A taxonomy for solar fuels generators
Adam C.
Nielander†
 ,
Matthew R.
Shaner†
,
Kimberly M.
Papadantonakis
,
Sonja A.
Francis
and
Nathan S.
Lewis
*
,
Matthew R.
Shaner†
,
Kimberly M.
Papadantonakis
,
Sonja A.
Francis
and
Nathan S.
Lewis
*
Beckman Institute, Kavli Nanoscience Institute, Joint Center for Artificial Photosynthesis, 210 Noyes Laboratory, 127-72 Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA. E-mail: nslewis@caltech.edu
First published on 19th September 2014
Abstract
A number of approaches to solar fuels generation are being developed, each of which has associated advantages and challenges. Many of these solar fuels generators are identified as “photoelectrochemical cells” even though these systems collectively operate based on a suite of fundamentally different physical principles. To facilitate appropriate comparisons between solar fuels generators, as well as to enable concise and consistent identification of the state-of-the-art for designs based on comparable operating principles, we have developed a taxonomy and nomenclature for solar fuels generators based on the source of the asymmetry that separates photogenerated electrons and holes. Three basic device types have been identified: photovoltaic cells, photoelectrochemical cells, and particulate/molecular photocatalysts. We outline the advantages and technological challenges associated with each type, and provide illustrative examples for each approach as well as for hybrid approaches.
Broader contextSolar fuels generators are devices that harness energy from sunlight to drive the synthesis of chemical fuels. A number of approaches to solar fuels generation are being pursued, many of which can be differentiated by the physical principles on which they are based. Herein, we propose a nomenclature and taxonomy based on three basic device types: photovoltaic cells, photoelectrochemical cells, and photoelectrosynthetic particulate/molecular photocatalysts. An understanding of the inherent operating principles and the advantages and challenges associated with each of these device types will facilitate clear comparisons between devices as well as help guide research efforts toward improving these devices and achieving the ultimate goal of sustainable fuel production. |
Introduction
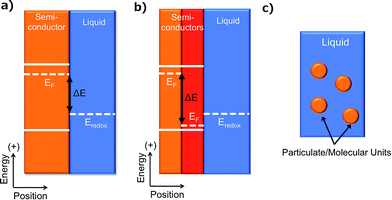
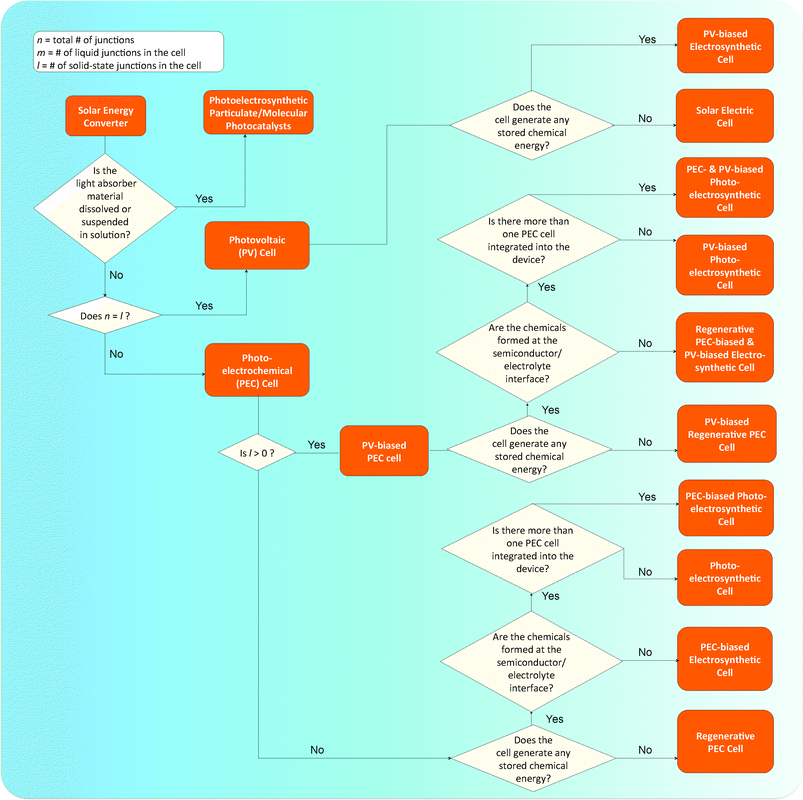
The development of an artificial photosynthetic process, whereby the energy from sunlight is captured and stored in the chemical bonds of a fuel, has been an active area of research for decades. This field of research, however, has recently undergone rapid expansion due to the promise of a scalable solar fuels generator that would provide a carbon-neutral source of energy capable of addressing concerns about the impact of carbon emissions on climate while providing a measure of environmental and energy security. Researchers have developed a diverse set of designs for solar fuels generators (Fig. 1), each of which presents unique challenges associated with the research and development required to obtain a fully operational system. Furthermore, the maturity of the technologies being implemented in the various designs varies widely. Despite these differences, a variety of solar fuels generators are often grouped together and denoted as “photoelectrochemical cells”. The focus of this Opinion is to establish a differentiating nomenclature and taxonomy for solar fuels generators that clearly identifies the principles underlying the designs. We hope that adoption of this taxonomy (Scheme 1) will bring clarity and precision to discussions and comparisons of solar fuels devices while facilitating concise and consistent identification of the research challenges and state-of-the-art for each type of system.All solar fuels generators require an electrical asymmetry to separate and transport photogenerated charge carriers vectorially.1–4 Without vectorial separation and transport, the charge carriers, and thus the chemical products, would have no net directionality and thus would undergo no net separation. Hence, deleterious recombination of charge carriers and/or a loss of chemical potential in the resulting fuel/oxidant mixture would result. The required vectorial separation can be effected by chemical and/or electrical potential gradients as well as by kinetic asymmetries at the interface between two unlike materials.1–4 We refer to this interface as a ‘junction’. We note that our usage of the term “junction” differentiates such an interface from an interface between two unlike materials that does not result in an asymmetry which produces a vectorial charge separation.5 We propose that the various solar-fuels generators can be differentiated at a fundamental level based on the underlying principles used to accomplish vectorial charge separation and by the method in which the separated charge is used to effect the synthesis of chemical fuels.
Photovoltaic cells
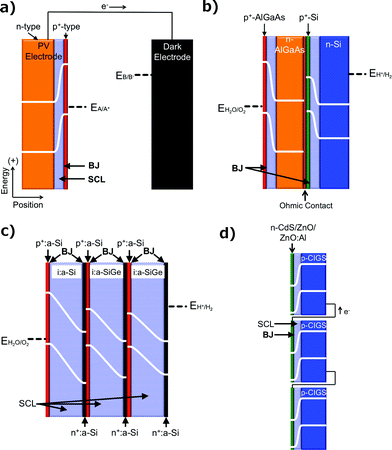
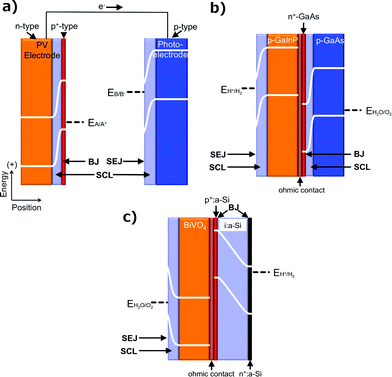
One fundamentally identifiable approach to charge separation in solar fuels devices is through the use of solid-state, or buried, junctions. Buried junctions are exclusively formed at the interface between two electronic conductors (as opposed to ionic conductors, vide infra) and are the basis for the operation of photovoltaic (PV) cells (Fig. 2a).6–8 In a device utilizing a buried junction, the photovoltage and photocurrent produced in the presence of illumination arise from charge separation mediated by a difference in electrochemical potential (Fig. 1b) and/or by a difference in charge-transfer kinetics between two unlike solids that are in mutual electrical contact. The photocurrent vs. voltage behavior of a PV cell is independent of the character of any solid/electrolyte interfaces in the system. Hence, measurements of the photocurrent–voltage characteristics of the PV cell can be performed independently of any electrochemical reaction, and can be used in concert with the current–voltage characteristics of various electrocatalysts to accurately predict the performance of a complete solar fuels generator that is based on a PV cell. PV cells will also produce the identical photocurrent–voltage behavior when both terminals of the device are contacted with wires connected to electrocatalysts vs. when all of the components of the structure (light absorbers and electrocatalysts) are integrated, contacted intimately, and immersed in an electrolyte solution. The operating principles of photovoltaic electrodes have been well documented for incorporation into full PV cells that either produce electricity or fuels.3PV cells that produce electricity are referred to as solar electric cells and are widely available commercially. PV cells that produce fuels are referred to as PV-biased electrosynthetic cells and can consist of any number of buried junctions arranged electrically in series with electrocatalysts submerged in an electrolyte. The electrocatalysts may or may not be in physical contact with the PV electrodes, but in all such systems the photovoltage generated by the structure is independent of the nature of the electrocatalyst/electrolyte interface. Examples of PV-biased electrosynthetic cells include AlGaAs/Si tandem structures,9 amorphous hydrogenated Si (a-Si:H) triple-junction structures,10–12 triple-junction structures based on CuInGaSe2 (Fig. 2b–d),13 and n-Si/SiOx/In-doped tin oxide (ITO) structures.14
The advantages of PV-based solar fuels generators are the high reported solar-to-fuels efficiencies and the independence of the power-producing junction with respect to the formal potential for the reactions of interest.15 The challenges associated with PV-based cells include achieving a cost advantage for a system with the functioning photovoltaic cell immersed in the electrolyte, relative to a system that utilizes a discrete photovoltaic cell in dry conditions wired to a discrete fuel-forming device, as well as finding catalyst/electrolyte interfaces that are transparent, conductive, and stable under operational, fuel-forming conditions.11,12,15–18 Thus, the key research needs involve the development of cost-competitive photovoltaic cells, the integration of components, discovery of materials, development of low-cost fabrication methods, and the stabilization of electrodes through the use of materials that act as transparent and conductive protecting layers.
Photoelectrochemical cells
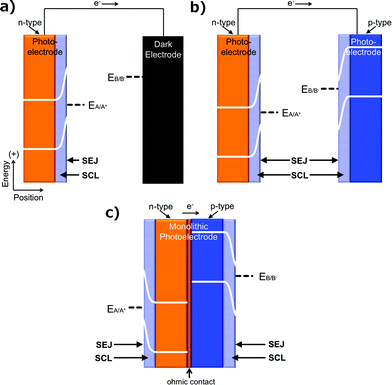
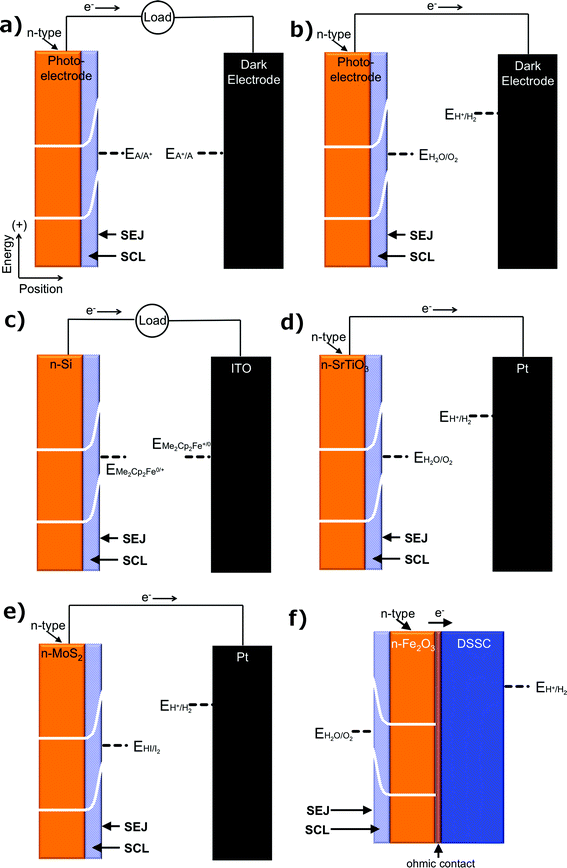
Another fundamentally identifiable approach to effect the separation of charge carriers is through the use of a solid/ionic-conductor junction. Devices utilizing solid/ionic-conductor junctions, also referred to as solid/electrolyte junctions, are called photoelectrochemical (PEC) cells (Fig. 3a). The solid in a PEC cell is commonly a semiconductor and may or may not have an attached photosensitizer. Other solids, including metals such as platinum and mercury, have also been observed to produce a photovoltage at a solid/electrolyte interface when the appropriate electrolyte is present.19,20 In a device utilizing a solid/electrolyte junction, the photovoltage and photocurrent produced in the presence of light arise from differences in the electrochemical potentials of the solid and the electrolyte as well as from asymmetries in the charge-transfer kinetics for electrons and holes across the junction. The operating principles of photoelectrodes have been well documented for incorporation into full PEC cells that either produce electricity or fuels.21 The properties of such photoelectrodes are determined routinely from a conventional three-electrode potentiostatic experiment using a half-cell configuration, with the understanding that the photoelectrode can be incorporated into an operational, two-electrode, full PEC cell. Unlike PV cells, for a given PEC-based solar fuels generator, photocurrent–voltage measurements cannot be made independently of the reaction of interest.PEC cells that utilize a semiconducting electrode can consist of one photoelectrode that has a semiconductor/electrolyte junction, in conjunction with a “dark” counter electrode (Fig. 3a); of two photoelectrodes, each with a semiconductor/electrolyte junction (Fig. 3b); or of a monolithically integrated combination of two photoelectrodes in a single structure that performs both the anodic and cathodic half-reactions simultaneously (Fig. 3c).
PEC cells that only produce electricity are referred to as regenerative photoelectrochemical cells (Fig. 4a), because the species that is reduced or oxidized at the working photoelectrode is regenerated at the counter electrode, ideally yielding zero net change in the composition of the solution.4,7,8,22 PEC cells that produce fuels at the semiconductor/electrolyte junction are referred to as photoelectrosynthetic cells (Fig. 4b).7,23,24 An example of a regenerative PEC cell is the n-Si/CH3OH-1,1′dimethylferrocene+/0/ITO cell (Fig. 4c, n-Si is the photoelectrode).25 Dye-sensitized solar cells (DSSCs) are also commonly operated as regenerative PEC cells.26 Examples of photoelectrosynthetic PEC cells include n-SrTiO3/NaOH(aq)/Pt cells for water splitting (Fig. 4d, SrTiO3 is the photoelectrode),27 n-MoS2 cells for the production of H2 and I2 from HI(aq) (Fig. 4e, n-MoS2 is the photoelectrode),28 and DSSC's including TiO2 photosensitized with a catalytic molecular [((PO3H2)2bpy)2Rua(4-Mebpy-4-bimpy)Rub(tpy)(OH2)]4+ unit for water splitting, as well as others.29–31
The product of coupling a regenerative PEC cell to metallic electrodes produces a PEC-biased electrosynthetic cell, whereas the product of coupling a regenerative PEC cell to a photoelectrosynthetic PEC cell is referred to as a PEC-biased photoelectrosynthetic cell. Photoelectrochemical cells, like photovoltaic cells, can be used to bias both PEC and PV cells to assist in fuel formation. An example of a PEC-biased photoelectrosynthetic cell is a DSSC placed electrically in series with an Fe2O3/electrolyte junction cell for water splitting (Fig. 4f).32 Here, the DSSC is a free-standing, two-terminal device whose photocurrent and photovoltage are independent of the fuel-forming reactions of interest, but which operates as a PEC nonetheless because the photocurrent and photovoltage are not independent of the solution at the interface of the two terminals of the DSSC itself.
The performance of photoelectrodes consisting of semiconductor/electrolyte junctions, in the absence of bulk semiconductor limitations, is determined by the energetics and kinetics of the semiconductor/electrolyte interface. The interfacial energetics determine the photovoltage through the difference between the formal potential of the fuel-forming reaction of interest and the electrochemical potential of the semiconductor,18,33,34 and also determine the driving force needed to produce a given current density. Commonly, an electrocatalyst is incorporated at the semiconductor/electrolyte interface to improve the interfacial charge-transfer kinetics; however, for the device to remain categorized as a PEC cell, the nature of the electrolyte must affect the performance of the cell.35 Examples of PEC cells with electrocatalysts incorporated at the semiconductor/electrolyte interface include H2-evolving photocathodes made from metal islands or thin metallic films on p-Si or p-InP photoelectrodes, because the work function of the metal, and thus the barrier height at the semiconductor surface, depends on the concentration of H2 in the electrolyte.36,37 Semiconductor/electrolyte junctions with ion-permeable, redox-active electrocatalysts would also be considered PEC cells because of the electrolyte-dependent behavior of the device.35 In addition, recent progress on stabilization schemes based on thin coatings on the surface of the semiconductor has produced examples of photoelectrodes in which the solution potential affects the photovoltage even though the photoelectrode is not in direct physical contact with the solution.38,39 Conversely, electrocatalysts deposited on semiconductors, such as CoPi on Fe2O3, are reported to convert what would otherwise be photoelectrosynthetic cells into photovoltaic electrosynthetic cells, by formation of a Schottky junction at the semiconductor/catalyst junction.40–42 Careful evaluation is often necessary to determine whether a device is a PV or PEC cell when electrocatalysts are present on the surface. Data including the current–voltage characteristics of the catalyst alone, the photocurrent–voltage characteristics of the semiconductor with and without the presence of the electrocatalyst, the photocurrent–voltage behavior of the semiconductor with and without electrocatalyst in contact with electrolytes of varying composition and electrochemical potential, and laser spectroscopic data on the electron–hole recombination mechanism in the presence or absence of electrocatalyst may be necessary to ascertain whether such a system is properly classified as a PV or PEC cell.
The principal advantages of PEC cells are their simplicity of fabrication and the finding that inexpensive polycrystalline semiconductor/electrolyte junctions can often perform nearly as well as their single crystalline counterparts.43–45 The challenges associated with PEC cells include obtaining a combination of materials that are operationally stable and also possess appropriate interfacial energetics and band gaps, as well as the development and integration of electrocatalysts into the semiconductor/electrolyte junction. Thus, the key research needs for solar fuels generators based on PEC cells involve the discovery and development of semiconducting materials that possess both the proper band gaps for effective sunlight absorption and well-positioned band energetics, and the development of methods for incorporating efficient electrocatalysts into semiconductor/electrolyte interfaces that are stable under operational, fuel-forming conditions.18,46–49
Photovoltaic-biased photoelectrochemical cells
The product of coupling a PV cell with a PEC cell, resulting in a cell that contains both a buried junction and a semiconductor/electrolyte junction, is a PV-biased PEC cell (Fig. 5a). In this hybrid approach, the advantages of both cells are combined through increased flexibility in materials availability. Like their parent cells, PV-biased PEC cells can produce electricity or fuel.PV-biased PEC cells that produce electricity are referred to as a PV-biased regenerative PEC cell. PV-biased PEC cells that produce fuels and that include at least one buried junction may fall into a number of categories, which are systematically named based on whether fuel formation occurs at a solid/electrolyte junction in the device and the presence or absence of additional two-terminal regenerative PEC cells. PV-biased photoelectrosynthetic cells are PV-biased PEC cells in which fuel formation occurs at the solid/electrolyte junction. PV-biased PEC cells that produce fuels that are formed away from a solid/electrolyte junction, but include at least one isolated regenerative PEC cell, are referred to as Regenerative PEC- and PV-biased electrosynthetic cells. PV-biased PEC cells that include at least one isolated regenerative PEC cell, but that produce fuels that are formed at a solid/electrolyte junction, are referred to as Regenerative PEC- and PV-biased photoelectrosynthetic cells. Examples of PV-biased PEC cells include the “Turner Cell”, a GaAs buried junction electrically in series and monolithically integrated with a p-GaInP2/electrolyte junction (Fig. 5b), as well as an a-Si:H PV cell electrically in series with a BiVO4/electrolyte junction (Fig. 5c) and the PEC cells often referred to as septum-based PEC cells.50–54
Photoelectrosynthetic particulate/molecular photocatalysts
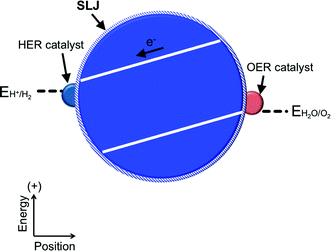
Both the buried junction and the semiconductor/electrolyte junction motifs can be employed when the semiconducting material is employed in a dispersed particulate form as opposed to a solid electrode (Fig. 6). In these particulate systems, the photovoltage and photocurrent that drive the interfacial electrochemical reactions in the presence of illumination are developed as a result of semiconductor/electrolyte and/or buried junctions in a single discrete particle unit that generally contains separate co-catalysts for each half-reaction.55 Although in theory one could distinguish between particles utilizing buried and semiconductor/electrolyte junctions in the same way as for the PEC and PV cells, in practice, these two types of systems are difficult to distinguish experimentally. A comparison of the photovoltage produced by a particle in solution with that measured across a particle removed from solution may be difficult or impossible to perform, due to the small size of the particles and the resulting effective absence of addressable electrodes. Indirect measurements of the photocurrent and/or photovoltage under varying conditions may be obtained by correlating changes in the amount of products formed by the light-driven reaction with various solution compositions, but accurate measurements of the products will be hindered by product crossover and incompatible catalysts. The particulate versions of PV and PEC cells, as well as the related photo-driven molecular photocatalysts wherein inorganic molecular compounds are dispersed in solution, share many of the same research challenges as their parent categories, with the added challenge of developing methods to physically separate the products of the fuel-forming reactions. The term cell does not apply to particulate schemes that employ neither addressable electrodes nor a built-in means to enforce the separation of products. For these reasons, we consider all three of these strategies to comprise members of the general category of photoelectrosynthetic particulate/molecular photocatalysts.7,56,57An example of photoelectrosynthetic particulate photocatalysts are CdS particles in contact with TiO2 particles, with an electrical asymmetry at the CdS/TiO2 interface.57–59 Other examples include a NiO–SrTiO3 photocatalyst capable of concomitantly evolving H2 and O2, as well as a number of metal nitrides, oxides, and oxynitrides (e.g. ZrO2, TaON, Ta3N5, WO3).57 Similarly, the performance of a photoelectrosynthetic molecular photocatalyst is based either on monomolecular photochemical processes or on coupled photoelectrochemical–photochemical or photochemical-dark reactions in an individual molecular unit. Examples of photoelectrosynthetic molecular cells include light-driven water splitting by UV irradiation of aqueous Ce(III)/Ce(IV) solutions;60 the use of molecular triads or tetrads coupled to nanoparticulate or molecular electrocatalysts for fuel production;61 the coupling of molecular catalysts to photoactive proteins;62,63 and related systems.64,65
The principal advantages of particulate/molecular photocatalysts are the simplicity of the photocatalysts relative to other approaches and the associated low predicted system cost, with a recent technoeconomic analysis suggesting that systems based on particulate/molecular photocatalysts could be significantly less expensive than electrode-based systems when deployed at scale.15 The challenges facing development of systems from photoelectrosynthetic particulate/molecular photocatalysts involve stabilizing all of the components; addressing safety concerns arising from the production of explosive mixtures of stoichiometric fuel products; and controlling undesired recombination processes to realize high steady-state quantum yields for net fuel production. Specific undesired processes include photogenerated electrons reducing key surface-bound intermediates, intermediates in solution, or products of the oxidation of water to O2, as well as photogenerated holes participating in analogous oxidation reactions, and the spontaneous recombination of the fuels facilitated by contact with the co-catalysts at any location in the system.
Discussion
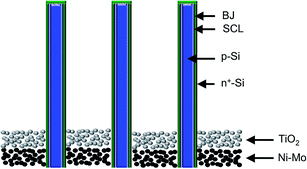
Both PV and PEC cells can be structured with multiple junctions to optimize the theoretical maximum efficiency for a given fuel-forming reaction.66 Single-junction cells are best suited for fuel-forming reactions that require operating voltages near or below the ∼1 V maximum power point of the single-junction devices that have the highest reported energy-conversion efficiency.67 Fuel-forming reactions that require larger voltages also require more junctions to better utilize the solar spectrum, with the optimal number of junctions being dependent on the voltage required for the operating current density. Hence, the maximum realizable efficiencies for water splitting are generally obtained with a tandem-absorber cell, where two light absorbers with appropriately tuned band gaps are arranged in series with respect to the incident light.9,53 Additional junctions can increase the efficiency of solar devices when the semiconductors have carefully selected band gaps.68 Triple-junction cells utilizing a single semiconductor or two semiconductors have also been used to effect solar-driven water splitting when related double junction devices were unable to generate sufficient voltage.11,12,51 When the same semiconductor is used to form multiple junctions, the cells suffer from a loss of current to achieve the necessary voltage for water splitting.Advanced structuring of PV- and PEC-based solar fuels generators can offer additional efficiency gains for systems, including those for which all of the components are in contact with the electrolyte. One example of advanced structuring is an array of p-Si microwires that have radial n+-doped emitter regions, with an electrocatalyst placed in specific physical locations between or along the surfaces of the microwires (Fig. 7).69 Some ambiguity exists regarding the classification of such a system. The mechanism of charge separation is a buried junction, and thus the device falls into the category of photovoltaic cells. However, although in concept a conformal electrical contact could be made to the microwires, with the resulting electrical current then passed to another identically microstructured conductive electrode that possessed the spatial distribution, loading, and resulting activity of the electrocatalyst in the integrated structure, separation of the integrated system into essentially identically functioning discrete components would be difficult to accomplish in practice. Because the performance of the device critically depends on the details of, and the presence of, the absorber/electrolyte junction, which acts in this case in a synergistic fashion with respect to one or more other components of the integrated system, designation of the device as a photoelectrochemical cell might seem reasonable. Furthermore, if the microwires are removed from the substrate and embedded in an immobilizing membrane, they may be deemed similar to a photoelectrosynthetic particulate photocatalyst. In this case, however, the uniform particle orientation and built-in barrier for product separation, would produce a photoelectrosynthetic particulate cell rather than a photoelectrosynthetic particulate photocatalyst. This discussion serves to emphasize that while some devices easily fall into a single taxonomic classification and therefore are subject to the research challenges associated with that classification, other devices may have characteristics of multiple classifications with some or all of the related challenges, advantages or disadvantages.
Conclusions
Although researchers have developed diverse designs for solar fuels generators based on a diverse set of underlying principles, solar fuels generators are often grouped together and denoted as “photoelectrochemical cells”. The purpose of this Opinion is not to favor, or establish a bias or preference towards, any specific design or approach. The different performance/cost/function trade-offs associated with each approach ultimately will determine which of these distinct technological approaches to the development of solar fuels generators will prove viable in the marketplace. Instead, we have described a taxonomy for solar fuels generators that is based on the operating principles underlying the designs, to bring clarity and precision to discussions of research in the field of artificial photosynthesis while facilitating concise and consistent identification of the research challenges and state-of-the-art for each type of system.Acknowledgements
ACN acknowledges the NSF, Grant CHE-1214152, and the National Science Foundation Graduate Research Fellowship for support. KMP acknowledges support from the DOE, Grant DE-FG02-03ER15483. MRS acknowledges the Resnick Sustainability Institute for a graduate fellowship. This material is based upon work performed by the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, supported through the Office of Science of the U.S. Department of Energy under Award Number DE-SC0004993.References
- A. J. Bard and M. A. Fox, Acc. Chem. Res., 1995, 28, 141–145 CrossRef CAS.
- S. J. Fonash, Solar Cell Device Physics, Elsevier Inc., Amsterdam, 2nd edn., 2010 Search PubMed.
- N. Serpone and E. Pelizzetti, Photocatalysis: Fundamentals and Applications, John Wiley & Sons, Inc., Hoboken, NJ, 1989 Search PubMed.
- M. X. Tan, P. E. Laibinis, S. T. Nguyen, J. M. Kesselman, C. E. Stanton and N. S. Lewis, in Prog. Inorg. Chem., ed. K. D. Karlin, John Wiley & Sons, Inc., 1994 Search PubMed.
- Within this Opinion, we specifically denote as ‘junctions’ those interfaces whose electrical asymmetry leads to the development of a photovoltage or photocurrent upon illumination. In other contexts, the interface between two materials that do not generate a photocurrent or photovoltage under illumination, such as ohmic contacts, may also be referred to as a ‘junction’.
- The term ‘photovoltaic’ has been used in a number of contexts, including to denote any device that generates a measurable photocurrent or photovoltage upon illumination, including specifically photoelectrochemical cells (ref. 7 and 8). This usage is defendable because both PV and PEC cells exhibit a photovolatic effect as understood by the strict definition of the term ‘photovoltaic’. However, as alluded to in ref. 8, photoelectrochemical cells are commonly differentiated from cells that utilize a junction between two electronic conductors, to emphasis the different underlying principles in the two different types of systems. The term ‘photovoltaic’ is therefore used herein to refer to only those devices that include a junction between electronic conductors.
- A. J. Bard, R. Memming and B. Miller, Pure Appl. Chem., 1991, 63, 569–596 CrossRef.
- Semiconductor Electrochemistry and Photoelectrochemistry, ed. S. Licht, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- S. Licht, B. Wang, S. Mukerji, T. Soga, M. Umeno and H. Tributsch, J. Phys. Chem. B, 2000, 104, 8920–8924 CrossRef CAS.
- W. Ayers, US Pat., 4,466,869, 1984.
- S. Y. Reece, J. A. Hamel, K. Sung, T. D. Jarvi, A. J. Esswein, J. J. H. Pijpers and D. G. Nocera, Science, 2011, 334, 645–648 CrossRef CAS PubMed.
- R. E. Rocheleau, E. L. Miller and A. Misra, Energy Fuels, 1998, 12, 3–10 CrossRef CAS.
- T. J. Jacobsson, V. Fjallstrom, M. Sahlberg, M. Edoff and T. Edvinsson, Energy Environ. Sci., 2013, 6, 3676–3683 CAS.
- G. Hodes, L. Thompson, J. DuBow and K. Rajeshwar, J. Am. Chem. Soc., 1983, 105, 324–330 CrossRef CAS.
- B. D. James, G. N. Baum, J. Perez and K. N. Baum, Technoeconomic Analysis of Photoelectrochemical (PEC) Hydrogen Production, 2009 Search PubMed.
- A. J. Bard and M. S. Wrighton, J. Electrochem. Soc., 1977, 124, 1706–1710 CrossRef CAS PubMed.
- R. Noufi, A. J. Frank and A. J. Nozik, J. Am. Chem. Soc., 1981, 103, 1849–1850 CrossRef CAS.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- M. Heyrovsky and R. G. W. Norrish, Nature, 1963, 200, 880–881 CrossRef CAS.
- W. J. Albery, Acc. Chem. Res., 1982, 15, 142–148 CrossRef CAS.
- B. Parkinson, Acc. Chem. Res., 1984, 17, 431–437 CrossRef CAS.
- In accordance with ref. 4 and 8, we have chosen to use the term ‘regenerative photoelectrochemical cell’ to denote a cell that utilizes a solid/electrolyte junction to generate electricity, with no net chemical conversion, under illumination. This usage is in contrast to that of ref. 7, which refers to these systems as ‘photovoltaic’ devices. As described above, we have reserved the term ‘photovoltaic’ to denote a system that utilizes a junction between two electronic conductors, in accord with broad usage and with the definition in many dictionaries of the term “photovoltaic”.
- In accordance with ref. 24, we have used the term ‘photoelectrosynthetic’ to encompass all devices in which a net endergonic chemical conversion takes place. We recognize that the term ‘photoelectrolytic’ has been used (e.g., ref. 7) in this context to refer to devices in which a net chemical conversion occurs with ΔG > 0, and the term ‘photocatalytic’ has been used to refer to devices with ΔG < 0. We chose our nomenclature to avoid confusion with the common usage of the term ‘photocatalyst’.
- Photochemical conversion and storage of solar energy, ed. J. Connolly, Academic Press, New York, 1981 Search PubMed.
- C. M. Gronet, N. S. Lewis, G. Cogan and J. Gibbons, Proc. Natl. Acad. Sci. U. S. A., 1983, 80, 1152–1156 CrossRef CAS.
- M. Grätzel, J. Photochem. Photobiol., C, 2003, 4, 145–153 CrossRef.
- M. S. Wrighton, A. B. Ellis, P. T. Wolczanski, D. L. Morse, H. B. Abrahamson and D. S. Ginley, J. Am. Chem. Soc., 1976, 98, 2774–2779 CrossRef CAS.
- C. Levy-Clement, A. Heller, W. A. Bonner and B. A. Parkinson, J. Electrochem. Soc., 1982, 129, 1701–1705 CrossRef CAS PubMed.
- L. Alibabaei, M. K. Brennaman, M. R. Norris, B. Kalanyan, W. Song, M. D. Losego, J. J. Concepcion, R. A. Binstead, G. N. Parsons and T. J. Meyer, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 20008–20013 CrossRef CAS PubMed.
- J. R. Swierk and T. E. Mallouk, Chem. Soc. Rev., 2013, 42, 2357–2387 RSC.
- K. J. Young, L. A. Martini, R. L. Milot, R. C. Snoeberger III, V. S. Batista, C. A. Schmuttenmaer, R. H. Crabtree and G. W. Brudvig, Coord. Chem. Rev., 2012, 256, 2503–2520 CrossRef CAS PubMed.
- J. Brillet, J.-H. Yum, M. Cornuz, T. Hisatomi, R. Solarska, J. Augustynski, M. Graetzel and K. Sivula, Nat. Photonics, 2012, 6, 824–828 CrossRef CAS.
- R. van de Krol, Y. Liang and J. Schoonman, J. Mater. Chem., 2008, 18, 2311–2320 RSC.
- H.-J. Lewerenz and L. Peter, Photoelectrochemical Water Splitting, The Royal Society of Chemistry, 2013 Search PubMed.
- F. Lin and S. W. Boettcher, Nat. Mater., 2014, 13, 81–86 CrossRef CAS PubMed.
- E. Aharon-Shalom and A. Heller, J. Electrochem. Soc., 1982, 129, 2865–2866 CrossRef CAS PubMed.
- R. N. Dominey, N. S. Lewis, J. A. Bruce, D. C. Bookbinder and M. S. Wrighton, J. Am. Chem. Soc., 1982, 104, 467–482 CrossRef CAS.
- N. C. Strandwitz, D. J. Comstock, R. L. Grimm, A. C. Nichols-Nielander, J. Elam and N. S. Lewis, J. Phys. Chem. C, 2013, 117, 4931–4936 CAS.
- A. C. Nielander, M. J. Bierman, N. Petrone, N. C. Strandwitz, S. Ardo, F. Yang, J. Hone and N. S. Lewis, J. Am. Chem. Soc., 2013, 135, 17246–17249 CrossRef CAS PubMed.
- M. Barroso, A. J. Cowan, S. R. Pendlebury, M. Grätzel, D. R. Klug and J. R. Durrant, J. Am. Chem. Soc., 2011, 133, 14868–14871 CrossRef CAS PubMed.
- M. Barroso, C. A. Mesa, S. R. Pendlebury, A. J. Cowan, T. Hisatomi, K. Sivula, M. Grätzel, D. R. Klug and J. R. Durrant, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15640–15645 CrossRef CAS PubMed.
- M. Barroso, S. R. Pendlebury, A. J. Cowan and J. R. Durrant, Chem. Sci., 2013, 4, 2724–2734 RSC.
- A. Heller, in Photoeffects at Semiconductor-Electrolyte Interfaces, American Chemical Society, 1981, vol. 146, ch. 4, pp. 57–77 Search PubMed.
- A. Heller, Acc. Chem. Res., 1981, 14, 154–162 CrossRef CAS.
- S. R. Lunt, L. G. Casagrande, B. J. Tufts and N. S. Lewis, J. Phys. Chem., 1988, 92, 5766–5770 CrossRef CAS.
- Basic Research Needs for Solar Energy Utilization, US Department of Energy, ed. N. S. Lewis and G. Crabtree, Office of Science, Washington, DC, 2005 Search PubMed.
- M. F. Weber and M. J. Dignam, J. Electrochem. Soc., 1984, 131, 1258–1265 CrossRef CAS PubMed.
- S. Hu, C. Xiang, S. Haussener, A. D. Berger and N. S. Lewis, Energy Environ. Sci., 2013, 6, 2984–2993 CAS.
- M. F. Weber and M. J. Dignam, Int. J. Hydrogen Energy, 1986, 11, 225–232 CrossRef CAS.
- Although the Turner Cell (ref. 53) is classified as a PV-biased PEC cell, the surface of the p-GaInP2 at the p-GaInP2/3M H2SO4(aq) interface is covered in a thin layer of Pt. This suggests the possibility that the I–V characteristics of this device may be independent of the nature of the electrolyte at the p-GaInP2/3M H2SO4(aq) interface, which would then result in the appropriate designation of this cell as a PV-biased electrosynthetic cell. However, as noted in ref. 35, the I–V behavior of electrodes consisting of thin layers of Pt on semiconductors is often dependent on the pH of the electrolyte. Thus, based on the available data, is not clear whether the Turner Cell is more appropriately designated as PV-biased photoelectrosynthetic cell or as a PV-biased electrosynthetic cell. A similar argument is relavent for the devices discussed in ref. 51.
- F. F. Abdi, L. Han, A. H. M. Smets, M. Zeman, B. Dam and R. van de Krol, Nat. Commun., 2013, 4, 2915 Search PubMed.
- A. J. Bard and T. E. Mallouk, J. Phys. Chem., 1993, 97, 7127–7128 CrossRef CAS.
- O. Khaselev and J. A. Turner, Science, 1998, 280, 425–427 CrossRef CAS.
- S. C. Kondapaneni, D. Singh and O. N. Srivastava, J. Phys. Chem., 1992, 96, 8094–8099 CrossRef CAS.
- K. Domen, S. Naito, M. Soma, T. Onishi and K. Tamaru, J. Chem. Soc., Chem. Commun., 1980, 543–544, 10.1039/c39800000543.
- We have used the term ‘photocatalyst’ to describe these systems, in accordance with common parlance and in accord with the usage of ref. 57. We recognize that ‘photocatalysis’ is often, however, used to refer specifically to the light-driven catalysis of a reaction for which ΔG < 0 (ref. 7).
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014 10.1039/c3cs60378d.
- K. R. Gopidas, M. Bohorquez and P. V. Kamat, J. Phys. Chem., 1990, 94, 6435–6440 CrossRef CAS.
- N. Serpone, E. Borgarello and M. Gratzel, J. Chem. Soc., Chem. Commun., 1984, 342–344, 10.1039/c39840000342.
- J. Weiss and D. Porret, Nature, 1937, 139, 1019–1020 CrossRef CAS.
- D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2009, 42, 1890–1898 CrossRef CAS PubMed.
- S. C. Silver, J. Niklas, P. Du, O. G. Poluektov, D. M. Tiede and L. M. Utschig, J. Am. Chem. Soc., 2013, 135, 13246–13249 CrossRef CAS PubMed.
- L. M. Utschig, S. C. Silver, K. L. Mulfort and D. M. Tiede, J. Am. Chem. Soc., 2011, 133, 16334–16337 CrossRef CAS PubMed.
- P. Du, J. Schneider, G. Luo, W. W. Brennessel and R. Eisenberg, Inorg. Chem., 2009, 48, 4952–4962 CrossRef CAS PubMed.
- B. D. Yuhas, A. L. Smeigh, A. P. Douvalis, M. R. Wasielewski and M. G. Kanatzidis, J. Am. Chem. Soc., 2012, 134, 10353–10356 CrossRef CAS PubMed.
- J. R. Bolton, S. J. Strickler and J. S. Connolly, Nature, 1985, 316, 495–500 CrossRef CAS.
- B. M. Kayes, H. Nie, R. Twist, S. G. Spruytte, F. Reinhardt, I. C. Kizilyalli and G. S. Higashi, Presented in part at the 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, 2011 Search PubMed.
- C. H. Henry, J. Appl. Phys., 1980, 51, 4494–4500 CrossRef CAS PubMed.
- E. L. Warren, J. R. McKone, H. A. Atwater, H. B. Gray and N. S. Lewis, Energy Environ. Sci., 2012, 5, 9653–9661 CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |