Microemulsion systems for catalytic reactions and processes
M.
Schwarze
*,
T.
Pogrzeba
,
I.
Volovych
and
R.
Schomäcker
Technische Universität Berlin, Department of Chemistry, Straße des 17. Juni 124, 10623 Berlin, Germany. E-mail: ms@chem.tu-berlin.de
First published on 10th October 2014
Abstract
Among the available green solvents, microemulsion systems show superior properties for catalytic reactions and processes which can be easily tuned by the selection of an appropriate surfactant. In this review, we show examples for bio-, homogeneous and heterogeneous catalysis and discuss the parameters which are important for successful implementation of these systems. Based on the kind of catalysis, they allow for the reuse of dissolved catalysts or even for a better solubilisation of hydrophobic reactants. However, their phase behaviour which strongly depends on composition and temperature as well as surfactant/catalyst interaction has to be studied in detail.
1. Introduction
The selection of a reaction medium to develop an industrial process is often done by a compromise between several selection criteria. Since nowadays an optimal yield on its own is not a satisfying objective, the selection criteria imply an integrated consideration of the process, including aspects of resource and energy efficiency as well as sustainability. The discussion of these aspects led to the development of selection criteria for the process conditions of green chemistry, which are avoidance of waste from side products and/or solvents, consequent execution of catalytic reactions instead of stoichiometric ones, and application of solvents with low hazard potentials and low energy costs in terms of supply and recycling. The evaluation of environmental indicators for commonly applied solvents recommends just a few of them for a green chemical process. Besides water, only easy producible hydrocarbons like heptane and cyclohexane as well as methanol, ethanol and acetic acid esters possess good scores.1 To overcome the disadvantage of a limited repertoire of solvents for process development and optimisation, several additives are available that permit to tune the properties of reaction media (and make them switchable at best) without affecting the process' sustainability. A great potential for process intensification can be unfolded, when these tuneable solvents are used in a subtle way. Well-known examples for tuneable media are supercritical media based on CO2 (ref. 2 and 3) or thermomorphic solvent mixtures.4 For reactions that can't be performed in a single solvent due to lack of solubility of the reactants, biphasic media can be applied as long as a low mutual solubility in all involved solvents is given.5 Two-phase systems are often evaluated better than homogeneous reaction media in an integrated consideration of reaction, product isolation, and catalyst recycling based on their minor energy costs. Ideally, a reaction medium should be homogeneous during the reaction to permit an optimal reaction process avoiding mass transport limitations and afterwards switchable to a two-phase system for the product isolation step.For water-soluble catalysts, aqueous solutions can be applied as tuneable reaction media for chemical processes by the use of surfactants. The addition of surfactant to an aqueous reaction mixture leads to the formulation of micellar solutions and microemulsions. As a result, solubility and mass transfer of the non-polar reactants in the polar water phase is increased.6 Furthermore, the surfactant reduces the interfacial tension and increases the interfacial area between both phases.7,8 In the case of microemulsion systems, recycling of the hydrophilic catalyst after reaction is easily done by temperature-induced phase separation.9
In this contribution we will give an overview about microemulsion systems as reaction media for catalytic reactions with a division into bio-, homogenous and heterogeneous catalysis based on the kind of catalyst added to the microemulsion system. We will summarize the aspects which are important to apply microemulsion systems for a whole catalytic process rather than for a single reaction only. In contrast to conventional catalytic processes in organic solvents, the use of microemulsion systems requires a higher degree of knowledge about the thermodynamics of these systems.
2. Chemical and physical characteristics of microemulsions
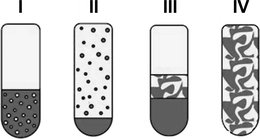
By adjusting a surfactant modified two-phase system for both a homogeneously catalyzed reaction and a subsequent product separation and catalyst recycling, it is important to choose a surfactant in respect of its phase behaviour. Furthermore, the distribution of reactants and catalyst between the aqueous and the organic phases based on the selected surfactant is important. The phase separation dynamics as well as reaction kinetics show a strong dependency on surfactant concentration and structure of the involved phases. By the addition of a nonionic surfactant to a mixture of water and oil, usually four different states can be observed that are named Winsor systems (I–IV). Common non-ionic surfactants are of the type CiEj. In CiEj, i is the number of hydrocarbon groups of the surfactant's hydrophobic part, and j is the number of ethoxylate groups of its hydrophilic part.In the case of a Winsor I system an oil-in-water (o/w) microemulsion exists in thermodynamic equilibrium with an organic excess phase, whereas for a Winsor II system a water-in-oil (w/o) microemulsion exists in equilibrium with a water excess phase. Both systems are macroscopically biphasic, which means that the added surfactant is solubilising only a part of the primary two-phase system's water or oil phase into the other phase. The Winsor III system is macroscopically triphasic with a water excess phase, a surfactant-rich middle-phase, and an oil excess phase, all in thermodynamic equilibrium. At last, a Winsor IV system occurs as a macroscopic clear one-phase solution called microemulsion that can be classified based on the oil content into (w/o) microemulsion, (o/w) microemulsion, and a bicontinuous structure where water and oil are dispersed into each other. The different Winsor systems are schematically illustrated in Fig. 1.
The setting of a state at a given temperature and surfactant concentration basically depends on the HLB value (HLB: hydrophilic–lipophilic balance) of surfactant and the water/oil ratio. The HLB value describes the mass ratio of hydrophilic to hydrophobic molecule fraction that regulates many characteristics of surfactants. However, it is not linearly representable for the surfactant's phase behaviour. By modification of the molecular structure (e.g. increasing the amount of ethoxylate groups), characteristics of nonionic surfactants can be modified in small steps and adapted to different applications, which has already been described in the literature.10
At a constant temperature and pressure the phase diagram of a ternary mixture is represented in a Gibbs phase triangle. Important parameters to characterize the mixture are α, γ, δ, w0, w1, and r1 [eqn (1)], where moil is the mass of oil, mwater is the mass of water, msurf is the mass of surfactant, mco-surf is the mass of co-surfactant, w0 is the water content calculated from the moles of water (nwater) and the moles of surfactant (nsurf) and w1 is the water content corrected by the cmc of the surfactant, and r1 is the size of the reverse micelles which is a function of w1 with f = (1,5…2,5) × 10−10 m.11
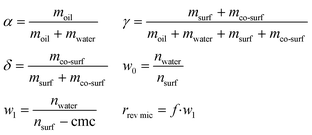 | (1) |
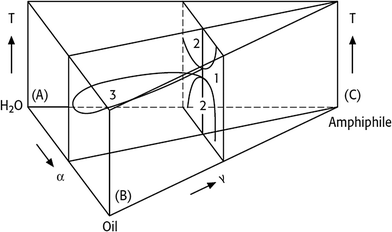
Consequentially, the temperature-dependent phase behaviour of ternary water–oil-nonionic surfactant systems is represented in a phase prism by stacking the isothermal Gibbs triangles on top of each other (Fig. 2). Each point in such a phase prism is clearly defined by the temperature T and the two parameters α and γ. The two characteristic vertical sections in a phase prism for a nonionic surfactant system are schematically shown in Fig. 3.
 | ||
| Fig. 2 Gibbs phase prism of a ternary mixture of water, oil, and nonionic surfactant showing the temperature-dependent phase behaviour. Modified from ref. 12. | ||
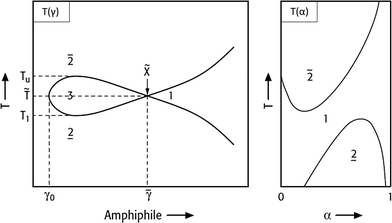 | ||
Fig. 3
T(γ)- and T(α)-section through the Gibbs phase prism. Left: Fish-type phase diagram for varying surfactant concentrations at a constant water/oil ratio (α). Right: Phase diagram for varying α while holding γ > ![[small gamma, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c4.gif) constant. Modified from ref. 12. constant. Modified from ref. 12. | ||
In Fig. 3 the left-hand diagram shows a T(γ)-section at a constant α value, where the phase boundaries resemble the shape of a fish. The sequence of phases as a function of temperature, the so-called phase inversion, is a result of a gradual change in the surfactant's solubility. At low temperatures the nonionic surfactant is mainly soluble in water, whereas it becomes better soluble in oil at higher temperatures. Thus, increasing the temperature switches a nonionic surfactant from hydrophilic into hydrophobic.
Starting with the binary water–oil system, small amounts of surfactant molecules are monomerically dissolved in both phases and preferentially adsorb at the macroscopic water/oil interface. At a mass fraction γ0 the two phases and the macroscopic interface are saturated with the surfactant and the amphiphilic molecules are forced to form microscopic water/oil interfaces. These microemulsions consist of oil-bearing micelles in a continuous water phase at low temperatures (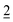 , o/w microemulsion) and water-bearing inverse micelles in a continuous oil phase at higher temperatures (
, o/w microemulsion) and water-bearing inverse micelles in a continuous oil phase at higher temperatures (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) , w/o microemulsion). Both microemulsions coexist with an excess phase, forming Winsor systems I and II as explained earlier. A three-phase region exists at intermediate temperatures (denoted as 3 or Winsor III). Here the nonionic surfactant is almost equally soluble in both solvents and forms a surfactant rich microemulsion phase in the middle of two excess phases. At the intersection of the three-phase and the one-phase system (1, Winsor IV) we find the so-called
, w/o microemulsion). Both microemulsions coexist with an excess phase, forming Winsor systems I and II as explained earlier. A three-phase region exists at intermediate temperatures (denoted as 3 or Winsor III). Here the nonionic surfactant is almost equally soluble in both solvents and forms a surfactant rich microemulsion phase in the middle of two excess phases. At the intersection of the three-phase and the one-phase system (1, Winsor IV) we find the so-called 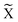 -point that marks the minimum mass fraction of surfactant needed to completely solubilise water and oil (denoted as
-point that marks the minimum mass fraction of surfactant needed to completely solubilise water and oil (denoted as ![[small gamma, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c4.gif) ), i.e. the efficiency of the surfactant, as well as the corresponding temperature
), i.e. the efficiency of the surfactant, as well as the corresponding temperature ![[T with combining tilde]](https://www.rsc.org/images/entities/i_char_0054_0303.gif) , which is a measure for the phase inversion temperature (PIT).12,13
, which is a measure for the phase inversion temperature (PIT).12,13
The right-hand diagram in Fig. 3 shows the vertical T(α)-section of the Gibbs phase prisms. It is obtained by varying α at a constant surfactant mass fraction γ > ![[small gamma, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c4.gif) . In this section a homogeneous single-phase channel can be observed that ascends from the water-rich to the oil-rich side with increasing temperature and α.12 The phase diagram is an important tool for process development, because the reaction and separation steps are based on it. However, phase behaviour strongly depends on the composition of the reaction mixture. The direction in which the phase behaviour of nonionic surfactant systems progresses as a function of increasing salinity, oil hydrophobicity and variations of HLB of the surfactant is exemplarily shown in Fig. 4.
. In this section a homogeneous single-phase channel can be observed that ascends from the water-rich to the oil-rich side with increasing temperature and α.12 The phase diagram is an important tool for process development, because the reaction and separation steps are based on it. However, phase behaviour strongly depends on the composition of the reaction mixture. The direction in which the phase behaviour of nonionic surfactant systems progresses as a function of increasing salinity, oil hydrophobicity and variations of HLB of the surfactant is exemplarily shown in Fig. 4.
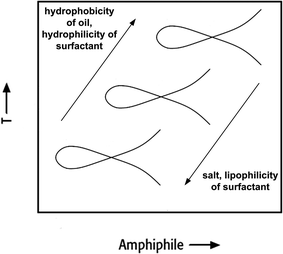 | ||
| Fig. 4 Qualitative effect of different additives on the phase behaviour of nonionic surfactant systems. | ||
For example, choosing an aliphatic reactant with an increased chain length shifts the PIT to higher temperatures, whereas a higher salinity decreases the PIT. To avoid an additional energy input after reaction in order to obtain the state where separation occurs, the composition and temperature should be selected carefully to obtain the desired state for separation at the end of reaction directly.
In general, each Winsor system can be applied as a reaction system for homogeneous catalysis. Compared to a binary system the reaction in a microemulsion system is carried out with considerably higher reaction rates, because the area for mass transfer is larger. It is also possible to exchange the homogeneous catalyst by an enzyme, e.g. a lipase, which is most likely dissolved in an aqueous phase and which converts hydrophobic reactants into the desired products. The large interfacial area between aqueous and oil domains provided by microemulsion systems allow for biocatalytic reactions with the possibility of enzyme recycling afterwards. If heterogeneous catalysts are applied in microemulsion systems, the situation becomes more complex, e.g. due to adsorption of the surfactant on the catalyst surface. On the one hand, the catalyst is easy to recycle after reaction and the microemulsion systems allow for a better solubilisation of the hydrophobic reactants only. On the other hand, depending on the location of the active species which can be inside the catalyst particle, e.g. as in sol–gel catalysts, or on the surface, the contact between the catalyst and the reactants could be hindered in the presence of surfactants. In the following section, we will give an overview of microemulsion systems for catalytic reactions/processes.
3. Catalyzed reactions in microemulsions
In this section, we will give an overview about reactions in microemulsion systems. We have subdivided this section based on the state of the selected catalyst to be an enzyme, a catalyst complex or a supported catalyst.3.1. Biocatalysis
Although enzymes allow for synthesis under mild reaction conditions, the application of biocatalysis on an industrial scale is still a challenge. The major problem is that enzymes prefer a well defined aqueous environment, e.g. wrong pH, high temperatures or a very hydrophobic ambience can lead to denaturation of the enzyme. However, most of the important organic transformations involve hydrophobic substrates. One approach to have contact between the enzyme and the substrate are conventional two-phase systems, with the hydrophobic reactant being dissolved in the oil and the enzyme in the aqueous phase. The reaction can take place at the interphase between the two phases. The organic solvent has to be selected carefully, because it can inhibit enzyme activity.14 Another approach is a w/o-microemulsion, where the enzyme is solubilised in the water-pool of the reverse micelles surrounded by the continuous oil phase with the substrate and, in contrast to a conventional two-phase system the micelles provide a much larger interfacial area for contact with the hydrophobic reactants. Many examples for enzymatic reactions, mostly carried out in w/o-microemulsions, have been published during the last years and a few of them are listed in Table 1.| Entry | Catalysis | ME | Discussed parameters | Ref. |
|---|---|---|---|---|
| a Candida cylindracea lipase and Humicola lanuginosa lipase. b Cutinase. c Wheat germ lipase. d Almonds β-glucosidase. e Mycobacterium M156. f Pseudomonas sp. esterase PF1-K. g Yeast alcohol dehydrogenase, horse liver alcohol dehydrogenase and Candida parapsilosis carbonyl reductase. | ||||
| 1 | Esterification of butan-1,3-diol + oleic acida | w/o | w 0 | 15 |
| 2 | Esterfirication of lauric acid + 1-pentanolb | w/o | w 0, [Enzyme], stability | 16 |
| 3 | Hydrolysis of Trioleinc | w/o | w 0, [Enzmye], stability, T | 17 |
| 4 | Synthesis of n-hexyl-β-d-glucopyranosided | bc | T, [Enzyme], stability | 18 |
| 5 | Epoxidation of allyl phenyl ethere | w/o | [surf], w0 | 19 |
| 6 | Hydrolysis of (R,S)-ketoprofen ethyl esterf | bc | [Enzyme], T | 20 |
| 7 | Reduction of 2-butanoneg | w/o | [Surf], w1 | 11 |
As already mentioned, the enzyme prefers a well defined environment. This is also true in the case of w/o-microemulsions as reaction media. It was found that activity and stability strongly depend on the water content w0. In most of the investigated reactions, a bell-shaped curve with highest activity at w0 values of about 10 is obtained. The explanation for the bell-shaped curve is not easy and usually the following effects play a role: hydration of the enzyme, diffusion of the reactant, and substrate concentration. Interestingly, but not in all cases, the highest enzyme stability is obtained for the same w0. At very high w0 values, fast enzyme deactivation is exhibited in stability tests, which is attributed to a denaturation effect of the used surfactant.16 Further parameters that influence the enzyme activity are the pH value and the temperature. Usually, the enzyme shows highest activity only for one pH value as shown by Park et al.21 and Fei et al.17 Both researchers also investigated enzyme activity as a function of temperature. For temperatures above 40 °C, enzyme denaturation is observed and activity decreases with increasing temperature. However, Park et al.21 showed that the enzyme is less temperature sensitive in w/o-microemulsions, because the surfactant suppresses the interaction between the enzyme and the organic solvent. Even if the reactions are carried out in w/o-microemulsions, different groups have shown that the reaction can be described by applying the Michaelis–Menten kinetics.
Although this article focuses on conventional microemulsion systems consisting of oil/water/surfactant/co-surfactant, we have to mention that in the last years also microemulsions based on ion liquids22–24 and supercritical carbon–dioxide (scCO2)25 have been investigated as media for biocatalysis. In these new microemulsion systems, the conventional oil is replaced by the ionic liquid or CO2.
To allow for continuous enzymatic processes based on w/o-microemulsions, our group studied the ultrafiltration of these systems with hydrophobic membranes made of polyamide (Berghof BM100/200). We obtained high enzyme recovery at low pressures, which makes ultrafiltration an ideal tool to recover enzymes from w/o-microemulsions.26 A combination of enzyme catalyzed reactions in w/o-microemulsions with an ultrafiltration process, as shown in Fig. 5, is a real alternative to conventional biocatalytic processes.
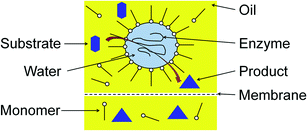 | ||
| Fig. 5 Enzymatic reaction in w/o-microemulsion with subsequent product/enzyme-separation via membrane filtration. | ||
3.2. Reactions with homogenous catalyst complexes
One major advantage of microemulsion systems in comparison to organic solvents is the possibility to recycle homogenous catalysts. If microemulsion systems are selected as solvents for homogeneous reactions, in general water-soluble catalyst complexes are added. Although the catalyst should be placed in the water phase of the individual states, we found that in many cases the catalyst follows the surfactant into the corresponding microemulsion phase. However, catalyst recycling can easily be done by phase separation after reaction – a general process concept is shown in Fig. 6.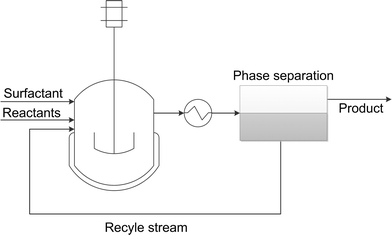 | ||
| Fig. 6 General process concept based on microemulsion systems. Recycle stream contains separated aqueous catalyst solution with surfactant. | ||
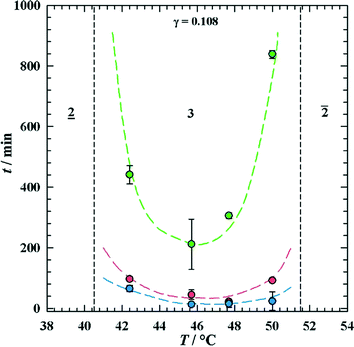
To separate the phases of a microemulsion system properly, the appropriate phase region of the phase diagram has to be chosen first. The composition of the reaction mixture and the kinetics of phase separation within this region are essential questions for process development. The kinetics of phase separation depends strongly on the temperature of the system and on the temperature distance to the phase boundaries.6 Near to the phase boundaries of the Winsor systems the phase separation often takes a long time due to low interfacial tension and high stability of the emulsified system. Here, the composition of the phases is quite similar and separation of the components will often be incomplete. In Fig. 7 the separation time for a three-phase H2O-n-decane-Lutensol ON30 microemulsion system (Lutensol ON 30 = RO(CH2CH2O)3H with R being the alkyl chain coming from a C10 alcohol) is illustrated for α = 0.5 as a function of temperature. It is shown that in the middle of the three-phase temperature range the separation is much faster than near to the boundaries. Furthermore, the separation of the excess phase is faster than the separation of the middle phase. Such a phase separation behavior is not limited to α = 0.5 and can be found for a broad range of α values as shown in Fig. 8. For efficient processes, time for separation should be as low as possible. From an engineering point of view the three-phase system has beneficial material properties because the viscosity is lower than in the two-phase regions and less energy is needed for efficient mixing as shown in Fig. 9. For further information about the study of microemulsions we refer to Kahlweit et al.27 However, the fact that reaction and separation are strongly dependent on temperature and composition makes the investigation of the phase behavior for each system mandatory.
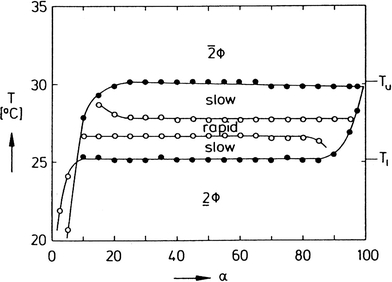 | ||
| Fig. 8 Dynamics of phase separation in the system H2O-n-tetradecane-C12E4. Shown is a section through the three-phase body at γ = 2 wt% with regions of slow and rapid phase separation. In the region between the empty points, a stirred mixture separates within less than 30 min after stirring.27 Reprinted with permission from Elsevier. | ||
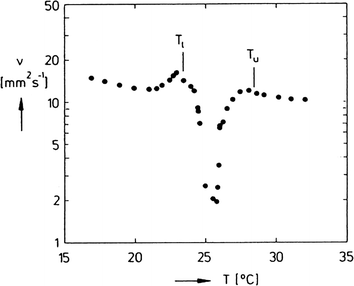 | ||
| Fig. 9 Kinematic viscosity v versus temperature T, measured in the three-phase body of the system H2O-n-tetradecane-C12E4 at α = 0.5 and γ = 2 wt%.27 Reprinted with permission from Elsevier. | ||
Which state of a microemulsion system is used for the reaction or a combined reaction and separation process can differ, depending on the applied reactants. Although microemulsion systems offer many opportunities for process management, in the published literature they are used as reaction media rather than for process development due to their complexity. Water-in-oil microemulsions are applicable reaction media for many different stoichiometric and catalytic reactions. Although the focus of this contribution lies on catalytic reactions in microemulsion systems, we would like to give some examples for stoichiometric reactions since their application is manifold. Sun et al.28 investigated the ring-opening polymerisation of octamethylcyclotetrasiloxane in microemulsions. Wielpütz et al.29 showed that the epoxidation of vitamin K is considerably enhanced when the reaction is performed in a bicontinuous microemulsion. Another example is the Diels–Alder reaction of N-ethylmaleimide and cyclopentadiene in w/o microemulsions.30
Compared to conventional two-phase systems the main advantage of w/o microemulsions is the highly enlarged interphase while retaining good solubility of hydrophobic reactants. Similar to the enzymes shown in section 3.1, hydrophilic catalyst complexes can also be embedded in the aqueous core of inverse micelles and thus be dispersed into the hydrophobic reactants. An example is the hydrogenation of dimethyl itaconate with Rh/TPPTS in a cyclohexane/TX-100/1-petanol/water-microemulsion (TX-100 = t-octylphenoxypolyethoxyethanol)31 (Table 2, entry 1). By changing the temperature, the microemulsion can be destabilized and either a W1 or W2 two-phase system is formed which enables product separation and the recycling of catalyst in its active form. If thermal stress leads to catalyst deactivation, a subsequent ultrafiltration process is possible for catalyst recycling in which the catalyst loaded micelles are rejected by the membrane. However, this separation method is mostly limited to normal micelles in oil-in-water microemulsions and time for separation is considerably longer than for optimized phase separation. In principal, o/w microemulsions are the mirror image of w/o microemulsions. Hydrophobic reactants are embedded in the nonpolar cores of micelles, which coexist with a continuous catalyst containing aqueous phase. A significant disadvantage of o/w microemulsions though is their low solubilisation capacity of hydrophobic substances. Examples for catalysis in o/w microemulsions are the hydroformylation of long-chained olefins by Rh/TPPTS with the addition of cationic gemini-surfactants,32 the epoxidation of 4-methylpentene with H2O2 by application of a palladium/chiraphos catalyst complex,33 and the catalytic hydroxylation of benzene on a [Fe(DDS)3] catalyst with H2O2.34
| Entry | Catalysis | ME | Catalyst complex | Ref. |
|---|---|---|---|---|
| a TPPTS = tri-(natrium-meta-sulfonatophenyl)-phosphan, SX = SulfoXantPhos, chiraphos = bis(diphenylphosphino)butan, Fe(DDS)3 = ferric sodium dodecane sulfonate. | ||||
| 1 | Hydrogenation of dimethyl itaconate and sunflower oil | w/o, WIII | Rh/TPPTS | 31 and 35 |
| 2 | Hydroformylation of long-chained olefins | WIII, o/w | Rh/SX, Rh/TPPTS | 32 and 36 |
| 3 | Suzuki coupling | WIII | Pd/TPPTS | 37 |
| 4 | Epoxidation of 4-methylpentene | o/w | Pt/chiraphos | 33 |
| 5 | Hydroxylation of benzene | o/w | Fe(DDS)3 | 34 |
A surfactant modified liquid/liquid two-phase system which forms a microemulsion of the Winsor III type enables interesting possibilities for reaction management. The three phases in equilibrium can be applied not only to separate the catalyst from the product, but also to discharge hydrophilic side-products as well, e.g. salts which are formed during reactions involving a base. The premise for a successful separation is a high affinity of the catalyst to the surfactant rich middle phase.
An example for catalysis in a triphasic microemulsion system is the Suzuki coupling of 2-bromobenzonitrile and 4-methylbenzeneboronic acid with Pd/TPPTS investigated by Nowothnick et al.37 The catalyst complex was used in a heptane/Novel 8/water-microemulsion and could be recycled four times. The salts which usually remain in the catalyst phase of a biphasic system and thus poison the catalyst were systematically separated by discharging the aqueous excess phase. Hamerla et al.36 showed that for the hydroformylation of 1-dodecene by Rh/SX higher reaction rates can be obtained when the reaction is performed in a triphasic microemulsion instead of W1 or W2 systems. Milano et al.35 studied the hydrogenation of sunflower oil and recycled the Rh/TPPTS catalyst up to three times by extracting the oil phase of a three-phase microemulsion system.
The difficulty of applying triphasic microemulsion systems is their smaller area of existence in the phase diagram compared to the other systems. Hence, this area is shifted much easier by changes in composition of the mixture, e.g. increasing product concentration. Thus, a detailed knowledge of the phase behaviour of a microemulsion system is necessary to achieve a stable reaction and separation process.
3.3. Reactions with heterogeneous micro- and mesoporous silica supported catalysts
In comparison to the reactions catalyzed by homogeneous metal complexes or enzymes, the application of multiphase microemulsion systems, e.g. Winsor III, to immobilize the catalyst, is not necessary if heterogeneous catalysts are used. The reactions can be processed in aqueous one phase Winsor IV microemulsions with >90% water, surfactant, propanol as the cosurfactant and the reactants as oil.38 The separation process of the catalyst and product after the reaction, which is schematically shown in Fig. 10, is very simple: (a) filtration and reuse of solid catalyst, (b) product separation through extraction with heptane and (c) separation and reuse of surfactant from water, e.g. by micellar enhanced ultra filtration (MEUF) or adsorption on silica or granite sand.39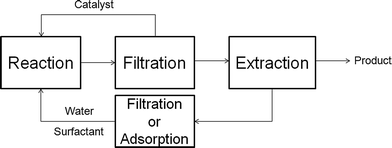 | ||
| Fig. 10 Workup procedure for heterogeneously catalyzed reactions in microemulsions. For more details see I. Volovych et al.39 | ||
The application of heterogeneous catalysts is very advantageous because of easy separation and high stability of the immobilized particles, whereas entrapment of active material during the synthesis and mass transport limitations during the reaction can lead to decreased activities.
There are several methods to immobilize catalysts, e.g. sol–gel process40 with/without surfactant as template41 or impregnation method.42 The distinction has to be drawn between mesoporous (d > 2 nm) and microporous (d < 2 nm) catalysts. Palladium, manganese and palladium–manganese catalysts were immobilized on hydrophilic and hydrophobically modified silica by a sol–gel method; palladium acetate was immobilized on commercially available mesoporous SBA-15 and Silica by impregnation method, and on mesoporous MCM-41 prepared by sol–gel method with surfactant as template. Dependent on catalyst precursor, solvent and surfactant, support material, additives, pore size, as well as immobilisation method, different activities and stabilities of the catalysts were obtained. The results of selected supported catalysts applied for the Heck reaction of styrene and bromo-/iodobenzene to trans-stilbene in microemulsions are summarized in Table 3. As can be seen from Table 3, the best results for the trans-stilbene synthesis from bromobenzene and styrene (Table 3, entry 2–3) or iodobenzene and styrene (Table 3, entry 9) were obtained with Pd(OAc)2 immobilized on hydrophobically modified silica PhSiO2 or OcSiO2 with tetramethyl orthosilicate and phenyltriethoxysilane or octyltrimethoxysilane as gel-building agent. The reaction rates are higher when using the hydrophobic support materials than the hydrophilic silica (Table 3, entry 1) because of hydrophobic interactions between the support surface and the substrates. Catalysts immobilized on hydrophilic surfaces are preferred for the reactions with hydrophilic substrates, e.g. enantioselective hydrogenation of itaconates with sol–gel immobilized Rh/BPPM@SiO2.43
| Entry | Catalyst precursor | Support | Surfactantc | Time [min] | Yield (%) |
|---|---|---|---|---|---|
| a Microemulsion: surfactant/propanol/H2O/reactants (styrene, bromobenzene). b Iodobenzene, entries 10–14 are unpublished results. c CTAB = cetyltrimethylammonium bromide, DTAB = dodecyltrimetylammonium bromide, TTAB = tetradecyltrimethylammonium bromide, SDS = sodiumdodecyl sulphate, TX-100 = t-octylphenoxypolyethoxyethanol. | |||||
| 1 | Pd(OAc)2 | SiO2 | CTAB | 394 | 60 |
| 2 | Pd(OAc)2 | OcSiO2 | CTAB | 300 | 81 |
| 3 | Pd(OAc)2 | PhSiO2 | CTAB | 396 | 96 |
| 4 | PdBr2 | PhSiO2 | CTAB | 386 | 66 |
| 5 | Pd(OAc)2 | PhSiO2 | TTAB | 405 | 54 |
| 6 | Pd(OAc)2 | PhSiO2 | DTAB | 371 | 58 |
| 7 | Pd(OAc)2 | PhSiO2 | SDS | 380 | 96 |
| 8 | Pd(OAc)2 | PhSiO2 | TX-100 | 388 | 92 |
| 9b | Pd(OAc)2 | PhSiO2 | CTAB | 300 | 100 |
| 10b | Mn(Acac)3 | PhSiO2 | CTAB | 300 | 40 |
| 11b | PdBr2-Mn(Acac)3 | PhSiO2 | CTAB | 60 | 100 |
| 12b | Pd(OAc)2 | MCM-41 | CTAB | 300 | 70 |
| 13b | Pd(OAc)2 | SBA-15 | CTAB | 350 | 90 |
| 14b | Pd(OAc)2 | Silica | CTAB | 350 | 50 |
Also the choice of catalyst precursor is very crucial for the reaction process. Usually this effect can be derived from analogue experiments with homogeneous catalysts as well as from literature. Of course the reaction rate is very low with Mn(Acac)3 as precursor (Table 3, entry 10). However, a synergistic effect between palladium and manganese species was observed (Table 3, entry 11) and the rate of reaction increases up to 5 times in comparison to Pd(OAc)2@PhSiO2 (Table 3, entry 9). Similar effects are already reported in the literature for arene hydrogenation with sol–gel immobilized [Rh(cod)Cl]2–Pd(OAc)2 catalyst44 and oxidative carbonylation of amine with polymer supported PdCl2–MnCl2 catalyst.45 However, the palladium–manganese catalyst is not very stable and leeching is observed.
Heterogeneously catalyzed Heck reaction takes place in the meso- and micropores of the immobilized catalysts where the metallic palladium particles are located. This is the reason why not only the activity of metal precursor influences the rate of reaction, but also the structure and stability of the immobilized catalysts, e.g. pore size. The application of mesoporous catalysts is attendant with less diffusion limited process and larger diffusion coefficients, but also with deactivation of metal precursor during the immobilisation process (Table 3, entry 12–14).
The application of commercially available heterogeneous catalysts, e.g. Pd@C or Pd@Al2O3 (ref. 39) is also possible and no extra synthesis process is necessary, but their reactivity is lower compared to the hydrophobically modified sol–gel catalysts. In addition, the strong difference in the behaviour of the commercial catalysts with certain substrates is unexpected and very difficult to predict.
Beside different parameters the solvent selection is also important for the reactions with micro- and mesoporous sol–gel immobilized Pd catalysts. As explained before, for the solubilization of the hydrophobic reactants in aqueous environment, surfactants have to be added to increase the solubility of the reactants. Heck coupling of bromobenzene and styrene was carried out in aqueous microemulsions with different anionic, nonionic and cationic surfactants, which can be characterized by their structures, critical micellar concentrations (cmc) and the average diameter of the micelles (dmicelle) or HLB value. Because of the small pores of microporous catalysts (dpore < 2 nm), the size of micellar aggregates should be also very small to avoid transport problems inside the pores.38 The conversion increased with increasing C-chain length, decreasing HLB value and decreasing cmc, if homologue series of cationic surfactants CTAB, TTAB and DTAB were used: C16 ≫ C14 ≈ C12. The most hydrophobic surfactant CTAB is preferred because of the more hydrophobic micelle cores (dmicelle) and largest storage capacity for the substrates (dmicelle = 5.39 nm > 3.840 nm > 3.34 nm (ref. 46)). Prediction cannot be done for the behaviour of the other surfactants in this coupling reaction because of their different structures; there is also no dependency between the type of the surfactant and the reaction rate.
The catalyst stability and reactivity depends not only on the metal precursor and support as it was shown before, but also on the additives and type of the reaction (Table 4).
| Entry | Catalyst | Reaction | Reference |
|---|---|---|---|
| a Microemulsion: surfactant/propanol/H2O/reactants. b Microemulsion:TX-100/PEG/butanol/H2O/heptane/reactants. c Microemulsion: TX-100/PG/butanol/H2O/heptane/reactants. d o/w-microemulsion: Brij 96 V/PG/H2O/dodecane, ethyl alcohol/chlorobenzene. | |||
| 1a | Pd(OAc)2@PhSiO2 | Heck, Suzuki or Stille coupling | 38, 39 and 47 |
| 2bc | Pd@C | Suzuki and Sonogoshira coupling | 48 and 49 |
| 3d | Pd@C | Homocoupling | 50 |
| 4a | RhCl3-Aliquat336@ OcSiO2 or PhSiO2 | Disproportionation | 51 |
| 5a | [Rh(cod)Cl]2-PR3 @EtSiO2 | Hydroformylation | 52–54 |
| 6a | Pd(OAc)2@PhSiO2 | Hydrogenation | 55 |
| 7a | Pd(OAc)2@PhSiO2 | Epoxidation | 55 |
| 8a | Pd(OAc)2@PhSiO2 | Decarbonylation | 56 |
The application of the sol–gel immobilized palladium and manganese catalysts in the epoxidation of styrene or trans-stilbene was much more difficult than in the Heck coupling reaction (Table 4, entry 7), because of the catalyst leaching into the reaction mixture caused by the addition of strong oxidizing agents, e.g. hydrogen peroxide.55 On the other hand reactions with Pd(OAc)2@PhSiO2, e.g. decarbonylation (Table 4, entry 8), coupling reactions (Table 4, entry 1) or hydrogenation (Table 4, entry 6) were carried out without catalyst destroying or poisoning additives and the catalyst could be recycled several times without leaching. In the case of regioselective hydroformylation of styrene (Table 4, entry 5), the catalyst precursor was air sensitive and the synthesis procedure and reaction was more complex and should be carried out under inert atmosphere to avoid catalyst deactivation. Also coupling reactions (Table 4, entry 2–3) were successfully carried out with commercial Pd@C catalysts in microemulsions. Due to severe surfactant/catalyst interaction, which is mainly based on surfactant adsorption on the catalyst surface and that can influence the catalytic activity to a large extent, surfactant screening is mandatory to find an appropriate candidate allowing for high yields in catalytic reactions. Furthermore, as mentioned above, porous catalyst supports have some drawbacks, e.g. mass-transport-limitations because of pore diffusion, but their stability and recyclability have a big impact on the overall efficiency ηov and under optimized conditions ηov can increase to values higher than 1 as shown by Volovych et al.39
All examples above have shown that microemulsion systems can be used for catalytic reactions. The selection of the microemulsion system depends on the type of reaction and the distribution of the reactants between organic and aqueous phases. This will also have influence on further purification steps after obtaining the desired product in the reaction. To these purification steps belongs also the removal of the used surfactant from the product. In case of hydrophobic products that will accumulate in the organic phase the three phase microemulsion system is the best choice for product separation afterwards. The surfactant concentration in the organic excess phase can't exceed the cmc of the surfactant, which is low for nonionic surfactants. However, in dependency on the surfactant type there are different separation techniques which are commonly used in industry for waste water treatment, such as micellar enhanced ultrafiltration (MEUF) or cloud point extraction. Most common is adsorption through, e.g. silica gel, clay, MCM-41 or granite sand.57 An appropriate surfactant removal step has to be selected based on the reaction system.
4. Summary and conclusions
Microemulsion systems offer different regions for chemical reactions which can be single-phase, biphasic or tri-phase in nature. With a profound knowledge of the thermodynamics of microemulsion systems and how the phase behaviour changes with composition and temperature, microemulsion systems can be used for process intensification. As catalyst recycling is the major drawback in homogenous catalysis, homogeneous catalyst complexes can be dispersed in one of the phases in microemulsion systems and easily recycled by phase separation for their further reuse in consecutive runs. Especially the three-phase region is of great interest because of its two excess phases allowing the separation of hydrophobic products and hydrophilic side-products in the same separation step. In the case of heterogeneous catalysts, the microemulsion systems act as superior solvents, but complex interactions between the surfactant, the catalyst and the reactants can have a huge impact on the catalytic activity. Also biocatalytic reactions can be performed in these media with better enzyme-substrate contact and higher enzyme stability. However, microemulsion systems are very complex and for successful implementation as reaction media in catalytic processes a lot of research is needed to adjust the reaction and separation steps.Acknowledgements
This work is part of the collaborative research centre InPROMPT (SFB/TRR 63). Financial support by the Deutsche Forschungsgemeinschaft (DFG) is gratefully acknowledged. We thank the group of Prof. R. Strey (University of Cologne) for providing the data of Fig. 7.References
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927 RSC.
- W. Leitner, Acc. Chem. Res., 2002, 35, 746 CrossRef CAS PubMed.
- P. G. Jessop, T. Ikariya and R. Noyori, Chem. Rev., 1999, 99, 475 CrossRef CAS PubMed.
- A. Behr, G. Henze and R. Schomäcker, Adv. Synth. Catal., 2006, 1485 CrossRef CAS.
- B. Cornils and W. A. Herrmann, Aqueous-Phase Organometallic Catalysis, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed.
- Microemulsions: Background, New Concepts, Applications, Perspectives, ed. R. Schomäcker, K. Holmberg and C. Stubenrauch, John Wiley & Sons, Chichester, 2009 Search PubMed.
- W. H. Wade, J. Morgan, R. S. Schechter, J. K. Jacobson and J. L. Salager, Soc. Pet. Eng. J., 1978, 18, 242–252 CrossRef CAS.
- T. Sottmann and R. Strey, Tenside, Surfactants, Deterg., 1998, 35, 35 Search PubMed.
- H. Nowothnick, A. Rost, T. Hamerla, R. Schomäcker, C. Müller and D. Vogt, Catal. Sci. Technol., 2013, 3, 600 CAS.
- W. C. Griffin, Off. Dig., Fed. Paint Varn. Prod. Clubs, 1956, 28, 446 CAS.
- B. Orlich, H. Berger, M. Lade and R. Schomäcker, Biotechnol. Bioeng., 2000, 70, 638 CrossRef CAS PubMed.
- T. Sottmann, M. Lade, M. Stolz and R. Schomäcker, Tenside, Surfactants, Deterg., 2002, 39, 1 Search PubMed.
- T. Sottmann and C. Stubenrauch, Microemulsions: Background, New Concepts, Applications, Perspectives, John Wiley & Sons, Chichester, 2009 Search PubMed.
- G. Carrea, Trends Biotechnol., 1984, 2, 102 CrossRef CAS.
- J. B. Macris, H. Stamatis and F. N. Kolisis, Appl. Microbiol. Biotechnol., 1996, 46, 521 CrossRef CAS.
- V. Papadimitriou, A. Xenakis, C. T. Cazianis and F. N. Kolisis, Colloid Polym. Sci., 1997, 275, 609 CAS.
- F. Jing, X. An and W. Shen, J. Mol. Catal. B: Enzym., 2003, 24–25, 53 CrossRef CAS.
- M. Sathishkumar, E. S. Jeong, S. E. Yun, S. P. Mun and J. F. Rusling, Enzyme Microb. Technol., 2008, 42, 252 CrossRef CAS.
- S. Prichanont, D. J. Leak and D. C. Stuckey, Enzyme Microb. Technol., 2000, 27, 134 CrossRef CAS PubMed.
- M. Sathishkumar, R. Jayabalan, S. P. Mun and S. E. Yun, Bioresour. Technol., 2010, 101, 7834 CrossRef CAS PubMed.
- K. M. Park, C. W. Kwon, S. J. Choi, Y.-H. Son, S. Lim, Y. Yoo and P.-S. Chang, J. Agric. Food Chem., 2013, 61, 9421 CrossRef CAS PubMed.
- M. Moniruzzaman, N. Kamiya, K. Nakashima and M. Goto, Green Chem., 2008, 10, 497 RSC.
- M. Moniruzzaman, N. Kamiya and M. Goto, Langmuir, 2009, 25, 977 CrossRef CAS PubMed.
- G.-P. Zhou, Y. Zhang, X.-R. Huang, C.-H. Shi, W.-F. Liu, Y.-Z. Li, Y.-B. Qu and P.-J. Gao, Colloids Surf., B, 2008, 66, 146 CrossRef CAS PubMed.
- J. D. Holmes, D. C. Steytler, G. D. Rees and B. H. Robinson, Langmuir, 1998, 14, 6371 CrossRef CAS.
- B. Orlich and R. Schomäcker, Chem. Eng. Technol., 1999, 22, 753 CrossRef CAS.
- M. Kahlweit, R. Strey, D. Haase, H. Kunieda, T. Schmeling, B. Faulhaber, M. Borkovec, H.-F. Eicke, G. Busse and F. Eggers, J. Colloid Interface Sci., 1987, 118, 436 CrossRef CAS.
- C.-N. Sun, M.-M. Shen, L.-L. Deng, J.-Q. Mo and B.-W. Zhou, Chin. Chem. Lett., 2014, 25, 621 CrossRef CAS.
- T. Wielpütz, T. Sottmann, R. Strey, F. Schmidt and A. Berkessel, Chem. – Eur. J., 2006, 12, 7565 CrossRef PubMed.
- J. B. F. N. Engberts, E. Fernández, L. García-Río and J. R. Leis, J. Org. Chem., 2006, 71, 4111 CrossRef CAS PubMed.
- J. S. Milano-Brusco, M. Schwarze, M. Djennad, H. Nowothnick and R. Schomäcker, Ind. Eng. Chem. Res., 2008, 47, 7586 CrossRef CAS.
- M. Li, H. Fu, M. Yang, H. Zheng, Y. He, H. Chen and X. Li, J. Mol. Catal. A: Chem., 2005, 235, 130 CrossRef CAS.
- M. Colladon, A. Scarso and G. Strukul, Adv. Synth. Catal., 2007, 349, 797 CrossRef CAS.
- H. Liu, Z. Fu, D. Yin, D. Yin and H. Liao, Catal. Commun., 2005, 6, 638 CrossRef CAS.
- J. S. Milano-Brusco and R. Schomäcker, Catal. Lett., 2009, 133, 273 CrossRef CAS.
- T. Hamerla, A. Rost, Y. Kasaka and R. Schomäcker, ChemCatChem, 2013, 5, 1854 CrossRef CAS.
- H. Nowothnick, J. Blum and R. Schomäcker, Angew. Chem., Int. Ed., 2011, 50, 1918 CrossRef CAS PubMed.
- A. R.-B. Baruch, D. Tsvelikhovsky, M. Schwarze, R. Schomäcker, M. Fanun and J. Blum, J. Mol. Catal. A: Chem., 2010, 323, 65 CrossRef CAS.
- I. Volovych, Y. Kasaka, M. Schwarze, Z. Nairoukh, J. Blum, M. Fanun, D. Avnir and R. Schomäcker, J. Mol. Catal. A: Chem., 2014, 393, 210 CrossRef CAS.
- M. V. Landau, Handbook of Heterogeneous Catalysis Vol. 2, Wiley-VCH, Weinheim, 2nd edn, 2008 Search PubMed.
- E. M. Rivera-Muñoz and R. Huirache-Acuña, Int. J. Mol. Sci., 2010, 11, 3069 CrossRef PubMed.
- C. Sener, T. Dogu and G. Dogu, Microporous Mesoporous Mater., 2006, 94, 89 CrossRef CAS.
- I. Volovych, M. Schwarze, T. Hamerla, J. Blum and R. Schomäcker, J. Mol. Catal. A: Chem., 2013, 366, 359 CrossRef CAS.
- R. Abu-Reziq, D. Avnir, I. Miloslavski, H. Schumann and J. Blum, J. Mol. Catal. A: Chem., 2002, 185, 179 CrossRef CAS.
- B. Wan, S. Liao and D. Yu, Appl. Catal., A, 1999, 183, 81 CrossRef CAS.
- K. Weidemaier, H. L. Tavernier and M. D. Fayer, J. Phys. Chem. B, 1997, 101, 9352 CrossRef CAS.
- D. Tsvelikhovsky and J. Blum, Eur. J. Org. Chem., 2008, 2008, 2417 CrossRef.
- J.-Z. Jiang and C. Cai, J. Dispers. Sci. Technol., 2008, 29, 453 CrossRef CAS.
- J.-Z. Jiang and C. Cai, Colloids Surf., A, 2006, 287, 212 CrossRef CAS.
- S. Mukhopadhyay, A. Yaghmur, M. Baidossi, B. Kundu and Y. Sasson, Org. Process Res. Dev., 2003, 7, 641 CrossRef CAS.
- T. Yosef, R. Schomäcker, M. Schwarze, M. Fanun, F. Gelman and J. Blum, J. Mol. Catal. A: Chem., 2011, 351, 46 CrossRef CAS.
- Z. Nairoukh and J. Blum, J. Mol. Catal. A: Chem., 2012, 358, 129 CrossRef CAS.
- K. Hamza and J. Blum, Eur. J. Org. Chem., 2007, 4706 CrossRef CAS.
- K. Hamza, H. Schumann and J. Blum, Eur. J. Org. Chem., 2009, 1502 CrossRef CAS.
- I. Volovych, M. Schwarze, Z. Nairoukh, J. Blum, M. Fanun and R. Schomäcker, Catal. Commun., 2014, 53, 1 CrossRef CAS.
- S. Dahoah, Z. Nairoukh, M. Fanun, M. Schwarze, R. Schomäcker and J. Blum, J. Mol. Catal. A: Chem., 2013, 380, 90 CrossRef CAS.
- S. Tripathi, R. Tyagi and B. Nandi, J. Dispersion Sci. Technol., 2013, 34, 1526 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |