interfaces, 2010, 2, 3141–3146 CAS.
- L. Zhang, J. J. Wu, Y. X. Wang, Y. H. Long, N. Zhao and J. Xu, J. Am. Chem. Soc., 2012, 134, 9879–9881 CrossRef CAS PubMed.
- P. Tempesti, M. Bonini, F. Ridi and P. Baglioni, J. Mater. Chem. A, 2014, 2, 1980–1984 CAS.
- S. W. Zhang, M. Y. Zeng, J. X. Li, J. Li, J. Z. Xu and X. K. Wang, J. Mater. Chem. A, 2014, 2, 4391–4397 CAS.
- Z. Zhang, G. Sèbe, D. Rentsch, T. Zimmermann and P. Tingaut, Chem. Mater., 2014, 26, 2659–2668 CrossRef CAS.
- X. T. Zhu, Z. Z. Zhang, G. N. Ren, J. Yang, K. Wang, X. H. Xu, X. H. Men and X. Y. Zhou, J. Mater. Chem., 2012, 22, 20146–20148 RSC.
- B. Ge, Z. Z. Zhang, X. T. Zhu, G. N. Ren, X. H. Men and X. Y. Zhou, Colloids Surf., A, 2013, 429, 129–133 CrossRef CAS PubMed.
- G. Hayase, K. Kanamori, M. Fukuchi, H. Kaji and K. Nakanishi, Angew. Chem., Int. Ed., 2013, 52, 1986–1989 CrossRef CAS PubMed.
- S. H. Wang, M. Li and Q. H. Lu, ACS Appl. Mater. Interfaces, 2010, 2, 677–683 CAS.
- M. Zhang, C. Y. Wang, S. L. Wang, Y. L. Shi and J. Li, Appl. Surf. Sci., 2012, 261, 764–769 CrossRef CAS PubMed.
- Z. X. Xue, Z. X. Sun, Y. Z. Cao, Y. N. Chen, L. Tao, K. Li, L. Feng, Q. Fu and Y. Wei, RSC Adv., 2013, 3, 23432–23437 RSC.
- M. J. Cheng, Y. F. Gao, X. P. Guo, Z. Y. Shi, J.-F. Chen and F. Shi, Langmiur, 2011, 27, 7371–7375 CrossRef CAS PubMed.
- M. J. Cheng, G. N. Ju, C. Jiang, Y. J. Zhang and F. Shi, J. Mater. Chem. A, 2013, 1, 13411–13416 CAS.
- J. Ge, Y. D. Ye, H. B. Yao, X. Zhu, X. Wang, L. Wu, J. L. Wang, H. Ding, N. Yong, L. H. He and S. H. Yu, Angew. Chem., Int. Ed., 2014, 53, 3612–3616 CrossRef CAS PubMed.
- M. J. Liu, S. T. Wang, Z. X. Wei, Y. L. Song and L. Jiang, Adv. Mater., 2009, 21, 665–669 CrossRef CAS.
- L.-P. Xu, J. Zhao, B. Su, X. L. Liu, J. T. Peng, Y. B. Liu, H. L. Liu, G. Yang, L. Jiang, Y. Q. Wen, X. J. Zhang and S. T. Wang, Adv. Mater., 2013, 25, 606–611 CrossRef CAS PubMed.
- M. Nosonovsky and B. Bhushan, Philos. Trans. R. Soc., A, 2009, 367, 1511–1539 CrossRef CAS PubMed.
- B. Wang, Z. G. Guo and W. M. Liu, RSC Adv., 2014, 4, 14684–14690 RSC.
- A. Raza, B. Ding, G. Zainab, M. El-Newehy, S. S. Al-Deyab and J. Y. Yu, J. Mater. Chem. A, 2014, 2, 10137–10145 CAS.
- L. B. Zhang, Y. J. Zhong, D. Cha and P. Wang, Sci. Rep., 2013, 3, 2326 Search PubMed.
- Z. X. Xue, S. T. Wang, L. Lin, L. Chen, M. J. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270–4273 CrossRef CAS.
- Y. Dong, J. Li, L. Shi, X. B. Wang, Z. G. Guo and W. M. Liu, Chem. Commun., 2014, 50, 5586–5589 RSC.
- Q. Wen, J. C. Di, L. Jiang, J. H. Yu and R. R. Xu, Chem. Sci., 2013, 4, 591–595 RSC.
- F. Zhang, W. B. Zhang, Z. Shi, D. Wang, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 4192–4198 CrossRef CAS PubMed.
- N. Liu, Y. N. Chen, F. Lu, Y. Z. Cao, Z. X. Xue, K. Li, L. Feng and Y. Wei, ChemPhysChem, 2013, 14, 3489–3494 CrossRef CAS PubMed.
- K. Liao, X. Y. Ye, P. C. Chen and Z. K. Xu, J. Appl. Polym. Sci., 2013 DOI:10.1002/APP.39897.
- J. Drelich and E. Chibowski, Langmuir, 2010, 26, 18621–18623 CrossRef CAS PubMed.
- S. Song, L. Q. Jing, S. D. Li, H. G. Fu and Y. B. Luan, Mater. Lett., 2008, 62, 3503–3505 CrossRef CAS PubMed.
- T. Darmanin and F. Guittard, J. Mater. Chem., 2009, 19, 7130 RSC.
- A. Steele, I. Bayer and E. Loth, Nano Lett., 2009, 9, 501–505 CrossRef CAS PubMed.
- J. W. Zeng, B. Wang, Y. B. Zhang, H. Zhu and Z. Guo, Chem. Lett., 2014, 43, 1566–1568 CrossRef CAS.
- S. J. Hutton, J. M. Crowther and J. P. S. Badyal, Chem. Mater., 2000, 12, 2282–2286 CrossRef CAS.
- J. A. Howarter and J. P. Youghblood, Adv. Mater., 2009, 19, 3838–3843 CrossRef.
- J. A. Howarter, K. L. Genson and J. P. Youghblood, ACS Appl. Mater. Interfaces, 2011, 3, 2022–2030 CAS.
- J. Yang, Z. Z. Zhang, X. H. Xu, X. T. Zhu, X. H. Men and X. Y. Zhou, J. Mater. Chem., 2012, 22, 2834–2837 RSC.
- A. K. Kota, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef PubMed.
- B. W. Xi and J. C. Hao, Chem. Soc. Rev., 2010, 39, 769–782 RSC.
- F. Xia, Y. Zhu, L. Feng and L. Jiang, Soft Matter, 2009, 5, 275–281 RSC.
- X. J. Feng, L. Feng, M. H. Jin, J. Zhai, L. Jiang and D. B. Zhu, J. Am. Chem. Soc., 2004, 126, 62–63 CrossRef CAS PubMed.
- T. L. Sun, G. J. Wang, L. Feng, B. Q. Liu, Y. M. Ma, L. Jiang and D. B. Zhu, Angew. Chem., Int. Ed., 2004, 43, 357–360 CrossRef CAS PubMed.
- L. Xu, W. Chen, A. Mulchandani and Y. Yan, Angew. Chem., Int. Ed., 2005, 44, 6009–6012 CrossRef CAS PubMed.
- X. Yu, Z. Q. Wang, Y. G. Jiang, F. Shi and X. Zhang, Adv. Mater., 2005, 17, 1289–1293 CrossRef CAS.
- M. J. Cheng, Q. Liu, G. N. Ju, Y. J. Zhang, L. Jiang and F. Shi, Adv. Mater., 2014, 26, 306–310 CrossRef CAS PubMed.
- B. Wang and Z. G. Guo, Chem. Commun., 2013, 49, 9416–9418 RSC.
- D. L. Tian, X. F. Zhang, Y. Tian, Y. Wu, X. Wang, J. Zhai and L. Jiang, J. Mater. Chem., 2012, 22, 19652–19657 RSC.
- A. J. Khopade and F. Caruso, Langmuir, 2002, 18, 7669–7676 CrossRef CAS.
- H. S. Lim, J. T. Han, D. Kwak, M. Jin and K. Cho, J. Am. Chem. Soc., 2006, 128, 14458–14459 CrossRef CAS PubMed.
- B. L. Xue, L. C. Gao, Y. P. Hou, Z. W. Liu and L. Jiang, Adv. Mater., 2013, 25, 273–277 CrossRef CAS PubMed.
- G. Kwon, A. K. Kota, Y. X. Li, A. Sohani, J. M. Mabry and A. Tuteja, Adv. Mater., 2012, 24, 3666–3671 CrossRef CAS PubMed.
- C. McAuliffe, J. Phys. Chem., 1966, 70, 1267–1275 CrossRef CAS.
- W. B. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2071–2076 CrossRef CAS PubMed.
- Z. Shi, W. B. Zhang, F. Zhang, X. Liu, D. Wang, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2422–2427 CrossRef CAS PubMed.
- Y. C. Wang, S. Y. Tao and Y. L. An, J. Mater. Chem. A, 2013, 1, 1701–1708 CAS.
- C.-F. Wang and S.-J. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8861–8864 CAS.
- Y. Z. Zhu, F. Zhang, D. Wang, X. F. Pei, W. B. Zhang and J. Jin, J. Mater. Chem. A, 2013, 1, 5758–5765 CAS.
- W. B. Zhang, Y. Z. Zhu, X. Liu, D. Wang, J. Y. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856–860 CrossRef CAS PubMed.
- H. C. Yang, K. J. Liao, H. Huang, Q. Y. Wu, L. S. Wan and Z. K. Xu, J. Mater. Chem. A, 2014, 2, 10225–10230 CAS.
- X. Wang, X. Chen, K. Yoon, D. Fang, B. S. Hsiao and B. Chu, Environ. Sci. Technol., 2005, 39, 7684–7691 CrossRef CAS.
- H. Li, Y. Cao, J. Xin, X. Jie, T. Wang, J. Liu and Q. Yuan, J. Membr. Sci., 2006, 279, 328–335 CrossRef CAS PubMed.
- B. Chakrabarty, A. K. Ghoshal and M. K. Purkait, J. Membr. Sci., 2008, 325, 427–437 CrossRef CAS PubMed.
- M. T. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 CAS.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef CAS PubMed.
- T. Young, Philos. Trans. R. Soc. London, 1805, 95, 65–87 CrossRef.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
- A. Cassie and S. Baxter, Trans. Faraday Soc., 1994, 40, 546–561 RSC.
- Z. L. Chu and S. Seeger, Chem. Soc. Rev., 2014, 43, 2784–2798 RSC.
- M. Nosonovsky and B. Bhushan, Langmuir, 2008, 24, 1525–1533 CrossRef CAS PubMed.
- P. Forsberg, F. Nikolajeff and M. Karlsson, Soft Matter, 2011, 7, 104–109 RSC.
- E. Bormashenko, R. Pogreb, G. Whyman and M. Erlich, Langmuir, 2007, 23, 6501–6503 CrossRef CAS PubMed.
- V. Bahadur and S. V. Garimella, Langmuir, 2007, 23, 4918–4924 CrossRef CAS PubMed.
- A. Lafuma and D. Quéré, Nat. Mater., 2003, 2, 457–460 CrossRef CAS PubMed.
- A. Tuteja, W. Choi, M. Ma, J. M. Mabry, S. A. Mazzella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618–1622 CrossRef CAS PubMed.
- A. Marmur, Langmuir, 2008, 24, 7573–7579 CrossRef CAS PubMed.
- G. Whyman and E. Bormashenko, Langmuir, 2011, 27, 8171–8176 CrossRef CAS PubMed.
- Y. C. Jung and B. Bhushan, Langmuir, 2009, 25, 14165–14173 CrossRef CAS PubMed.
Footnote |
| † Ben Wang and Weixin Liang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |